Composites of Rigid Polyurethane Foams Reinforced with POSS
Abstract
:1. Introduction
2. Experimental
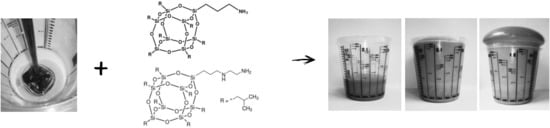
2.1. Materials and Manufacturing

2.2. Characterization Techniques
3. Results and Discussion
3.1. Characterization of POSS-Modified Polyol Premixes
3.2. Apparent Density of RPUFs
3.3. Foaming Kinetic
3.4. Cell. Structure of RPUFs
3.5. Compressive Strength of RPUFs
3.6. Impact Strength of RPUFs
3.7. Flexural Strength of RPUFs
3.8. Dynamic Mechanical Analysis (DMA)
3.9. Thermogravimetric Analysis (TGA)
3.10. Contact Angle and Water Absorption
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tan, S.; Abraham, T.; Ference, D.; Macosko, C.W. Rigid polyurethane foams from a soybean oil-based Polyol. Polymer 2011, 52, 2840–2846. [Google Scholar] [CrossRef]
- Nikje, M.M.A.; Noruzian, M.; Moghaddam, S.T. Investigation of Fe3O4/AEAP supermagnetic nanoparticles on the morphological, thermal and magnetite behavior of polyurethane rigid foam nanocomposites. Polimery 2015, 60, 26–32. [Google Scholar] [CrossRef]
- Xie, H.; Yang, W.; Yuen, A.C.Y.; Xie, C.; Xie, J.; Lu, H.; Yeoh, G.H. Study on flame retarded flexible polyurethane foam/alumina aerogel composites with improved fire safety. Chem. Eng. J. 2017, 311, 310–317. [Google Scholar] [CrossRef]
- Yang, C.; Fischer, L.; Maranda, S.; Worlitschek, J. Rigid polyurethane foams incorporated with phase change materials: A state-of-the-art review and future research pathways. Energy Build. 2015, 87, 25–36. [Google Scholar] [CrossRef]
- Zieleniewska, M.; Leszczyński, M.K.; Kurańska, M.; Prociak, A.; Szczepkowski, L.; Krzyżowska, M.; Ryszkowska, J. Preparation and characterisation of rigid polyurethane foams using a rapeseed oil-based polyol. Ind. Crop. Prod. 2015, 74, 887–897. [Google Scholar] [CrossRef]
- Yan, D.-X.; Xu, L.; Chen, C.; Tang, J.; Ji, X.; Li, Z. Enhanced mechanical and thermal properties of rigid polyurethane foam composites containing graphene nanosheets and carbon nanotubes. Polym. Int. 2012, 61, 1107–1114. [Google Scholar] [CrossRef]
- Widya, T.; Macosko, C.W. Nanoclay-Modified Rigid Polyurethane Foam. J. Macromol. Sci. 2005, 44, 897–908. [Google Scholar] [CrossRef]
- Madaleno, L.; Pyrz, R.; Crosky, A.; Jensen, L.R.; Rauhe, J.C.M.; Dolomanova, V.; de Barros Timmons, A.M.M.V.; Pinto, J.J.C.; Norman, J. Processing and characterization of polyurethane nanocomposite foam reinforced with montmorillonite–carbon nanotube hybrids. Composites 2013, 44, 1–7. [Google Scholar] [CrossRef]
- Thirumal, M.; Khastgir, D.; Singha, N.K.; Manjunath, B.S.; Naik, Y.P. Effect of a Nanoclay on the Mechanical, Thermal and Flame Retardant Properties of Rigid Polyurethane Foam. J. Macromol. Sci. 2009, 46, 704–712. [Google Scholar] [CrossRef]
- Modesti, M.; Lorenzetti, A.; Besco, S. Influence of nanofillers on thermal insulating properties of polyurethane nanocomposites foams. Polym. Eng. Sci. 2007, 47, 1351–1358. [Google Scholar] [CrossRef]
- Dolomanova, V.; Rauhe, J.C.M.; Jensen, L.R.; Pyrz, R.; Timmons, A.B. Mechanical properties and morphology of nano-reinforced rigid PU foam. J. Cell. Plast. 2011, 47, 81–93. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, M.; Zhou, Y.; Hu, L. The study of mechanical behavior and flame retardancy of castor oil phosphate-based rigid polyurethane foam composites containing expanded graphite and triethyl phosphate. Poly. Degrad. Stab. 2013, 98, 2784–2794. [Google Scholar] [CrossRef]
- Cao, X.; Lee, L.J.; Widya, T.; Macosko, C. Polyurethane/clay nanocomposites foams: Processing, structure and properties. Polymer 2005, 46, 775–783. [Google Scholar] [CrossRef]
- Cai, D.; Jin, J.; Yusoh, K.; Rafiq, R.; Song, M. High performance polyurethane/functionalized graphene nanocomposites with improved mechanical and thermal properties. Compos. Sci. Technol. 2012, 72, 702–707. [Google Scholar] [CrossRef]
- Pauzi, N.N.P.N.; Majid, R.A.; Dzulkifli, M.H.; Yahya, M.Y. Development of rigid bio-based polyurethane foam reinforced with nanoclay. Composites 2014, 67, 521–526. [Google Scholar] [CrossRef]
- Uthaman, N.; Majeed, A. Pandurangan Impact Modification of Polyoxymethylene (POM). e-Polymers 2006, 6, 1–9. [Google Scholar] [CrossRef]
- Liu, H.; Zheng, S. Polyurethane Networks Nanoreinforced by Polyhedral Oligomeric Silsesquioxane. Macromol. Rapid Commun. 2005, 26, 196–200. [Google Scholar] [CrossRef]
- Hernandez, R.; Weksler, J.; Padsalgikar, A.; Runt, J. Microstructural Organization of Three-Phase Polydimethylsiloxane-Based Segmented Polyurethanes. Macromolecules 2007, 40, 5441–5449. [Google Scholar] [CrossRef]
- Raftopoulos, K.N.; Koutsoumpis, S.; Jancia, M.; Lewicki, J.P.; Kyriakos, K.; Mason, H.E.; Harley, S.J.; Hebda, E.; Papadakis, C.M.; Pielichowski, K. Reduced Phase Separation and Slowing of Dynamics in Polyurethanes with Three-Dimensional POSS-Based Cross-Linking Moieties. Macromolecules 2015, 48, 1429–1441. [Google Scholar] [CrossRef]
- Paul, D.; Robeson, L. Polymer nanotechnology: Nanocomposites. Polymer 2008, 49, 3187–3204. [Google Scholar] [CrossRef]
- Song, X.Y.; Geng, H.P.; Li, Q.F. The synthesis and characterization of polystyrene/magnetic polyhedral oligomeric silsesquioxane (POSS) nanocomposites. Polymer 2006, 47, 3049–3056. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Huang, J.-M.; Kuo, S.-W.; Lu, J.-S.; Chang, F.-C. Polyimide and polyhedral oligomeric silsesquioxane nanocomposites for low-dielectric applications. Polymer 2005, 46, 173–181. [Google Scholar] [CrossRef]
- Liu, Y.; Tseng, M.; Fangchiang, M. Polymerization and nanocomposites properties of multifunctional methylmethacrylate POSS. J. Polym. Sci. 2008, 46, 5157–5166. [Google Scholar] [CrossRef]
- Rashid, E.S.A.; Ariffin, K.; Kooi, C.C.; Akil, H.M. Preparation and properties of POSS/epoxy composites for electronic packaging applications. Mater. Des. 2009, 30, 1–8. [Google Scholar] [CrossRef]
- Liu, L.; Tian, M.; Zhang, W.; Zhang, L.; Mark, J.E. Crystallization and morphology study of polyhedral oligomeric silsesquioxane (POSS)/polysiloxane elastomer composites prepared by melt blending. Polymer 2007, 48, 3201–3212. [Google Scholar] [CrossRef]
- Pellice, S.A.; Fasce, D.P.; Williams, R.J.J.; Pellice, S. Properties of epoxy networks derived from the reaction of diglycidyl ether of bisphenol A with polyhedral oligomeric silsesquioxanes bearing OH–functionalized organic substituents. J. Polym. Sci. 2003, 41, 1451–1461. [Google Scholar] [CrossRef]
- Matějka, L.; Strachota, A.; Pleštil, J.; Whelan, P.; Steinhart, M.; Šlouf, M. Epoxy networks reinforced with polyhedral oligomeric silsesquioxanes (POSS). Structure and morphology. Macromolecules 2004, 37, 9449–9456. [Google Scholar] [CrossRef]
- Prządka, D.; Jęczalik, J.; Andrzejewska, E.; Marciniec, B.; Dutkiewicz, M.; Szłapka, M.; Jȩczalik, J. Novel hybrid polyurethane/POSS materials via bulk polymerization. React. Funct. Polym. 2013, 73, 114–121. [Google Scholar] [CrossRef]
- Raftopoulos, K.N.; Janowski, B.; Apekis, L.; Pissis, P.; Pielichowski, K. Direct and indirect effects of POSS on the molecular mobility of polyurethanes with varying segment Mw. Polymer 2013, 54, 2745–2754. [Google Scholar] [CrossRef]
- Seifalian, A.; Ghanbari, H. Cardiovascular application of polyhedral oligomeric silsesquioxane nanomaterials: A glimpse into prospective horizons. Int. J. Nanomed. 2011, 6, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.; Soler-Illia, G.J.D.A.A.; Ribot, F.; Lalot, T.; Mayer, C.R.; Cabuil, V. Designed Hybrid Organic–Inorganic Nanocomposites from Functional Nanobuilding Blocks. Chem. Mater. 2001, 13, 3061–3083. [Google Scholar] [CrossRef]
- Hebda, E.; Ozimek, J.; Raftopoulos, K.N.; Michałowski, S.; Pielichowski, J.; Jancia, M.; Pielichowski, K. Synthesis and morphology of rigid polyurethane foams with POSS as pendant groups or chemical crosslinks. Polym. Adv. Technol. 2015, 26, 932–940. [Google Scholar] [CrossRef]
- Michałowski, S.; Pielichowski, K. 1,2-Propanediolizobutyl POSS as a co-flame retardant for rigid polyurethane foams. J. Therm. Anal. Calorim. 2018, 134, 1351–1358. [Google Scholar] [CrossRef]
- Michałowski, S.; Hebda, E.; Pielichowski, K. Thermal stability and flammability of polyurethane foams chemically reinforced with POSS. J. Therm. Anal. Calorim. 2017, 130, 155–163. [Google Scholar] [CrossRef]
- Mráz, J.; Simek, P.; Chvalová, D.; Nohová, H.; Šmigolová, P. Studies on the methyl isocyanate adducts with globin. Chem. Biol. Interact. 2004, 148, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Schwetlick, K.; Noack, R.; Stebner, F. Three fundamental mechanisms of base-catalysed reactions of isocyanates with hydrogen-acidic compounds. J. Chem. Soc. Perkin Trans. 2 1994, 2, 599. [Google Scholar] [CrossRef]
- Arnold, R.G.; Nelson, J.A.; Verbanc, J.J. Recent Advances in Isocyanate Chemistry. Chem. Rev. 1957, 57, 47–76. [Google Scholar] [CrossRef]
- Guo, C.; Zhou, L.; Lv, J. Effects of Expandable Graphite and Modified Ammonium Polyphosphate on the Flame-Retardant and Mechanical Properties of Wood Flour-Polypropylene Composites. Polym. Polym. Compos. 2013, 21, 449–456. [Google Scholar] [CrossRef]
- Kairytė, A.; Vaitkus, S.; Vėjelis, S.; Girskas, G.; Balčiūnas, G. Rapeseed-based polyols and paper production waste sludge in polyurethane foam: Physical properties and their prediction models. Ind. Crop Prod. 2018, 112, 119–129. [Google Scholar] [CrossRef]
- Amin, M.; Najwa, K. Cellulose Nanocrystals Reinforced Thermoplastic Polyurethane Nanocomposites. Ph.D. Thesis, Australian Institute for Bioengineering and Nanotechnology, The University of Queensland, Brisbane, Australia, 2016. [Google Scholar] [CrossRef]
- Michalowski, S.; Cabulis, U.; Kirpluks, M.; Prociak, A.; Kuranska, M. Microcellulose as a natural filler in polyurethane foams based on the biopolyol from rapeseed oil. Polimery 2016, 61, 625–632. [Google Scholar]
- Malewska, E.; Prociak, A. The effect of nanosilica filler on the foaming process and properties of flexible polyurethane foams obtained with rapeseed oil-based polyol. Polimery 2015, 60, 472–479. [Google Scholar] [CrossRef]
- Farkas, A.; Strohm, P.F. Mechanism of Amine-Catalyzed Reaction of Isocyanates with Hydroxyl Compounds. Ind. Eng. Chem. Fund. 1965, 4, 32–38. [Google Scholar] [CrossRef]
- Silva, A.L.; Bordado, J.C. Recent Developments in Polyurethane Catalysis: Catalytic Mechanisms Review. Catal. Rev. 2004, 46, 31–51. [Google Scholar] [CrossRef]
- Ni, H.; Nash, H.A.; Worden, J.G.; Soucek, M.D. Effect of catalysts on the reaction of an aliphatic isocyanate and water. J. Polym. Sci. 2002, 40, 1677–1688. [Google Scholar] [CrossRef]
- Kairytė, A.; Kizinievič, O.; Kizinievič, V.; Kremensas, A. Synthesis of biomass-derived bottom waste ash based rigid biopolyurethane composite foams: Rheological behaviour, structure and performance characteristics. Composites 2019, 117, 193–201. [Google Scholar] [CrossRef]
- Gama, N.V.; Silva, R.; Mohseni, F.; Davarpanah, A.; Amaral, V.; Ferreira, A.; Barros-Timmons, A. Enhancement of physical and reaction to fire properties of crude glycerol polyurethane foams filled with expanded graphite. Polym. Test. 2018, 69, 199–207. [Google Scholar] [CrossRef]
- Kurańska, M.; Prociak, A.; Cabulis, U.; Kirpluks, M.; Ryszkowska, J.; Auguścik, M. Innovative porous polyurethane-polyisocyanurate foams based on rapeseed oil and modified with expandable graphite. Ind. Crop. Prod. 2017, 95, 316–323. [Google Scholar] [CrossRef]
- Takahashi, J.A.; Chaussy, D.; Silva, G.G.; Silva, M.C.; Belgacem, M.N. Composites of rigid polyurethane foam and cellulose fiber residue. J. Appl. Polym. Sci. 2010, 117, 3665–3672. [Google Scholar]
- Song, Z.-L.; Ma, L.-Q.; Wu, Z.-J.; He, D.-P. Effects of viscosity on cellular structure of foamed aluminum in foaming process. J. Mater. Sci. 2000, 35, 15–20. [Google Scholar] [CrossRef]
- John, J.; Bhattacharya, M.; Turner, R.B. Characterization of polyurethane foams from soybean oil. J. Appl. Polym. Sci. 2002, 86, 3097–3107. [Google Scholar] [CrossRef]
- Jin, J.F.; Chen, Y.L.; Wang, D.N.; Hu, C.P.; Zhu, S.; VanOverloop, L.; Randall, D. Structures and physical properties of rigid polyurethane foam prepared with rosin-based polyol. J. Appl. Polym. Sci. 2002, 84, 598–604. [Google Scholar] [CrossRef]
- Sung, G.; Kim, J.H. Influence of filler surface characteristics on morphological, physical, acoustic properties of polyurethane composite foams filled with inorganic fillers. Compos. Sci. Technol. 2017, 146, 147–154. [Google Scholar] [CrossRef]
- Gu, R.; Khazabi, M.; Sain, M. Fiber reinforced soy-based polyurethane spray foam insulation. Part 2: Thermal and mechanical properties. BioResources 2011, 6, 3775–3790. [Google Scholar]
- Tian, H.; Rajulu, A.; Zhang, S.; Xiang, A. Water-Blown Castor Oil-Based Polyurethane Foams with Soy Protein as a Reactive Reinforcing Filler. J. Polym. Environ. 2016, 26, 15–22. [Google Scholar]
- Wolska, A.; Goździkiewicz, M.; Ryszkowska, J. Thermal and mechanical behaviour of flexible polyurethane foams modified with graphite and phosphorous fillers. J. Mater. Sci. 2012, 47, 5627–5634. [Google Scholar] [CrossRef]
- Formela, K.; Hejna, A.; Zedler, Ł.; Przybysz, M.; Ryl, J.; Saeb, M.R.; Piszczyk, Ł. Structural, thermal and physico-mechanical properties of polyurethane/brewers’ spent grain composite foams modified with ground tire rubber. Ind. Crop Prod. 2017, 108, 844–852. [Google Scholar] [CrossRef]
- Verdejo, R.; Stämpfli, R.; Alvarez-Lainez, M.; Mourad, S.; Rodriguez-Perez, M.; Brühwiler, P.; Shaffer, M. Enhanced acoustic damping in flexible polyurethane foams filled with carbon nanotubes. Compos. Sci. Technol. 2009, 69, 1564–1569. [Google Scholar] [CrossRef]
- Maharsia, R.R.; Jerro, H.D. Enhancing tensile strength and toughness in syntactic foams through nanoclay reinforcement. Mater. Sci. Eng. 2007, 454, 416–422. [Google Scholar] [CrossRef]
- Saint-Michel, F.; Chazeau, L.; Cavaillé, J.-Y.; Chabert, E. Mechanical properties of high density polyurethane foams: I. Effect of the density. Compos. Sci. Technol. 2006, 66, 2700–2708. [Google Scholar] [CrossRef]
- Nazeran, N.; Moghaddas, J. Synthesis and characterization of silica aerogel reinforced rigid polyurethane foam for thermal insulation application. J. Non-Cryst. Solids 2017, 461, 1–11. [Google Scholar] [CrossRef]
- Gu, R.; Konar, S.; Sain, M. Preparation and Characterization of Sustainable Polyurethane Foams from Soybean Oils. J. Am. Oil Chem. Soc. 2012, 89, 2103–2111. [Google Scholar] [CrossRef]
- Ciecierska, E.; Jurczyk-Kowalska, M.; Bazarnik, P.; Gloc, M.; Kulesza, M.; Krauze, S.; Lewandowska, M.; Kowalski, M. Flammability, mechanical properties and structure of rigid polyurethane foams with different types of carbon reinforcing materials. Compos. Struct. 2016, 140, 67–76. [Google Scholar] [CrossRef]
- Jiao, L.; Xiao, H.; Wang, Q.; Sun, J. Thermal degradation characteristics of rigid polyurethane foam and the volatile products analysis with TG-FTIR-MS. Polym. Degrad. Stab. 2013, 98, 2687–2696. [Google Scholar] [CrossRef]
- Levchik, S.V.; Weil, E.D. Thermal decomposition, combustion and fire-retardancy of polyurethanes—A review of the recent literature. Polym. Int. 2004, 53, 1585–1610. [Google Scholar] [CrossRef]
- Septevani, A.A.; Evans, D.A.; Chaleat, C.; Martin, D.J.; Annamalai, P.K.; Evans, D.A.C. A systematic study substituting polyether polyol with palm kernel oil based polyester polyol in rigid polyurethane foam. Ind. Crop Prod. 2015, 66, 16–26. [Google Scholar] [CrossRef]
- Chattopadhyay, D.; Webster, D.C. Thermal stability and flame retardancy of polyurethanes. Prog. Polym. Sci. 2009, 34, 1068–1133. [Google Scholar] [CrossRef]
- Pagacz, J.; Hebda, E.; Michałowski, S.; Ozimek, J.; Sternik, D.; Pielichowski, K. Polyurethane foams chemically reinforced with POSS—Thermal degradation studies. Thermochim. Acta 2016, 642, 95–104. [Google Scholar] [CrossRef]
- Chen, S.; Guo, L.; Du, D.; Rui, J.; Qiu, T.; Ye, J.; Li, X. Waterborne POSS-silane-urethane hybrid polymer and the fluorinated films. Polymer 2016, 103, 27–35. [Google Scholar] [CrossRef]
- Hojiyev, R.; Ulcay, Y.; Hojamberdiev, M.; Çelik, M.S.; Carty, W.M. Hydrophobicity and polymer compatibility of POSS-modified Wyoming Na-montmorillonite for developing polymer-clay nanocomposites. J. Colloid Interface Sci. 2017, 497, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Badri, K.H.; Ahmad, S.H.; Zakaria, S. Production of a high-functionality RBD palm kernel oil-based polyester polyol. J. Appl. Polym. Sci. 2001, 81, 384–389. [Google Scholar] [CrossRef]















| Sample Codes | Dynamic Viscosity η [mPa·s] | Fitting Equation | Power Law Index (n) | R2 | ||
|---|---|---|---|---|---|---|
| 0.5 RPM | 5 RPM | 10 RPM | ||||
| PU-0 | 852 | 423 | 379 | y = −0.058 + 0.335 | 0.335 | 0.970 |
| PU-APIB-0.5 | 1887 | 878 | 765 | y = −0.061 + 0.320 | 0.320 | 0.968 |
| PU-APIB-1.5 | 3285 | 1542 | 821 | y = −0.059 + 0.315 | 0.315 | 0.971 |
| PU-APIB-5 | 6875 | 4250 | 1358 | y = −0.059 + 0.295 | 0.295 | 0.972 |
| PU-AEAPIB-0.5 | 2831 | 1317 | 1148 | y = −0.058 + 0.325 | 0.325 | 0.979 |
| PU-AEAPIB-1.5 | 4523 | 2015 | 1105 | y = −0.060 + 0.315 | 0.315 | 0.971 |
| PU-AEAPIB-5 | 7540 | 5250 | 1955 | y = −0.062 + 0.310 | 0.310 | 0.976 |
| Sample Codes | T [°C] | Cream Time [s] | Extension Time [s] | Tack-Free Time [s] | Cell Size [µm] | Wall Thickness [µm] | Apparent Density [kg m−3] |
|---|---|---|---|---|---|---|---|
| PU-0 | 110 | 43 ± 4 | 277 ± 10 | 341 ± 14 | 472 ± 10 | 62 ± 4 | 38 ± 1 |
| PU-APIB-0.5 | 135 | 41 ± 2 | 312 ± 11 | 320 ± 12 | 390 ± 8 | 66 ± 2 | 40 ± 2 |
| PU-APIB-1.5 | 128 | 46 ± 1 | 358 ± 12 | 265 ± 10 | 274 ± 7 | 68 ± 3 | 41 ± 1 |
| PU-APIB-5 | 120 | 48 ± 2 | 370 ± 10 | 260 ± 12 | 209 ± 8 | 70 ± 2 | 43 ± 1 |
| PU-AEAPIB-0.5 | 130 | 50 ± 2 | 332 ± 12 | 335 ± 15 | 364 ± 9 | 68 ± 5 | 42 ± 2 |
| PU-AEAPIB-1.5 | 126 | 57 ± 4 | 360 ± 12 | 277 ± 14 | 320 ± 8 | 69 ± 4 | 44 ± 2 |
| PU-AEAPIB-5 | 112 | 69 ± 3 | 384 ± 18 | 270 ± 16 | 184 ± 10 | 74 ± 4 | 40 ± 3 |
| Sample Code | Tg | T5 | T10 | T50 | T70 | Char Residue |
|---|---|---|---|---|---|---|
| [°C] | [°C] | [°C] | [°C] | [°C] | [%] | |
| APIB-POSS | 267 | 280 | 342 | 531 | 21.6 | |
| AEAPIB-POSS | 260 | 287 | 350 | 449 | 18.4 | |
| PU-0 | 126 | 220 | 265 | 454 | 591 | 27.9 |
| PU-APIB-0.5 | 126 | 205 | 245 | 418 | 596 | 29.0 |
| PU-APIB-1.5 | 129 | 206 | 247 | 406 | 590 | 28.6 |
| PU-APIB-5 | 142 | 206 | 247 | 394 | 590 | 27.6 |
| PU-AEAPIB-0.5 | 126 | 206 | 248 | 418 | 595 | 29.2 |
| PU-AEAPIB-1.5 | 129 | 212 | 254 | 444 | 593 | 28.2 |
| PU-AEAPIB-5 | 122 | 209 | 251 | 415 | 590 | 27.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Członka, S.; Strąkowska, A.; Strzelec, K.; Adamus-Włodarczyk, A.; Kairytė, A.; Vaitkus, S. Composites of Rigid Polyurethane Foams Reinforced with POSS. Polymers 2019, 11, 336. https://doi.org/10.3390/polym11020336
Członka S, Strąkowska A, Strzelec K, Adamus-Włodarczyk A, Kairytė A, Vaitkus S. Composites of Rigid Polyurethane Foams Reinforced with POSS. Polymers. 2019; 11(2):336. https://doi.org/10.3390/polym11020336
Chicago/Turabian StyleCzłonka, Sylwia, Anna Strąkowska, Krzysztof Strzelec, Agnieszka Adamus-Włodarczyk, Agnė Kairytė, and Saulius Vaitkus. 2019. "Composites of Rigid Polyurethane Foams Reinforced with POSS" Polymers 11, no. 2: 336. https://doi.org/10.3390/polym11020336