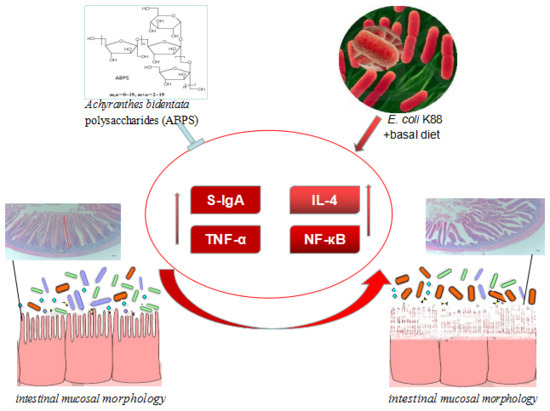
Effects of Achyranthes bidentata Polysaccharides on Intestinal Morphology, Immune Response, and Gut Microbiome in Yellow Broiler Chickens Challenged with Escherichia coli K88
Abstract
:1. Introduction
2. Materials and Methods
2.1. Birds, Diets, and Experimental Design
2.1.1. Preparation of ABPS and E. coli K88
2.1.2. Animals
2.1.3. Diets and Experimental Design
2.2. Data and Sampling Collection
2.2.1. Growth Performance
2.2.2. Blood Samples
2.2.3. Fecal and Jejunal Mucosa Samples
2.2.4. Duodenum, Jejunum, and Ileum Samples
2.3. Mucosal Cytokines and S-IgA
2.4. Measurement of Intestinal Mucosal Morphology
2.5. Jejunum Immunohistochemistry (IHC)
2.6. Cecal Microbiota Analysis
2.7. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Serum Biochemistry Indices
3.3. Immune Responses
3.4. Response of NF-κB and EKR1/2 in the Jejunum
3.5. The Change in Intestinal Mucosal Morphology
3.6. Cecal Microbial Community Structure
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mead, P.S.; Slutsker, L.; Dietz, V.; McCaig, L.F.; Bresee, J.S.; Shapiro, C.; Griffin, P.M.; Tauxe, R.V. Food-Related Illness and Death in the United States. Emerg. Infect. Dis. 1999, 5, 607–625. [Google Scholar] [CrossRef] [PubMed]
- He, C.L.; Fu, B.D.; Shen, H.Q.; Jiang, X.L.; Zhang, C.S.; Wu, S.C.; Zhu, W.; Wei, X.B. Xiang-Qi-Tang Increases Avian Pathogenic Escherichia coli-Induced Survival Rate and Regulates Serum Levels of Tumor Necrosis Factor Alpha, Interleukin-1 and Soluble Endothelial Protein C Receptor in Chicken. Biol. Pharm. Bull. 2011, 34, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Alonso, M.Z.; Padola, N.L.; Parma, A.E.; Lucchesi, P.M.A. Enteropathogenic Escherichia coli contamination at different stages of the chicken slaughtering process. Poult. Sci. 2011, 90, 2638–2641. [Google Scholar] [CrossRef] [PubMed]
- Baurhoo, B.; Letellier, A.; Zhao, X.; Ruiz-Feria, C.A. Cecal Populations of Lactobacilli and Bifidobacteria and Escherichia coli Populations after In Vivo Escherichia coli Challenge in Birds Fed Diets with Purified Lignin or Mannanoligosaccharides. Poult. Sci. 2007, 86, 2509–2516. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cao, G.T.; Zeng, X.F.; Zhou, L.; Ferket, P.R.; Xiao, Y.P.; Chen, A.G.; Yang, C.M. Effects of Clostridium butyricum on growth performance, immune function, and cecal microflora in broiler chickens challenged with Escherichia coli K88. Poult. Sci. 2013, 93, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Mohana Devi, S.; Lee, S.I.; Kim, I.H. Effect of phytogenics on growth performance, fecal score, blood profiles, fecal noxious gas emission, digestibility, and intestinal morphology of weanling pigs challenged with Escherichia coli K88. Pol. J. Vet. Sci. 2015, 18, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, L.; Chen, Z.; Ma, X.; Yang, X.; Zhang, J.; Jiang, Z. In Vitro Evaluation of Swine-Derived Lactobacillus reuteri: Probiotic Properties and Effects on Intestinal Porcine Epithelial Cells Challenged with Enterotoxigenic Escherichia coli K88. J. Microbiol. Biotechnol. 2016, 26, 1018–1025. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, L.; Zhan, X.; Zeng, X.; Zhou, L.; Cao, G.; Chen, A.G.; Yang, C. Effects of dietary supplementation of probiotic, Clostridium butyricum, on growth performance, immune response, intestinal barrier function, and digestive enzyme activity in broiler chickens challenged with Escherichia coli K88. J. Anim. Sci. Biotechnol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Puyalto, M.; Chamba, F.; Ortiz, A.; Torrealba, H.; Mallo, J.J.; Riboty, R. Effect of Partially Protected Sodium Butyrate on Performance, Digestive Organs, Intestinal Villi and E. coli Development in Broilers Chickens. Int. J. Poult. Sci. 2014, 13, 390–396. [Google Scholar] [CrossRef]
- Jiang, M.-H.; Zhu, L.; Jiang, J.-G. Immunoregulatory actions of polysaccharides from Chinese herbal medicine. Expert Opin. Ther. Targets 2010, 14, 1367–1402. [Google Scholar] [CrossRef] [PubMed]
- Vasudeva Rao, Y.; Das, B.K.; Jyotyrmayee, P.; Chakrabarti, R. Effect of Achyranthes aspera on the immunity and survival of Labeo rohita infected with Aeromonas hydrophila. Fish Shellfish Immunol. 2006, 20, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Meng, J.; Chen, W.; Liu, J.; Li, X.; Li, W.; Lu, C.; Shan, F. Modulation of phenotypic and functional maturation of murine dendritic cells (DCs) by purified Achyranthes bidentata polysaccharide (ABP). Int. Immunopharmacol. 2011, 11, 1103–1108. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Liu, Y.; Fan, W.; Han, J.; Hou, Y.; Yin, Y.; Zhu, H.; Qu, Y. Effects of Achyranthes Bidentata Polysaccharide on Growth Performance, Immunological, Adrenal, and Somatotropic Responses of Weaned Pigs Challenged with Escherichia coli Lipopolysaccharide. Asian-australas. J. Anim. Sci. 2008, 21, 1189–1195. [Google Scholar] [CrossRef]
- Kang, P.; Xiao, H.L.; Hou, Y.Q.; Ding, B.Y.; Liu, Y.L.; Zhu, H.L.; Hu, Q.Z.; Hu, Y.; Yin, Y.L. Effects of Astragalus Polysaccharides, Achyranthes bidentata Polysaccharides, and Acantbepanax senticosus Saponin on the Performance and Immunity in Weaned Pigs. Asian-Australas. J. Anim. Sci. 2010, 23, 750–756. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, Z.; He, J. Achyranthes bidentatapolysaccharide enhances immune response in weaned piglets. Immunopharmacol. Immunotoxicol. 2009, 31, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Liu, Z.; He, J.; Zhao, Y.; Wu, X. Achyranthes bidentata polysaccharide enhances growth performance and health status in weaned piglets. Food Agric. Immunol. 2011, 22, 17–29. [Google Scholar] [CrossRef]
- Chen, Q.H.; Ding, Z.; Qiu, W.; He, J.H. Achyranthes bidentata polysaccharide decreases inflammatory cytokine secretion in weaned piglets after LPS challenge. Asian J. Anim. Vet. Adv. 2014, 13, 454–459. [Google Scholar]
- Liu, Z.Y.; Wang, X.L.; Ou, S.Q.; Fu, C.X.; Hou, D.X.; He, J.H. Effect of Sanguinarine and Achyranthes Bidentata Polysaccharides on Performance and Immunity of Yellow Broilers. J. Poult. Sci. 2018. under review. [Google Scholar]
- Chen, X.M.; Xu, Y.J.; Tian, G.Y. Physical-chemical properties and structure elucidation of AbPS isolated from the root of Achyranthes bidentata. Acta Pharm. Sin. 2005, 40, 32–35. (In Chinese) [Google Scholar]
- Xia, M.S.; Hu, C.H.; Xu, Z.R. Effects of copper-bearing montmorillonite on growth performance, digestive enzyme activities, and intestinal microflora and morphology of male broilers. Poult. Sci. 2004, 83, 1868–1875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.R.; Hu, C.H.; Xia, M.S.; Zhan, X.A.; Wang, M.Q. Effects of dietary fructooligosaccharide on digestive enzyme activities, intestinal microflora and morphology of male broilers. Poult. Sci. 2003, 82, 1030–1036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agha-Hosseini, F.; Khalili, M.; Rohani, B. Immunohistochemistry Analysis of P53 and Ki-67 Proteins in Oral Lichen Planus and Normal Oral Mucosa. Iran. J. Public Health 2009, 38, 37–43. [Google Scholar]
- Zhu, L.; Baker, S.S.; Gill, C.; Liu, W.; Alkhouri, R.; Baker, R.D.; Gill, S.R. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: A connection between endogenous alcohol and NASH. Hepatology 2013, 57, 601–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, G.T.; Zeng, X.F.; Chen, A.G.; Zhou, L.; Zhang, L.; Xiao, Y.P.; Yang, C.M. Effects of a probiotic, Enterococcus faecium, on growth performance, intestinal morphology, immune response, and cecal microflora in broiler chickens challenged with Escherichia coli K88. Poult. Sci. 2013, 92, 2949–2955. [Google Scholar] [CrossRef] [PubMed]
- Jing, M.; Munyaka, P.M.; Tactacan, G.B.; Rodriguez-Lecompte, J.C.; O, K.; House, J.D. Performance, serum biochemical responses, and gene expression of intestinal folate transporters of young and older laying hens in response to dietary folic acid supplementation and challenge with Escherichia coli lipopolysaccharide. Poult. Sci. 2013, 93, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Chen, X.; Lu, J.; Jin, L. Protective effect of sulfated Achyranthes bidentata polysaccharides on streptozotocin-induced oxidative stress in rats. Carbohydr. Polym. 2009, 75, 415–419. [Google Scholar] [CrossRef]
- Yang, X.D.; Zhang, J.; Shen, M.S. Effect of Achyranthes Bidentata Polysaccharides on Expression of Adipose Differentiation-Related Protein Gene in Type 2 Diabetes Mellitus Rats. Pharm. Biotechnol. 2010, 17, 334–336. (In Chinese) [Google Scholar]
- He, K.; Li, X.; Chen, X.; Ye, X.; Huang, J.; Jin, Y.; Li, P.; Shu, H. Evaluation of antidiabetic potential of selected traditional Chinese medicines in STZ-induced diabetic mice. J. Ethnopharmacol. 2011, 137, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.M.; Fridovich, I. Superoxide radical and the oxygen enhancement of the toxicity of paraquat in Escherichia coli. J. Biol. Chem. 1978, 253, 8143–8148. [Google Scholar] [PubMed]
- Cuevas-Ramos, G.; Petit, C.R.; Marcq, I.; Boury, M.; Oswald, E.; Nougayrede, J.-P. Escherichia coli induces DNA damage in vivo and triggers genomic instability in mammalian cells. Proc. Natl. Acad. Sci. USA 2010, 107, 11537–11542. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhao, Y.; Liu, X. Path analysis of the relationship between serum protein contents and the growth traits in chicks. Acta Vet. Zootech. Sin. 1994, 25, 301–305. (In Chinese) [Google Scholar]
- Lee, D.N.; Shen, T.F.; Yen, H.T.; Weng, C.F.; Chen, B.J. Effects of Chromium Supplementation and Lipopolysaccharide Injection on the Immune Responses of Weanling Pigs. Asian-Australas. J. Anim. Sci. 2000, 13, 1414–1421. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, J.; Hou, Y.; Zhu, H.; Zhao, S.; Ding, B.; Yin, Y.; Yi, G.; Shi, J.; Fan, W. Dietary arginine supplementation alleviates intestinal mucosal disruption induced by Escherichia coli lipopolysaccharide in weaned pigs. Br. J. Nutr. 2008, 100, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Muir, W.I.; Bryden, W.L.; Husband, A.J. Evaluation of the efficacy of intraperitoneal immunization in reducing Salmonella typhimurium infection in chickens. Poult. Sci. 1998, 77, 1874–1883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.; Pan, Y.; Zheng, L.; Cui, L.; Cao, Y. Polysaccharides from the Chinese medicinal herb Achyranthes bidentata enhance anti-malarial immunity during Plasmodium yoelii 17XL infection in mice. Malar. J. 2012, 11, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ou, N.; Sun, Y.; Zhou, S.; Gu, P.; Liu, Z.; Bo, R.; Hu, Y.; Liu, J.; Wang, D. Evaluation of optimum conditions for Achyranthes bidentata polysaccharides encapsulated in cubosomes and immunological activity in vitro. Int. J. Biol. Macromol. 2018, 109, 748–760. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, M.W.; Ma, J.; Chen, Q.H. Achyranthes bidentata Polysaccharides:Regulation on Immunological Stress in Piglet Intestinal Epithelial Cells and Its Action Mechanism. Chin. J. Anim. Sci. 2017, 29, 4116–4122. [Google Scholar]
- Yi, G.F.; Carroll, J.A.; Allee, G.L.; Gaines, A.M.; Kendall, D.C.; Usry, J.L.; Toride, Y.; Izuru, S. Effect of glutamine and spray-dried plasma on growth performance, small intestinal morphology, and immune responses of Escherichia coli K88+-challenged weaned pigs. J. Anim. Sci. 2005, 83, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Olnood, C.G.; Beski, S.S.M.; Iji, P.A.; Choct, M. Delivery routes for probiotics: Effects on broiler performance, intestinal morphology and gut microflora. Anim Nutr. 2015, 1, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Roofchaei, A.; Rezaeipour, V.; Vatandour, S.; Zaefarian, F. Influence of dietary carbohydrases, individually or in combination with phytase or an acidifier, on performance, gut morphology and microbial population in broiler chickens fed a wheat-based diet. Anim. Nutr. 2017. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Bäckhed, F.; Fulton, L.; Gordon, J.I. Diet-Induced Obesity Is Linked to Marked but Reversible Alterations in the Mouse Distal Gut Microbiome. Cell Host Microbe 2008, 3, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Koliada, A.; Syzenko, G.; Moseiko, V.; Budovska, L.; Puchkov, K.; Perederiy, V.; Gavalko, Y.; Vaiserman, A. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol. 2017, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Krajmalnik-Brown, R.; Ilhan, Z.E.; Kang, D.W.; DiBaise, J. K Effects of Gut Microbes on Nutrient Absorption and Energy Regulation. Nutr. Clin. Pract. 2012, 27, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Madigan, T.M.; Martinko, J.M. Brock Biology of Microorganisms, 11th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2005; pp. 122–185. ISBN 0-13-144329-1. [Google Scholar]
- Fisher, K.; Phillips, C. The ecology, epidemiology and virulence of Enterococcus. Microbiology 2009, 155, 1749–1757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, H.B.; Zou, Y.; Liu, L.L.; Yang, Y.S.; He, J.H. Effects of Botanical Polysaccharide on Growth Performance and Intestinal Environment of Weaned Piglets. Chin. J. Anim. Nutr. 2018, 30, 2662–2671. [Google Scholar]
- Flint, H.J.; Bayer, E.A.; Rincon, M.T.; Lamed, R.; White, B.A. Polysaccharide utilization by gut bacteria: Potential for new insights from genomic analysis. Nat. Rev. Microbiol. 2008, 6, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Young, P.; Holtrop, G.; Flint, H.J. Diversity of human colonic butyrate-producing bacteria revealed by analysis of the butyryl-CoA:acetate CoA-transferase gene. Environ. Microbiol. 2010, 12, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Meehan, C.J.; Beiko, R.G. A Phylogenomic View of Ecological Specialization in the Lachnospiraceae, a Family of Digestive Tract-Associated Bacteria. Genome Biol. Evol. 2014, 6, 703–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oelschlaeger, T.A. Mechanisms of probiotic action—A review. Int. J. Med. Microbiol. 2010, 300, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.H.; Park, J.H.; Jeon, W.M.; Han, K.S. Butyrate modulates bacterial adherence on LS174T human colorectal cells by stimulating mucin secretion and MAPK signaling pathway. Nutr. Res. Pract. 2005, 9, 343. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, G.T.; Macfarlane, S. Bacteria, Colonic Fermentation, and Gastrointestinal Health. J. AOAC Int. 2012, 95, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.; He, G.Q.; Jia, J.L.; Zhu, Q.L.; Ruan, H. Oral Administration of Clostridium butyricum for Modulating Gastrointestinal Microflora in Mice. Curr. Microbiol. 2010, 62, 512–517. [Google Scholar] [CrossRef] [PubMed]




| Item | Basal Diet (0 to 4 weeks) |
|---|---|
| Ingredient, % | |
| Ground yellow maize | 56.65 |
| Soybean meal | 36 |
| Soybean oil | 3.0 |
| Dicalcium phosphate | 1.8 |
| Limestone | 1.0 |
| NaCl | 0.3 |
| DL-Met 3 | 0.1 |
| Choline chloride | 0.15 |
| Premix 1 | 1.0 |
| Nutrient level 2 | |
| ME 3, MJ/kg | 12.22 |
| CP 3, % | 20.10 |
| Lys, % | 1.02 |
| Met, % | 0.42 |
| Cys,% | 0.32 |
| Ca,% | 1.11 |
| Available P, % | 0.54 |
| Item | −E. coli | +E. coli | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| NG | ABP | CNG | CABP | ABPS | E. coli | ABPS * E. coli | ||
| BW (g) | 635.17 a,b | 651.1 a | 579.5 c | 627.33 b | 6.49 | <0.001 | <0.001 | 0.038 |
| ADG (g) | 21.6 a,b | 22.16 a | 19.61 c | 21.32 b | 0.13 | <0.001 | <0.001 | 0.038 |
| ADFI (g) | 44.83 | 44.57 | 42.58 | 44.55 | 0.39 | 0.298 | 0.168 | 0.172 |
| FCR (g:g) 1 | 2.08 | 2.01 | 2.17 | 2.09 | 0.02 | 0.002 | <0.001 | 0.658 |
| Mortality (%) | 5.00 | 3.33 | 8.33 | 5.00 | 1.03 | 0.242 | 0.242 | 0.692 |
| Item | −E. coli | +E. coli | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| NG | ABP | CNG | CABP | ABPS | E. coli | ABPS * E. coli | ||
| ALB (g/L) | 13.98 | 14.66 | 13.23 | 14.18 | 0.24 | 0.104 | 0.198 | 0.763 |
| TP 1 (g/L) | 35.28 | 42.42 | 32.15 | 40.75 | 1.01 | <0.001 | 0.008 | 0.375 |
| GLB 2 (g/L) | 21.28 | 27.77 | 19.31 | 26.57 | 0.91 | <0.001 | 0.091 | 0.669 |
| GLU (mmol/L) 3 | 14.49 | 13.15 | 13.66 | 12.39 | 0.17 | <0.001 | <0.001 | 0.744 |
| UA(μmol/L) 4 | 273.19 | 242.56 | 295.55 | 279.49 | 4.35 | <0.001 | <0.001 | 0.059 |
| Taxonomy Units | -E. coli % Abundance | +E. coli % Abundance | abp Value | SEM | ||||
|---|---|---|---|---|---|---|---|---|
| NG | ABP | CNG | CABP | ABPS | E. coli | ABPS * E. coli | ||
| p__Firmicutes; c__Clostridia; o__Clostridiales; f__Lachnospiraceae; g__Coprococcus_1; s__unidentified(OTU 2) | 15.87 ab | 21.08 a | 9.31 b | 13.20 b | 0.849 | 0.007 | 0.038 | 0.015 |
| p__Bacteroidetes; c__Bacteroidia; o__Bacteroidales; f__Bacteroidaceae; g__Bacteroides; s__unidentified (OTU 6) | 2.13 | 3.14 | 4.31 | 4.81 | 0.056 | 0.001 | 0.474 | 0.003 |
| p__Bacteroidetes; c__Bacteroidia; o__Bacteroidales; f__Bacteroidaceae; g__Bacteroides(OTU11) | 2.97 | 4.28 | 3.98 | 5.16 | 0.015 | 0.003 | 0.848 | 0.003 |
| p__Proteobacteria;c__Gammaproteobacteria; o__Enterobacteriales;f__Enterobacteriaceae; g__Escherichia-Shigella; s__Escherichia_coli(OTU 4) | 0.19 b | 0.85 b | 12.34 a | 3.41 b | 0.055 | 0.004 | 0.031 | 0.016 |
| p__Firmicutes; c__Bacilli; o__Lactobacillales; f__Enterococcaceae; g__Enterococcus; s__unidentified(OTU 27) | 0.20 b | 0.01 b | 3.15 a | 0.04 b | 0.001 | 0.001 | 0.001 | 0.004 |
| p__Firmicutes; c__Clostridia; o__Clostridiales; f__Lachnospiraceae; g__Eisenbergiella; s__unidentified(OTU 32) | 0.58 | 1.31 | 0.35 | 0.21 | 0.003 | 0.003 | 0.027 | 0.001 |
| p__Firmicutes; c__Clostridia; o__Clostridiales; f__Ruminococcaceae; g__Ruminococcaceae_UCG-014; s__unidentified(OTU201) | 0.32 b | 1.17 a | 0.31 b | 0.34 b | 0.037 | 0.005 | 0.005 | 0.001 |
| p__Firmicutes; c__Clostridia; o__Clostridiales; f__Ruminococcaceae(OTU 56) | 0.08 | 0.51 | 0.05 | 0.31 | 0.028 | 0.522 | 0.294 | 0.0004 |
| p__Firmicutes; c__Clostridia; o__Clostridiales; f__Ruminococcaceae; g__Ruminococcaceae_UCG-014; s__unidentified(OTU63) | 0.00 | 0.43 | 0.00 | 0.11 | 0.07 | 0.189 | 0.271 | 0.001 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Wang, X.; Ou, S.; Arowolo, M.A.; Hou, D.-X.; He, J. Effects of Achyranthes bidentata Polysaccharides on Intestinal Morphology, Immune Response, and Gut Microbiome in Yellow Broiler Chickens Challenged with Escherichia coli K88. Polymers 2018, 10, 1233. https://doi.org/10.3390/polym10111233
Liu Z, Wang X, Ou S, Arowolo MA, Hou D-X, He J. Effects of Achyranthes bidentata Polysaccharides on Intestinal Morphology, Immune Response, and Gut Microbiome in Yellow Broiler Chickens Challenged with Escherichia coli K88. Polymers. 2018; 10(11):1233. https://doi.org/10.3390/polym10111233
Chicago/Turabian StyleLiu, Zhuying, Xiaolong Wang, Shuqi Ou, Muhammed Adebayo Arowolo, De-Xing Hou, and Jianhua He. 2018. "Effects of Achyranthes bidentata Polysaccharides on Intestinal Morphology, Immune Response, and Gut Microbiome in Yellow Broiler Chickens Challenged with Escherichia coli K88" Polymers 10, no. 11: 1233. https://doi.org/10.3390/polym10111233