Composite Biopolymer-Based Wafer Dressings Loaded with Microbial Biosurfactants for Potential Application in Chronic Wounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
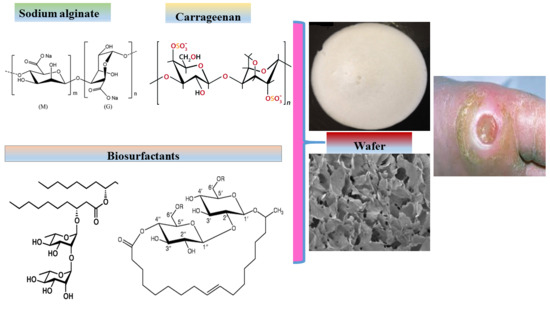
2.3. Scanning Electron Microscopy
2.4. X-ray Diffraction
2.5. Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy (ATR-FTIR)
2.6. Mechanical Strength (‘Hardness’)
2.7. Fluid Handling Properties
2.7.1. Swelling Studies
2.7.2. Pore Analysis
2.7.3. Water Absorption (Aw) and Equilibrium Water Content (EWC)
2.7.4. Evaporative Water Loss (EWL)
2.7.5. Water Vapor Transmission Rate (WVTR)
2.8. Thermogravimetric Analysis (TGA)
2.9. In Vitro Adhesion Studies
2.10. Statistical Analysis of Data
3. Results and Discussion
3.1. Preliminary Formulation Development and Optimization
3.2. Scanning Electron Microscopy (SEM)
3.3. Mechanical Strength (‘Hardness’)
3.4. X-ray Diffraction (XRD)
3.5. Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy (ATR-FTIR)
3.6. Fluid Handling Properties
3.6.1. Swelling
3.6.2. Pore Analysis
3.6.3. Water Absorption (Aw) and Equilibrium Water Content (EWC)
3.6.4. Evaporative Water Loss (EWL)
3.6.5. Water Vapor Transmission Rate
3.6.6. Thermogravimetric Analysis (TGA)
3.6.7. In Vitro Adhesion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dhivya, S.; Padma, V.; Santhini, E. Wound dressings—A review. Biomedicine. 2015, 5, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Beanes, S.; Dang, C.; Soo, C.K. Skin repair and scar formation: The central role of TGF-beta. Expert Rev. Mol. Med. 2003, 5, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Boateng, J.S.; Catanzano, O. Advanced therapeutic dressings for effective wound healing—A review. J. Pharm. Sci. 2015, 104, 3653–3680. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.; Gray, D. Using PHMB antimicrobial to prevent wound infection. Wounds UK 2007, 3, 96–102. [Google Scholar]
- Boateng, J.S.; Matthews, K.; Stevens, H.; Eccleston, G. Wound healing dressings and drug delivery systems: A review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Posthauer, M.; Dorner, B.; Redovian, V.; Maloney, M. Pressure ulcer healing with a concentrated, fortified, collagen protein hydrolysate supplement: A randomised controlled trial. Adv. Skin Wound Care 2006, 19, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Regan, P. The impact of cancer and its treatment on wound healing. Wounds UK 2007, 3, 144–148. [Google Scholar]
- Vuolo, J. Current options for managing the problem of excess wound exudate. Prof. Nurs. 2004, 19, 487–491. [Google Scholar]
- Thakur, R.A.; Florek, C.A.; Kohn, J.; Michniak, B.B. Electrospun nanofibrous polymeric scaffold with targeted drug release profiles for potential application as wound dressing. Int. J. Pharm. 2008, 364, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Matthews, K.; Stevens, H.; Auffret, A.; Humphrey, M.; Eccleston, G. Lyophilized wafers as a drug delivery system for wound healing containing methylcellulose as a viscosity modifier. Int. J. Pharm. 2005, 289, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Matthews, K.H.; Stevens, H.N.E.; Auffret, A.D.; Humphrey, M.J.; Eccleston, G.M. Gamma-irradiation of lyophilized wound healing wafers. Int. J. Pharm. 2006, 313, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Boateng, J.S.; Auffret, A.; Matthews, K.H.; Humphrey, M.J.; Stevens, H.N.E.; Eccleston, G.M. Characterisation of freeze-dried wafers and solvent evaporated films as potential drug delivery systems to mucosal surfaces. Int. J. Pharm. 2010, 389, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Ayensu, I.; Mitchell, J.; Boateng, J.S. Development and physico-mechanical characterization of lyophilized chitosan wafers as potential protein drug delivery systems via the buccal mucosa. Coll. Surf. B 2012, 91, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Boateng, J.S.; Pawar, H.; Tetteh, J. Evaluation of in vitro wound adhesion characteristics of composite film and wafer based dressings using texture analysis and FTIR spectroscopy: A chemometrics factor analysis approach. RSC Adv. 2015, 5, 107064–107075. [Google Scholar] [CrossRef]
- Paşcalău, V.; Popescu, V.; Popescu, G.; Dudescu, M.; Borodi, G.; Dinescu, A.; Moldovan, M. Obtaining and characterizing alginate/k-carrageenan hydrogel cross-linked with adipic dihydrazide. Adv. Mater. Sci. Eng. 2013, 2013, 1–12. [Google Scholar] [CrossRef]
- Roh, Y.H.; Shin, C.S. Preparation and characterization of alginate–carrageenan L complex films. J. Appl. Polym. Sci. 2006, 99, 3483–3490. [Google Scholar] [CrossRef]
- Popa, E.G.; Gomes, M.E.; Reis, R.L. Cell delivery systems using alginate–carrageenan hydrogel beads and fibers for regenerative medicine applications. Biomacromolecules 2011, 12, 3952–3961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paşcalău, V.; Popescu, V.; Popescu, G.; Dudescu, M.; Borodi, G.; Dinescu, A.; Perhaiţa, I.; Paul, M. The alginate/k-carrageenan ratio’s influence on the properties of the cross-linked composite films. J. Alloy. Compd. 2012, 536, 4184–4494. [Google Scholar] [CrossRef]
- Pawar, H.; Boateng, J.S.; Ayensu, I.; Tetteh, J. Multifunctional medicated lyophilized wafer dressing for effective chronic wound healing. J. Pharm. Sci. 2014, 103, 1720–1733. [Google Scholar] [CrossRef] [PubMed]
- Langer, R. Polymeric delivery systems for controlled drug release. Chem. Eng. Commun. 1980, 6, 1–48. [Google Scholar] [CrossRef]
- Seydlová, G.; Svobodová, J. Review of surfactin chemical properties and the potential biomedical applications. Open Med. 2008, 3, 123–133. [Google Scholar] [CrossRef] [Green Version]
- Magalhães, L.; Nitschke, M. Anti-microbial activity of rhamnolipids against Listeria monocytogenes and their synergistic interaction with nisin. Food Contr. 2013, 29, 138–142. [Google Scholar] [CrossRef]
- Sotirova, A.; Spasova, D.; Galabova, D.; Karpenko, E.; Shulga, A. Rhamnolipid biosurfactant permeabilising effects on Gram-positive and Gram-negative bacterial strains. Curr. Microbiol. 2008, 56, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Ju, L.; Dashtbozorg, S.; Vongpanish, N. Wound Dressings with Enhanced Gas Permeation and Other Beneficial Properties. U.S. Patent No. 9,468,700, 18 October 2016. [Google Scholar]
- Stipcevic, T.; Piljac, A.; Piljac, G. Enhanced healing of full-thickness burn wounds using dirhamnolipid. Burns 2006, 32, 24–34. [Google Scholar] [CrossRef] [PubMed]
- McClure, C.; Schiller, N. Inhibition of macrophage phagocytosis by Pseudomonas aeruginosa rhamnolipids in vitro and in vivo. Curr. Microbiol. 1996, 33, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Joshi-Navare, K.; Prabhune, A. A biosurfactant-sophorolipid acts in synergy with antibiotics to enhance their efficiency. BioMed. Res. Int. 2013, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.; Doncel, G.F.; Seyoum, T.; Eaton, K.M.; Zalenskaya, I.; Hagver, R.; Azim, A.; Gross, R. Sophorolipids, microbial glycolipids with anti-human immunodeficiency virus and sperm immobilizing activities. Antimicrob. Agents Chemother. 2005, 49, 4093–4100. [Google Scholar] [CrossRef] [PubMed]
- Concaix, F.B. Use of Sophorolipids Comprising Diacetyl Lactones as Agent for Stimulating Skin Fibroblast Metabolism. U.S. Patent No. 6,596,265, 22 July 2003. [Google Scholar]
- Akiyode, O.; George, D.; Getti, G.; Boateng, J.S. Systematic comparison of the functional physico-chemical characteristics and biocidal activity of microbial derived biosurfactants on blood-derived and breast cancer cells. J. Coll. Interface Sci. 2016, 479, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, I.; Faustino, C.; Guerreiro, P.; Frade, R.; Bronze, M.; Castro, M.; Ribeiro, M. Development of novel sophorolipids with improved cytotoxic activity toward MDA-MB-231 breast cancer cells. J. Mol. Recognit. 2015, 28, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Bluth, M.H.; Kandil, E.; Mueller, C.M.; Shah, V.; Lin, Y.Y.; Zhang, H.; Dresner, L.; Lempert, L.; Nowakowski, M.; Gross, R.; et al. Sophorolipids block lethal effects of septic shock in rats in a cecal ligation and puncture model of experimental sepsis. Crit. Care Med. 2006, 34, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Hardin, R.; Pierre, J.; Schulze, R.; Mueller, C.M.; Fu, S.L.; Wallner, S.R.; Stanek, A.; Shah, V.; Gross, R.A.; Weedon, J.; et al. Sophorolipids improve sepsis survival: Effects of dosing and derivatives. J. Surg. Res. 2007, 142, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Catanzano, O.; Docking, R.; Schofield, P.A.; Boateng, J.S. Advanced multi-targeted composite biomaterial dressing for pain and infection control in chronic leg ulcers. Carbohydr. Polym. 2017, 172, 40–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chavda, H.; Patel, R.; Modhia, I.; Patel, C. Preparation and characterization of super porous hydrogel based on different polymers. Int. J. Pharm. Investig. 2012, 2, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Yoo, M.; Seo, J.; Park, S.; Na, H.; Lee, H.; Kim, S.; Cho, C. Evaluation of semi-interpenetrating polymer networks composed of chitosan and poloxamer for wound dressing application. Int. J. Pharm. 2007, 341, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Markl, D.; Wang, P.; Ridgway, C.; Karttunen, A.P.; Chakraborty, M.; Bawuah, P.; Pääkkönen, P.; Gane, P.; Ketolainen, J.; Peiponen, K.; et al. Characterisation of the pore structure of functionalised calcium carbonate tablets by terahertz time-domain spectroscopy and X-ray computed microtomography. J. Pharm. Sci. 2017, 106, 1586–1595. [Google Scholar] [CrossRef] [PubMed]
- Siafaka, P.I.; Zisi, A.; Exindari, M.; Karantas, I.D.; Bikiaris, D.N. Porous dressings of modified Chitosan with poly(2-hydroxyethyl acrylate) for topical wound delivery of Levofloxacin. Carbohydr. Polym. 2016, 143, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.A.; Peh, K.K.; Ch’ng, H.S. Mechanical, bioadhesive strength and biological evaluations of chitosan films for wound dressing. J. Pharm. Sci. 2000, 3, 303–311. [Google Scholar]
- Sachan, N.; Pushkar, S.; Jha, A.; Bhattcharya, A. Sodium alginate: The wonder polymer for controlled drug delivery. J. Pharm. Res. 2009, 2, 1191–1199. [Google Scholar]
- Prasad, K.; Kaneko, Y.; Kadokawa, J.I. Novel gelling systems of κ-, ι-and λ-carrageenans and their composite gels with cellulose using ionic liquid. Macromol. Biosci. 2009, 9, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Prabaharan, M.; Grailer, J.J.; Steerber, D.A.; Gong, S. Stimuli-responsive chitosan-graft-poly(N-vinylcaprolactam) as a promising material for controlled hydrophobic drug delivery. Macromol. Biosci. 2008, 8, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, C.; Jin, H.; Jiang, B.; Zhu, X.; Zhou, Y.; Lu, Z.; Yan, D. A supramolecular Janus hyperbranched polymer and its photoresponsive self-assembly of vesicles with narrow size distribution. J. Am. Chem. Soc. 2013, 135, 4765–4770. [Google Scholar] [CrossRef] [PubMed]
- Caykara, T.; Demirci, S.; Eroğlu, M.S.; Güven, O. Poly (ethylene oxide) and its blends with sodium alginate. Polymer 2005, 46, 10750–10757. [Google Scholar] [CrossRef]
- Król, Ż.; Malik, M.; Marycz, K.; Jarmoluk, A. Physicochemical properties of biopolymer hydrogels treated by direct electric current. Polymers 2016, 8, 248. [Google Scholar] [CrossRef]
- Ghori, M.; Conway, B. Hydrophilic matrices for oral control drug delivery. Am. J. Pharmacol. Sci. 2015, 3, 103–109. [Google Scholar] [CrossRef]
- Dyson, M.; Young, S.; Pendle, C.L.; Webster, D.F.; Lang, S.M. Comparison of the effects of moist and dry conditions on dermal repair. J. Investig. Dermatol. 1988, 9, 434–439. [Google Scholar] [CrossRef]
- Queen, D.; Gaylor, J.; Evans, J.; Courtney, J.; Reid, W. The preclinical evaluation of the water vapour transmission rate through burn wound dressings. Biomaterials 1987, 8, 367–371. [Google Scholar] [CrossRef]
- Momoh, F.; Boateng, J.S.; Richardson, S.; Chowdhry, B.; Mitchell, J. Development and functional characterization of alginate dressing as potential protein delivery system for wound healing. Int. J. Biol. Macromol. 2015, 81, 137–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tobyn, M.; Johnson, J.; Dettmar, P. Factors affecting in vitro gastric mucoadhesion IV. Influence of tablet excipients, surfactants and salts on the observed mucoadhesion of polymers. Eur. J. Pharm. Biopharm. 1997, 43, 65–71. [Google Scholar] [CrossRef]










| Starting Material | CARR:SA (BLK) (mg) | CARR:SA (0.1% RL) (mg) | CARR:SA (0.1% SL) (mg) | CARR:SA (0.2% RL) (mg) | CARR:SA (5% SL) (mg) |
|---|---|---|---|---|---|
| CARR | 375.0 | 375.0 | 375.0 | 375.0 | 375.0 |
| SA | 1125.0 | 1125.0 | 1125.0 | 1125.0 | 1125.0 |
| RL | - | 1.5 | 1.5 | - | - |
| SL | - | 1.5 | 1.5 | - | |
| RL | - | - | - | 3.0 | - |
| SL | - | - | - | - | 75.0 |
| (a) | |||||||
| Gel Content (% w/w) | Hardness of BLK Single Wafers CARR:SA (N) | Hardness of BLK Composite Wafers CARR:SA (N) | |||||
| 1% | 1:0 | 0:1 | 1:1 | 1:2 | 1:3 | 2:1 | 3:1 |
| 1 | 1.55(±0.2) | 0.61(±0.1) | 0.24(±0.1) | 0.87(±0.1) | 0.23(±0.0) | 0.40(±0.0) | 0.13(±0.0) |
| 2 | 1.53(±0.2) | 0.63(±0.2) | 0.32(±0.1) | 0.70(±0.2) | 0.18(±0.0) | 0.39(±0.0) | 0.11(±0.0) |
| 3 | 1.48(±0.2) | 0.57(±0.1) | 0.28(±0.0) | 0.71(±0.2) | 0.29(±0.0) | 0.47(±0.1) | 0.11(±0.0) |
| 4 | 1.29(±0.2) | 0.61(±0.1) | 0.48(±0.1) | 0.87(±0.2) | 0.28(±0.0) | 0.43(±0.1) | 0.13(±0.0) |
| 1.5% | 1:0 | 0:1 | |||||
| 1 | 0.40(±0.1) | 2.16(±0.2) | 4.02(±0.6) | 4.33(±0.4) | 4.50(±0.6) | 0.80(±0.1) | 0.44(±0.1) |
| 2 | 0.35(±0.1) | 2.08(±0.3) | 4.33(±0.5) | 4.11(±0.4) | 3.89(±0.6) | 0.73(±0.1) | 0.39(±0.1) |
| 3 | 0.46(±0.1) | 1.94(±0.3) | 4.68(±0.1) | 3.78(±0.5) | 3.90(±0.6) | 0.74(±0.1) | 0.44(±0.1) |
| 4 | 0.33(±0.1) | 1.83(±0.4) | 4.12(±0.6) | 3.59(±0.4) | 4.03(±0.8) | 0.65(±0.1) | 0.44(±0.1) |
| 2% | 1:0 | 0:1 | |||||
| 1 | 0.60(±0.1) | 4.40(±1.0) | 8.34(±1.5) | 4.77(±0.3) | 7.72(±1.3) | 0.74(±0.2) | 3.75(±0.2) |
| 2 | 0.67(±0.2) | 6.22(±1.1) | 8.52(±0.5) | 4.26(±0.5) | 7.65(±0.8) | 0.47(±0.1) | 3.02(±0.3) |
| 3 | 0.42(±0.1) | 5.04(±1.0) | 8.30(±0.5) | 4.82(±0.5) | 8.29(±1.3) | 0.62(±0.3) | 3.66(±0.4) |
| 4 | 0.60(±0.1) | 4.44(±1.0) | 9.81(±0.4) | 4.79(±0.5) | 8.39(±1.0) | 0.52(±0.1) | 2.99(±0.3) |
| 2.5% | 1:0 | 0:1 | |||||
| 1 | 0.83(±0.2) | - | - | - | - | - | - |
| 2 | 0.86(±0.0) | - | - | - | - | - | - |
| 3 | 0.97(±0.1) | - | - | - | - | - | - |
| 4 | 0.90(±0.1) | - | - | - | - | - | - |
| 3% | 1:0 | 0:1 | |||||
| 1 | 1.76(±0.3) | - | - | - | - | - | - |
| 2 | 1.12(±0.2) | - | - | - | - | - | - |
| 3 | 1.51(±0.2) | - | - | - | - | - | - |
| 4 | 1.39(±0.3) | - | - | - | - | - | - |
| (b) | |||||||
| 1.5% (1:3) DL Wafers | 0.1% RL | 0.2% RL | 0.1% SL | 5% SL N | |||
| 1 | 2.77(±0.5) | 2.78(±0.3) | 2.86(±0.3) | 3.43(±0.3) | |||
| 2 | 2.63(±0.4) | 2.81(±0.3) | 2.94(±0.4) | 3.14(±0.3) | |||
| 3 | 2.60(±0.5) | 3.09(±0.2) | 3.18(±0.5) | 3.40(±0.2) | |||
| 4 | 2.81(±0.4) | 3.09(±0.4) | 2.97(±0.4) | 3.93(±0.6) | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akiyode, O.; Boateng, J. Composite Biopolymer-Based Wafer Dressings Loaded with Microbial Biosurfactants for Potential Application in Chronic Wounds. Polymers 2018, 10, 918. https://doi.org/10.3390/polym10080918
Akiyode O, Boateng J. Composite Biopolymer-Based Wafer Dressings Loaded with Microbial Biosurfactants for Potential Application in Chronic Wounds. Polymers. 2018; 10(8):918. https://doi.org/10.3390/polym10080918
Chicago/Turabian StyleAkiyode, Olufunke, and Joshua Boateng. 2018. "Composite Biopolymer-Based Wafer Dressings Loaded with Microbial Biosurfactants for Potential Application in Chronic Wounds" Polymers 10, no. 8: 918. https://doi.org/10.3390/polym10080918