Photo Processing for Biomedical Hydrogels Design and Functionality: A Review
Abstract
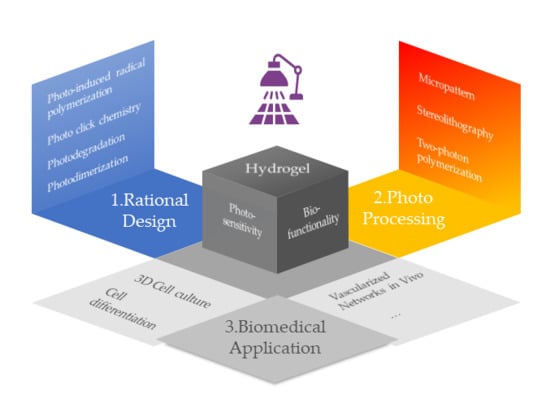
:1. Introduction
2. Rational Design of Hydrogel for Photo-Induced Construction and Modification
2.1. Light Curing Hydrogels Design
2.1.1. Photo initiated Polymerization
2.1.2. Photoclick Chemistry
2.2. Photodegradable Hydrogels Design
2.3. Photodimerization Hydrogel Design
3. Processing
3.1. Micropatterning
3.1.1. Working Principles and Traits of Micropattern
3.1.2. Hydrogel Feasibility and Limitations for Micropattern
3.2. Stereolithography (SLA)
3.2.1. Working Principles and Traits of SLA
3.2.2. Hydrogel Feasibility and Limitations for SLA
3.3. Two-Photon Polymerizations (TPP)
3.3.1. Working Principles and Traits of TPP
3.3.2. Hydrogel Feasibility and Limitations for TPP
4. Biomedical Applications
4.1. Three-Dimensional Cell Culture and Cell Behavior Research
4.2. Cell Differentiation
4.3. Vascularized Networks In Vivo
5. Summary and Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rosales, A.M.; Anseth, K.S. The design of reversible hydrogels to capture extracellular matrix dynamics. Nat. Rev. Mater. 2016, 1, 15012. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.R.; Bae, H.; Cha, J.M.; Mun, J.Y.; Chen, Y.C.; Tekin, H.; Shin, H.; Farshchi, S.; Dokmeci, M.R.; Tang, S.; et al. Carbon nanotube reinforced hybrid microgels as scaffold materials for cell encapsulation. ACS Nano 2012, 6, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Yuk, H.; Zhang, T.; Parada, G.A.; Koo, H.; Yu, C.; Zhao, X. Stretchable Hydrogel Electronics and Devices. Adv. Mater. 2016, 28, 4497–4505. [Google Scholar] [CrossRef] [PubMed]
- Green, J.J.; Elisseeff, J.H. Mimicking biological functionality with polymers for biomedical applications. Nature 2016, 540, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Klotz, B.J.; Gawlitta, D.; Rosenberg, A.J.W.P.; Malda, J.; Melchels, F.P.W. Gelatin-Methacryloyl Hydrogels: Towards Biofabrication-Based Tissue Repair. Trends Biotechnol. 2016, 34, 394–407. [Google Scholar] [CrossRef] [PubMed]
- Yue, K.; Li, X.; Schrobback, K.; Sheikhi, A.; Annabi, N.; Leijten, J.; Zhang, W.; Zhang, Y.S.; Hutmacher, D.W.; Klein, T.J.; et al. Structural analysis of photocrosslinkable methacryloyl-modified protein derivatives. Biomaterials 2017, 139, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Olsen, B.D.; Khademhosseini, A. Gellan gum microgel-reinforced cell-laden gelatin hydrogels. J. Mater. Chem. B. Mater. Biol. Med. 2014, 2, 2508–2516. [Google Scholar] [CrossRef] [PubMed]
- Desai, R.M.; Koshy, S.T.; Hilderbrand, S.A.; Mooney, D.J.; Joshi, N.S. Versatile click alginate hydrogels crosslinked via tetrazine-norbornene chemistry. Biomaterials 2015, 50, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Wu, B.; Raymond, J.E.; Zeng, Y.; Fang, X.; Wooley, K.L.; Liu, W.R. A genetically encoded acrylamide functionality. ACS Chem. Biol. 2013, 8, 1664–1670. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kong, Y.P.; Niedzielski, S.M.; Singh, R.K.; Putnam, A.J.; Shikanov, A. Characterization of the crosslinking kinetics of multi-arm poly(ethylene glycol) hydrogels formed via Michael-type addition. Soft Matter 2016. [Google Scholar] [CrossRef] [PubMed]
- Aubin, H.; Nichol, J.W.; Hutson, C.B.; Bae, H.; Sieminski, A.L.; Cropek, D.M.; Akhyari, P.; Khademhosseini, A. Directed 3D cell alignment and elongation in microengineered hydrogels. Biomaterials 2010, 31, 6941–6951. [Google Scholar] [CrossRef] [PubMed]
- Nikkhah, M.; Eshak, N.; Zorlutuna, P.; Annabi, N.; Castello, M.; Kim, K.; Dolatshahi-Pirouz, A.; Edalat, F.; Bae, H.; Yang, Y.; et al. Directed endothelial cell morphogenesis in micropatterned gelatin methacrylate hydrogels. Biomaterials 2012, 33, 9009–9018. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.T.; García, J.R.; Paez, J.I.; Singh, A.; Phelps, E.A.; Weis, S.; Shafiq, Z.; Shekaran, A.; del Campo, A.; García, A.J. Light-triggered in vivo activation of adhesive peptides regulates cell adhesion, inflammation and vascularization of biomaterials. Nat. Mater. 2014, 14, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.Z.; Chen, Y.C.; Moreno-Luna, R.; Khademhosseini, A.; Melero-Martin, J.M. Transdermal regulation of vascular network bioengineering using aphotopolymerizable methacrylated gelatin hydrogel. Biomaterials 2013, 34, 6785–6796. [Google Scholar] [CrossRef] [PubMed]
- Bin Imran, A.; Seki, T.; Takeoka, Y.; Imran, A. Bin Recent advances in hydrogels in terms of fast stimuli responsiveness and superior mechanical performance. Polym. J. 2010, 42, 839–851. [Google Scholar] [CrossRef]
- Weber, W.; Fussenegger, M. Emerging biomedical applications of synthetic biology. Nat. Rev. Genet. 2012, 13, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Burdick, J.A.; Murphy, W.L. Moving from static to dynamic complexity in hydrogel design. Nat. Commun. 2012, 3, 1269. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat. Rev. Drug Discov. 2014, 13, 813–827. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zhang, Y.S.; Santiago, G.T.; Alvarez, M.M.; Ribas, J.; Jonas, S.J.; Weiss, P.S.; Andrews, A.M.; Aizenberg, J.; Khademhosseini, A. Interplay between materials and microfluidics. Nat. Rev. Mater. 2017, 2, 17016. [Google Scholar] [CrossRef]
- Xing, J.-F.; Zheng, M.-L.; Duan, X.-M. Two-photon polymerization microfabrication of hydrogels: An advanced 3D printing technology for tissue engineering and drug delivery. Chem. Soc. Rev. 2015, 44, 5031–5039. [Google Scholar] [CrossRef] [PubMed]
- Nichol, J.W.; Koshy, S.T.; Bae, H.; Hwang, C.M.; Yamanlar, S.; Khademhosseini, A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 2010, 31, 5536–5544. [Google Scholar] [CrossRef] [PubMed]
- Van Den Steen, P.E.; Dubois, B.; Nelissen, I.; Rudd, P.M.; Dwek, R.A.; Opdenakker, G. Biochemistry and molecular biology of gelatinase B matrix metalloproteinase-9 (MMP-9). Crit. Rev. Biochem. Mol. Biol. 2002, 37, 375–536. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Gungor-Ozkerim, P.S.; Zhang, Y.S.; Yue, K.; Zhu, K.; Liu, W.; Pi, Q.; Byambaa, B.; Dokmeci, M.R.; Shin, S.R.; et al. Direct 3D bioprinting of perfusable vascular constructs using a blend bioink. Biomaterials 2016, 106, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Hoch, E.; Hirth, T.; Tovar, G.E.M.; Borchers, K. Chemical tailoring of gelatin to adjust its chemical and physical properties for functional bioprinting. J. Mater. Chem. B 2013, 1, 5675. [Google Scholar] [CrossRef]
- Kolesky, D.B.; Homan, K.A.; Skylar-Scott, M.A.; Lewis, J.A. Three-dimensional bioprinting of thick vascularized tissues. Proc. Natl. Acad. Sci. USA 2016, 113, 3179–3184. [Google Scholar] [CrossRef] [PubMed]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 2011, 23, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Seidlits, S.K.; Schmidt, C.E.; Shear, J.B. High-resolution patterning of hydrogels in three dimensions using direct-write photofabrication for cell guidance. Adv. Funct. Mater. 2009, 19, 3543–3551. [Google Scholar] [CrossRef]
- Yu, F.; Cao, X.; Li, Y.; Chen, X. Diels-Alder Click-Based Hydrogels for Direct Spatiotemporal Postpatterning via Photoclick Chemistry. ACS Macro Lett. 2015, 4, 289–292. [Google Scholar] [CrossRef]
- Knall, A.-C.; Slugovc, C. Inverse electron demand Diels-Alder (iEDDA)-initiated conjugation: A (high) potential click chemistry scheme. Chem. Soc. Rev. 2013, 42, 5131–5142. [Google Scholar] [CrossRef] [PubMed]
- Azagarsamy, M.A.; Marozas, I.A.; Spaans, S.; Anseth, K.S. Photoregulated Hydrazone-Based Hydrogel Formation for Biochemically Patterning 3D Cellular Microenvironments. ACS Macro Lett. 2016, 5, 19–23. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Bowman, C.N. Thiol-ene click chemistry. Angew. Chem. Int. Ed. 2010, 49, 1540–1573. [Google Scholar] [CrossRef] [PubMed]
- Shih, H.; Fraser, A.K.; Lin, C.C. Interfacial thiol-ene photoclick reactions for forming multilayer hydrogels. ACS Appl. Mater. Interfaces 2013, 5, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Ki, C.S.; Shih, H. Thiol-norbornene photoclick hydrogels for tissue engineering applications. J. Appl. Polym. Sci. 2015, 132, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Olsen, B.D.; Khademhosseini, A. The mechanical properties and cytotoxicity of cell-laden double-network hydrogels based on photocrosslinkable gelatin and gellan gum biomacromolecules. Biomaterials 2012, 33, 3143–3152. [Google Scholar] [CrossRef] [PubMed]
- Dubruel, P.; Unger, R.; Van Vlierberghe, S.; Cnudde, V.; Jacobs, P.J.S.; Schacht, E.; Kirkpatrick, C.J. Porous gelatin hydrogels: 2. In vitro cell interaction study. Biomacromolecules 2007, 8, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Brigo, L.; Urciuolo, A.; Giulitti, S.; Della Giustina, G.; Tromayer, M.; Liska, R.; Elvassore, N.; Brusatin, G. 3D high-resolution two-photon crosslinked hydrogel structures for biological studies. Acta Biomater. 2017, 55, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Skardal, A.; Sarker, S.F.; Crabbé, A.; Nickerson, C.A.; Prestwich, G.D. The generation of 3-D tissue models based on hyaluronan hydrogel-coated microcarriers within a rotating wall vessel bioreactor. Biomaterials 2010, 31, 8426–8435. [Google Scholar] [CrossRef] [PubMed]
- Dicker, K.T.; Gurski, L.A.; Pradhan-Bhatt, S.; Witt, R.L.; Farach-Carson, M.C.; Jia, X. Hyaluronan: A simple polysaccharide with diverse biological functions. Acta Biomater. 2014, 10, 1558–1570. [Google Scholar] [CrossRef] [PubMed]
- Caliari, S.R.; Burdick, J.A. A practical guide to hydrogels for cell culture. Nat. Methods 2016, 13, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.E.; Barth, T.A.; Bencherif, S.A.; Washburn, N.R. Complex fluids based on methacrylated hyaluronic acid. Biomacromolecules 2010, 11, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Schweller, R.M.; West, J.L. Encoding Hydrogel Mechanics via Network Cross-Linking Structure. ACS Biomater. Sci. Eng. 2015, 1, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.T.; Marchesan, S.; Evans, R.A.; Styan, K.E.; Such, G.K.; Postma, A.; McLean, K.M.; Muir, B.W.; Caruso, F. Photoinitiated alkyne-azide click and radical cross-linking reactions for the patterning of PEG hydrogels. Biomacromolecules 2012, 13, 889–895. [Google Scholar] [CrossRef] [PubMed]
- DeForest, C.A.; Anseth, K.S. Cytocompatible click-based hydrogels with dynamically tunable properties through orthogonal photoconjugation and photocleavage reactions. Nat. Chem. 2011, 3, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Fu, Y.; Chung, W.; Zheng, X.; Cui, Y.; Hsu, I.C.; Kao, W.J. Thiol-ene-based biological/synthetic hybrid biomatrix for 3-D living cell culture. Acta Biomater. 2012, 8, 2504–2516. [Google Scholar] [CrossRef] [PubMed]
- Alge, D.L.; Azagarsamy, M.A.; Donohue, D.F.; Anseth, K.S. Synthetically tractable click hydrogels for three-dimensional cell culture formed using tetrazine-norbornene chemistry. Biomacromolecules 2013, 14, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Shih, H.; Lin, C.C. Cross-linking and degradation of step-growth hydrogels formed by thiol-ene photoclick chemistry. Biomacromolecules 2012, 13, 2003–2012. [Google Scholar] [CrossRef] [PubMed]
- Shih, H.; Lin, C.C. Photoclick hydrogels prepared from functionalized cyclodextrin and poly(ethylene glycol) for drug delivery and in situ cell encapsulation. Biomacromolecules 2015, 16, 1915–1923. [Google Scholar] [CrossRef] [PubMed]
- Greene, T.; Lin, C.C. Modular Cross-Linking of Gelatin-Based Thiol-Norbornene Hydrogels for in Vitro 3D Culture of Hepatocellular Carcinoma Cells. ACS Biomater. Sci. Eng. 2015, 1, 1314–1323. [Google Scholar] [CrossRef]
- Truong, V.X.; Tsang, K.M.; Ercole, F.; Forsythe, J.S. Red Light Activation of Tetrazine-Norbornene Conjugation for Bioorthogonal Polymer Cross-Linking across Tissue. Chem. Mater. 2017, 29, 3678–3685. [Google Scholar] [CrossRef]
- Karimi, M.; Sahandi Zangabad, P.; Baghaee-Ravari, S.; Ghazadeh, M.; Mirshekari, H.; Hamblin, M.R. Smart Nanostructures for Cargo Delivery: Uncaging and Activating by Light. J. Am. Chem. Soc. 2017, 139, 4584–4610. [Google Scholar] [CrossRef] [PubMed]
- Norris, S.C.P.; Tseng, P.; Kasko, A.M. Direct Gradient Photolithography of Photodegradable Hydrogels with Patterned Stiffness Control with Submicrometer Resolution. ACS Biomater. Sci. Eng. 2016, 2, 1309–1318. [Google Scholar] [CrossRef]
- Cheng, W.; Wu, D.; Liu, Y. Michael Addition Polymerization of Trifunctional Amine and Acrylic Monomer: A Versatile Platform for Development of Biomaterials. Biomacromolecules 2016, 17, 3115–3126. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Jie, K.; Huang, F. Supramolecular Amphiphiles Based on Host-Guest Molecular Recognition Motifs. Chem. Rev. 2015, 115, 7240–7303. [Google Scholar] [CrossRef] [PubMed]
- Wosnick, J.H.; Shoichet, M.S. Three-dimensional chemical patterning of transparent hydrogels. Chem. Mater. 2008, 20, 55–60. [Google Scholar] [CrossRef]
- Mahmoodi, M.M.; Fisher, S.A.; Tam, R.Y.; Goff, P.C.; Anderson, R.B.; Wissinger, J.E.; Blank, D.A.; Shoichet, M.S.; Distefano, M.D. 6-Bromo-7-hydroxy-3-methylcoumarin (mBhc) is an efficient multi-photon labile protecting group for thiol caging and three-dimensional chemical patterning. Org. Biomol. Chem. 2016, 14, 8289–8300. [Google Scholar] [CrossRef] [PubMed]
- Tam, R.Y.; Smith, L.J.; Shoichet, M.S. Engineering Cellular Microenvironments with Photo- and Enzymatically Responsive Hydrogels: Toward Biomimetic 3D Cell Culture Models. Acc. Chem. Res. 2017, 50, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi, M.M.; Abate-Pella, D.; Pundsack, T.J.; Palsuledesai, C.C.; Goff, P.C.; Blank, D.A.; Distefano, M.D. Nitrodibenzofuran: A One-and Two-Photon Sensitive Protecting Group That Is Superior to Brominated Hydroxycoumarin for Thiol Caging in Peptides. J. Am. Chem. Soc. 2016, 138, 5848–5859. [Google Scholar] [CrossRef] [PubMed]
- Wells, L.A.; Brook, M.A.; Sheardown, H. Generic, Anthracene-Based Hydrogel Crosslinkers for Photo-controllable Drug Delivery. Macromol. Biosci. 2011, 11, 988–998. [Google Scholar] [CrossRef] [PubMed]
- Ihmels, H.; Luo, J. The reversible [4 + 4] photocycloaddition of acridizinium derivatives. J. Photochem. Photobiol. A Chem. 2008, 200, 3–9. [Google Scholar] [CrossRef]
- Huq, N.A.; Ekblad, J.R.; Leonard, A.T.; Scalfani, V.F.; Bailey, T.S. Phototunable Thermoplastic Elastomer Hydrogel Networks. Macromolecules 2017, 50, 1331–1341. [Google Scholar] [CrossRef]
- Froimowicz, P.; Frey, H.; Landfester, K. Towards the generation of self-healing materials by means of a reversible photo-induced approach. Macromol. Rapid Commun. 2011, 32, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Truong, V.X.; Li, F.; Forsythe, J.S. Versatile Bioorthogonal Hydrogel Platform by Catalyst-Free Visible Light Initiated Photodimerization of Anthracene. ACS Macro Lett. 2017, 657–662. [Google Scholar] [CrossRef]
- Zdobinsky, T.; Sankar Maiti, P.; Klajn, R. Support curvature and conformational freedom control chemical reactivity of immobilized species. J. Am. Chem. Soc. 2014, 136, 2711–2714. [Google Scholar] [CrossRef] [PubMed]
- Claus, T.K.; Telitel, S.; Welle, A.; Bastmeyer, M.; Vogt, A.P.; Delaittre, G.; Barner-Kowollik, C. Light-driven reversible surface functionalization with anthracenes: Visible light writing and mild UV erasing. Chem. Commun. 2017, 53, 1599–1602. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Sun, Y.; Kaplan, J.A.; Grinstaff, M.W.; Parquette, J.R. Photo-crosslinking of a self-assembled coumarin-dipeptide hydrogel. New J. Chem. 2015, 39, 3225–3228. [Google Scholar] [CrossRef]
- Azagarsamy, M.A.; McKinnon, D.D.; Alge, D.L.; Anseth, K.S. Coumarin-based photodegradable hydrogel: Design, synthesis, gelation, and degradation kinetics. ACS Macro Lett. 2014, 3, 515–519. [Google Scholar] [CrossRef]
- Huang, Q.; Bao, C.; Ji, W.; Wang, Q.; Zhu, L. Photocleavable coumarin crosslinkers based polystyrene microgels: Phototriggered swelling and release. J. Mater. Chem. 2012, 22, 18275. [Google Scholar] [CrossRef]
- Annabi, N.; Tamayol, A.; Uquillas, J.A.; Akbari, M.; Bertassoni, L.E.; Cha, C.; Camci-Unal, G.; Dokmeci, M.R.; Peppas, N.A.; Khademhosseini, A. 25th anniversary article: Rational design and applications of hydrogels in regenerative medicine. Adv. Mater. 2014, 26, 85–124. [Google Scholar] [CrossRef] [PubMed]
- Tsang, V.L.; Bhatia, S.N. Fabrication of three-dimensional tissues. Adv. Biochem. Eng. Biotechnol. 2006, 103, 189–205. [Google Scholar] [CrossRef]
- Fernandez, J.G.; Khademhosseini, A. Micro-masonry: Construction of 3D structures by microscale self-assembly. Adv. Mater. 2010, 22, 2538–2541. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Lo, E.; Vidula, M.K.; Khabiry, M.; Khademhosseini, A. Method of Bottom-Up Directed Assembly of Cell-Laden Microgels. Cell. Mol. Bioeng. 2008, 1, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Tsang, V.L.; Chen, A.A.; Cho, L.M.; Jadin, K.D.; Sah, R.L.; DeLong, S.; West, J.L.; Bhatia, S.N. Fabrication of 3D hepatic tissues by additive photopatterning of cellular hydrogels. FASEB J. 2007, 21, 790–801. [Google Scholar] [CrossRef] [PubMed]
- Cells, L. Hybrid Bio/Artificial Microdevices: Three-Dimensional Photopatterning of Hydrogels Containing Living Cells. Biomed. Microdevices 2002, 4, 257–266. [Google Scholar] [CrossRef]
- Albrecht, D.R.; Tsang, V.L.; Sah, R.L.; Bhatia, S.N. Photo- and electropatterning of hydrogel-encapsulated living cell arrays. Lab Chip 2005, 5, 111. [Google Scholar] [CrossRef] [PubMed]
- Annabi, N.; Mithieux, S.M.; Zorlutuna, P.; Camci-Unal, G.; Weiss, A.S.; Khademhosseini, A. Engineered cell-laden human protein-based elastomer. Biomaterials 2013, 34, 5496–5505. [Google Scholar] [CrossRef] [PubMed]
- Ovsianikov, A.; Deiwick, A.; Van Vlierberghe, S.; Pflaum, M.; Wilhelmi, M.; Dubruel, P.; Chichkov, B. Laser fabrication of 3D gelatin scaffolds for the generation of bioartificial tissues. Materials 2010, 4, 288–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kufelt, O.; El-Tamer, A.; Sehring, C.; Schlie-Wolter, S.; Chichkov, B.N. Hyaluronic acid based materials for scaffolding via two-photon polymerization. Biomacromolecules 2014, 15, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Melchels, F.P.W.; Feijen, J.; Grijpma, D.W. A review on stereolithography and its applications in biomedical engineering. Biomaterials 2010, 31, 6121–6130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suwandi, J.S.; Toes, R.E.M.; Nikolic, T.; Roep, B.O. Inducing tissue specific tolerance in autoimmune disease with tolerogenic dendritic cells. Clin. Exp. Rheumatol. 2015, 33, 97–103. [Google Scholar] [CrossRef]
- Khademhosseini, A.; Langer, R. Microengineered hydrogels for tissue engineering. Biomaterials 2007, 28, 5087–5092. [Google Scholar] [CrossRef] [PubMed]
- Hutmacher, D.W.; Sittinger, M.; Risbud, M.V. Scaffold-based tissue engineering: Rationale for computer-aided design and solid free-form fabrication systems. Trends Biotechnol. 2004, 22, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Shoichet, M.S. A photolabile hydrogel for guided three-dimensional cell growth and migration. Nat. Mater. 2004, 3, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Dhariwala, B.; Hunt, E.; Boland, T. Rapid Prototyping of Tissue-Engineering Constructs, Using Photopolymerizable Hydrogels and Stereolithography. Tissue Eng. 2004, 10, 1316–1322. [Google Scholar] [CrossRef] [PubMed]
- Ovsianikov, A.; Gruene, M.; Pflaum, M.; Koch, L.; Maiorana, F.; Wilhelmi, M.; Haverich, A.; Chichkov, B. Laser Printing of Cells into 3D Scaffolds 1 M Pflaum. Biofabrication 2010, 2, 014104. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, S.; Hoch, E.; Borchers, K.; Meyer, W.; Krüger, H.; Tovar, G.E.M.; Gillner, A. Fabrication of 2D protein microstructures and 3D polymer–protein hybrid microstructures by two-photon polymerization. Biofabrication 2011, 3, 25003. [Google Scholar] [CrossRef] [PubMed]
- Madaghiele, M.; Piccinno, A.; Saponaro, M.; Maffezzoli, A.; Sannino, A. Collagen- and gelatine-based films sealing vascular prostheses: Evaluation of the degree of crosslinking for optimal blood impermeability. J. Mater. Sci. Mater. Med. 2009, 20, 1979–1989. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.X.F.; Mo, X.M.; Teoh, S.H.; Hutmacher, D.W. Scaffold development using 3D printing with a starch-based polymer. Mater. Sci. Eng. C 2002, 20, 49–56. [Google Scholar] [CrossRef]
- Zorlutuna, P.; Annabi, N.; Camci-Unal, G.; Nikkhah, M.; Cha, J.M.; Nichol, J.W.; Manbachi, A.; Bae, H.; Chen, S.; Khademhosseini, A. Microfabricated biomaterials for engineering 3D tissues. Adv. Mater. 2012, 24, 1782–1804. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; He, J.; Nichol, J.W.; Wang, L.; Hutson, C.B.; Wang, B.; Du, Y.; Fan, H.; Khademhosseini, A. Synthesis and characterization of photocrosslinkable gelatin and silk fibroin interpenetrating polymer network hydrogels. Acta Biomater. 2011, 7, 2384–2393. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Lo, E.; Ali, S.; Khademhosseini, A. Directed assembly of cell-laden microgels for fabrication of 3D tissue constructs. Proc. Natl. Acad. Sci. USA 2008, 105, 9522–9527. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.Y.; Griffin, D.R.; Reed, J.; Kasko, A.M. Photodegradable hydrogels to generate positive and negative features over multiple length scales. Macromolecules 2010, 43, 2824–2831. [Google Scholar] [CrossRef]
- Jain, G.; Ford, A.J.; Rajagopalan, P. Opposing Rigidity-Protein Gradients Reverse Fibroblast Durotaxis. ACS Biomater. Sci. Eng. 2015, 1, 621–631. [Google Scholar] [CrossRef]
- García, S.; Sunyer, R.; Olivares, A.; Noailly, J.; Atencia, J.; Trepat, X. Generation of stable orthogonal gradients of chemical concentration and substrate stiffness in a microfluidic device. Lab Chip 2015, 15, 2606–2614. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.L.; Khetan, S.; Baker, B.M.; Chen, C.S.; Burdick, J.A. Chondrogenesis through Mechanical and Adhesive Cues. Biomaterials 2014, 34, 5571–5580. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Mao, Z.; Tan, H.; Han, L.; Ren, T.; Gao, C. Gradient biomaterials and their influences on cell migration. Interface Focus 2012, 2, 337–355. [Google Scholar] [CrossRef] [PubMed]
- Bahney, C.S.; Lujan, T.J.; Hsu, C.W.; Bottlang, M.; West, J.L.; Johnstone, B. Visible light photoinitiation of mesenchymal stem cell-laden bioresponsive hydrogels. Eur. Cells Mater. 2011, 22, 43–55. [Google Scholar] [CrossRef]
- Nguyen, K.T.; West, J.L. Photopolymerizable hydrogels for tissue engineering applications. Biomaterials 2002, 23, 4307–4314. [Google Scholar] [CrossRef]
- Bigi, A.; Cojazzi, G.; Panzavolta, S.; Roveri, N.; Rubini, K. Stabilization of gelatin films by crosslinking with genipin. Biomaterials 2002, 23, 4827–4832. [Google Scholar] [CrossRef]
- Stratakis, E.; Ranella, A.; Farsari, M.; Fotakis, C. Laser-based micro/nanoengineering for biological applications. Prog. Quantum Electron. 2009, 33, 127–163. [Google Scholar] [CrossRef]
- Billiet, T.; Vandenhaute, M.; Schelfhout, J.; Van Vlierberghe, S.; Dubruel, P. A review of trends and limitations in hydrogel-rapid prototyping for tissue engineering. Biomaterials 2012, 33, 6020–6041. [Google Scholar] [CrossRef] [PubMed]
- Cooke, M.N.; Fisher, J.P.; Dean, D.; Rimnac, C.; Mikos, A.G. Use of stereolithography to manufacture critical-sized 3D biodegradable scaffolds for bone ingrowth. J. Biomed. Mater. Res. 2003, 64B, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Arcaute, K.; Mann, B.K.; Wicker, R.B. Stereolithography of three-dimensional bioactive poly(ethylene glycol) constructs with encapsulated cells. Ann. Biomed. Eng. 2006, 34, 1429–1441. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Kang, T.; Rhie, J.W.; Cho, D.W. Development of three-dimensional hybrid scaffold using chondrocyte-encapsulated alginate hydrogel. Sens. Mater. 2007, 19, 445–451. [Google Scholar]
- Lee, S.J.; Kang, H.W.; Park, J.K.; Rhie, J.W.; Hahn, S.K.; Cho, D.W. Application of microstereolithography in the development of three-dimensional cartilage regeneration scaffolds. Biomed. Microdevices 2008, 10, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Mapili, G.; Suhali, G.; Chen, S.; Roy, K. A digital micro-mirror device-based system for the microfabrication of complex, spatially patterned tissue engineering scaffolds. J. Biomed. Mater. Res. Part A 2006, 77, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Zorlutuna, P.; Jeong, J.H.; Kong, H.; Bashir, R. Stereolithography-based hydrogel microenvironments to examine cellular interactions. Adv. Funct. Mater. 2011, 21, 3642–3651. [Google Scholar] [CrossRef]
- Arcaute, K.; Mann, B.; Wicker, R. Stereolithography of spatially controlled multi-material bioactive poly(ethylene glycol) scaffolds. Acta Biomater. 2010, 6, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Melchels, F.P.W.; Bertoldi, K.; Gabbrielli, R.; Velders, A.H.; Feijen, J.; Grijpma, D.W. Mathematically defined tissue engineering scaffold architectures prepared by stereolithography. Biomaterials 2010, 31, 6909–6916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Claeyssens, F.; Hasan, E.A.; Gaidukeviciute, A.; Achilleos, D.S.; Ranella, A.; Reinhardt, C.; Ovsianikov, A.; Shizhou, X.; Fotakis, C.; Vamvakaki, M.; et al. Three dimensional biodegradable structures fabricated by two photon polymerization. Langmuir 2009, 25, 3219–3223. [Google Scholar] [CrossRef] [PubMed]
- Wylie, R.G.; Ahsan, S.; Aizawa, Y.; Maxwell, K.L.; Morshead, C.M.; Shoichet, M.S. Spatially controlled simultaneous patterning of multiple growth factors in three-dimensional hydrogels. Nat. Mater. 2011, 10, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.; Hoop, M.; Pané, S.; Nelson, B.J.; Hierold, C. Degradable Magnetic Composites for Minimally Invasive Interventions: Device Fabrication, Targeted Drug Delivery, and Cytotoxicity Tests. Adv. Mater. 2016, 28, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Nanoscribe GmbH Data Sheet Nanoscribe Photonic Professional GT. 2010. Available online: http://www.nanoscribe.de/files/4514/8179/1302/DataSheet_PPGT_V04_2016_web.pdf (accessed on 22 December 2017).
- Kufelt, O.; El-Tamer, A.; Sehring, C.; Meißner, M.; Schlie-Wolter, S.; Chichkov, B.N. Water-soluble photopolymerizable chitosan hydrogels for biofabrication via two-photon polymerization. Acta Biomater. 2015, 18, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Van Hoorick, J.; Gruber, P.; Markovic, M.; Tromayer, M.; Van Erps, J.; Thienpont, H.; Liska, R.; Ovsianikov, A.; Dubruel, P.; Van Vlierberghe, S. Cross-Linkable Gelatins with Superior Mechanical Properties Through Carboxylic Acid Modification: Increasing the Two-Photon Polymerization Potential. Biomacromolecules 2017, 18, 3260–3272. [Google Scholar] [CrossRef] [PubMed]
- Nicodemus, G.D.; Bryant, S.J. Cell Encapsulation in Biodegradable Hydrogels for Tissue Engineering Applications. Tissue Eng. Part B Rev. 2008, 14, 149–165. [Google Scholar] [CrossRef] [PubMed]
- Gittard, S.D.; Nguyen, A.; Obata, K.; Koroleva, A.; Narayan, R.J.; Chichkov, B.N. Fabrication of microscale medical devices by two-photon polymerization with multiple foci via a spatial light modulator. Biomed. Opt. Express 2011, 2, 3167. [Google Scholar] [CrossRef] [PubMed]
- Wylie, R.G.; Shoichet, M.S. Two-photon micropatterning of amines within an agarose hydrogel. J. Mater. Chem. 2008, 18, 2716–2721. [Google Scholar] [CrossRef]
- Aizawa, Y.; Shoichet, M.S. The role of endothelial cells in the retinal stem and progenitor cell niche within a 3D engineered hydrogel matrix. Biomaterials 2012, 33, 5198–5205. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Highley, C.B.; Rodell, C.B.; Sun, W.; Burdick, J.A. 3D Printing of Shear-Thinning Hyaluronic Acid Hydrogels with Secondary Cross-Linking. ACS Biomater. Sci. Eng. 2016, 2, 1743–1751. [Google Scholar] [CrossRef]
- Ouyang, L.; Highley, C.B.; Sun, W.; Burdick, J.A. A Generalizable Strategy for the 3D Bioprinting of Hydrogels from Nonviscous Photo-crosslinkable Inks. Adv. Mater. 2016, 1604983. [Google Scholar] [CrossRef] [PubMed]
- Pantani, R.; Turng, L.S. 3D bioprinting of photocrosslinkable hydrogel constructs. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Tsurkan, M.V.; Jungnickel, C.; Schlierf, M.; Werner, C. Forbidden Chemistry: Two-Photon Pathway in [2+2] Cycloaddition of Maleimides. J. Am. Chem. Soc. 2017, 139, 10184–10187. [Google Scholar] [CrossRef] [PubMed]
- Yuk, H.; Zhang, T.; Parada, G.A.; Liu, X.; Zhao, X. Skin-inspired hydrogel-elastomer hybrids with robust interfaces and functional microstructures. Nat. Commun. 2016, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Frenzel, T.; Kadic, M.; Wegener, M. Three-dimensional mechanical metamaterials with a twist. Science 2017, 358, 1072–1074. [Google Scholar] [CrossRef] [PubMed]
- Sydney Gladman, A.; Matsumoto, E.A.; Nuzzo, R.G.; Mahadevan, L.; Lewis, J.A. Biomimetic 4D printing. Nat. Mater. 2016, 15, 413–418. [Google Scholar] [CrossRef] [PubMed]











| Hydrogel Backbones | Acylated-Based Resources | Advantages | Drawbacks | Applications | References |
|---|---|---|---|---|---|
| Gelatin | Methacrylic anhydride, methacrylamide |
|
|
| [21,24,34,35,36,37] |
| Hyaluronic acid | methacrylic anhydride, Glycidyl Methacrylate |
|
|
| [26,27,38,39,40,41] |
| PEG | methacrylic anhydride |
|
|
| [23,42,43] |
| Mechanism | Nomenclature | Features | References |
|---|---|---|---|
 | thiol-vinyl or thiol-(meth)acrylate | The presence of initiators increases the polymerization rates and acrylate conversion percentage. | [44,45] |
 | thiol-norbornene | Radical-mediated step-growth and biorthogonal polymerization | [32,46,47,48,49] |
| Techniques | Hydrogel Materials | Photon Chemistry | Advantages | Drawbacks | Resolution | References |
|---|---|---|---|---|---|---|
| Micropattern | PEGDA PEGDM GelMA HA MeTro | Crosslinking Degradation |
|
| 100 μm | [21,26,69,75,76,77] |
| (μ)SLA | GelMA HA HEMA | Crosslinking |
|
| 5–30 μm | [78,79,80,81,82,83,84,85] |
| Two photon polymerizations (2PP) | Fibronectin Bovine Serum Albumin (BSA) PEG-DA | Crosslinking Degradation |
|
| 0.5–1 μm | [85,86,87,88] |
| Modified Aspect | Hydrogel Applied | Operations | Results | Technology Improvement | References |
|---|---|---|---|---|---|
| Material | (Gel-MOD)/(Gel-MA) | Modify the primary amines as well as the carboxylic acids from gelatin with photo-cross-linkable moieties | Superior and tunable mechanical properties | Strengthen the stiffness of single cured voxel, leading to a higher precision and efficiency | [115] |
| Material | HA | HA is covalently cross-linked with poly (ethylene glycol) diacrylate (PEGDA) in situ | Modulate the physical and chemical properties | Generate porous scaffold More efficiency | [78,116] |
| Technology | Resin | Involve holographic data with a spatial light modulator (SLM) | Multiple foci 2PP technique | Higher precision; Shorter fabrication times | [117] |
| Material | Chitosan | n-succinylated followed by a conjugation with glycidyl methacrylate | Convert in a water-soluble species | Higher precision; Generate a diversity of tailor-made scaffold shapes | [114] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, H.; Wang, J.; Mi, S. Photo Processing for Biomedical Hydrogels Design and Functionality: A Review. Polymers 2018, 10, 11. https://doi.org/10.3390/polym10010011
Yao H, Wang J, Mi S. Photo Processing for Biomedical Hydrogels Design and Functionality: A Review. Polymers. 2018; 10(1):11. https://doi.org/10.3390/polym10010011
Chicago/Turabian StyleYao, Hongyi, Jieqiong Wang, and Shengli Mi. 2018. "Photo Processing for Biomedical Hydrogels Design and Functionality: A Review" Polymers 10, no. 1: 11. https://doi.org/10.3390/polym10010011