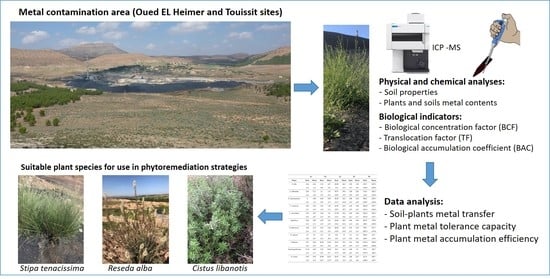
Screening of Native Plants Growing on a Pb/Zn Mining Area in Eastern Morocco: Perspectives for Phytoremediation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Soil and Plant Analysis
2.3. Soil Pollution Index (PI)
[metal]soil: concentration in mg kg−1
2.4. Soil Enrichment Factor (EF)
2.5. Bioconcentration Factor, Translocation Factor, and Biological Accumulation Coefficient
2.6. Data Analysis
3. Results and Discussions
3.1. Site Description
3.2. Soil Properties and Metals Concentrations
3.3. Screening of Native Plant Species in Oued el Heimer and Touissite Areas
3.4. Heavy Metals Concentration in Plants
3.4.1. Arsenic (As)
3.4.2. Cadmium (Cd)
3.4.3. Copper (Cu)
3.4.4. Nickel (Ni)
3.4.5. Lead (Pb)
3.4.6. Zinc (Zn)
3.4.7. Antimony (Sb)
3.5. Plants Polymetallic Accumulation Ability
3.6. Suitable Plant Species for Use in Phytoremediation Strategies
3.6.1. Stipa tenacissima Suitable Plant for Cd/Cu Phytostabilization and Pb Phytoextraction
3.6.2. A. herba-alba Suitable Plant for Cu/Zn Phytostabilization and Pb Phytoextraction
3.6.3. Reseda alba a Powerful Plant Species for Pb Phytoextraction
3.6.4. Cistus libanotis a Good Candidate for Pb Phytoextraction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baize, D.; Tercé, M. Les Éléments Traces Métalliques Dans Les Sols: Approches Fonctionnelles et Spatiales; Editions Quae: Versailles, France, 2002. [Google Scholar]
- Adriano, D.C. Trace Elements in Terrestrial Environments; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2001; pp. 219–261. [Google Scholar]
- Singh, A.; Sharma, R.K.; Agrawal, S.B.; Marshall, F.M. Health risk assessment of heavy metals via dietary intake of foodstuffs from the wastewater irrigated site of a dry tropical area of India. Food Chem. Toxicol. 2010, 48, 611–619. [Google Scholar] [CrossRef]
- Saxena, G.; Purchase, D.; Mulla, S.I.; Saratale, G.D.; Bharagava, R.N. Phytoremediation of Heavy Metal-Contaminated Sites: Eco-environmental Concerns, Field Studies, Sustainability Issues, and Future Prospects. Rev. Environ. Contam. and Toxicol. Vol. 201 2019, 249, 71–131. [Google Scholar] [CrossRef] [Green Version]
- Sanyaolu, V.; Adeniran, A. Determination of Heavy Metal Fallout on the Surrounding Flora and Aquifer: Case Study of a Scrap Metal Smelting Factory in Odogunyan Area, Ikorodu, Lagos-State, Nigeria. Int. Res. J. Environ. Sci. 2014, 3, 93–100. [Google Scholar]
- Angelovičová, L.; Fazekašová, D. Contamination of the soil and water environment by heavy metals in the former mining area of Rudňany (Slovakia). Soil Water Res. 2014, 9, 18–24. [Google Scholar] [CrossRef] [Green Version]
- Lenart-Boroń, A.; Wolny-Koładka, K. Heavy metal concentration and the occurrence of selected microorganisms in soils of a steelworks area in Poland. Plant, Soil Environ. 2016, 61, 273–278. [Google Scholar] [CrossRef] [Green Version]
- Midhat, L.; Ouazzani, N.; Hejjaj, A.; Ouhammou, A.; Mandi, L. Accumulation of heavy metals in metallophytes from three mining sites (Southern Centre Morocco) and evaluation of their phytoremediation potential. Ecotoxicol. Environ. Saf. 2019, 169, 150–160. [Google Scholar] [CrossRef]
- Cao, R.X.; Ma, L.Q.; Chen, M.; Singh, S.P.; Harris, W.G. Phosphate-induced metal immobilization in a contaminated site. Environ. Pollut. 2003, 122, 19–28. [Google Scholar] [CrossRef]
- Mulligan, C.; Yong, R.; Gibbs, B. Remediation technologies for metal-contaminated soils and groundwater: An evaluation. Eng. Geol. 2001, 60, 193–207. [Google Scholar] [CrossRef]
- Mahar, A.; Wang, P.; Ali, A.; Awasthi, M.K.; Lahori, A.H.; Wang, Q.; Li, R.; Zhang, Z. Challenges and opportunities in the phytoremediation of heavy metals contaminated soils: A review. Ecotoxicol. Environ. Saf. 2016, 126, 111–121. [Google Scholar] [CrossRef] [PubMed]
- McGrath, S.; Zhao, F.-J. Phytoextraction of metals and metalloids from contaminated soils. Curr. Opin. Biotechnol. 2003, 14, 277–282. [Google Scholar] [CrossRef]
- Lorestani, B.; Cheraghi, M.; Yousefi, N. Phytoremediation potential of native plants growing on a heavy metals contaminated soil of copper mine in Iran. World Acad. Sci. Eng. Technol. 2011, 77, 377–382. [Google Scholar]
- Baker, A.J.; Brooks, R. Terrestrial higher plants which hyperaccumulate metallic elements. A review of their distribution, ecology and phytochemistry. Biorecovery 1989, 1, 81–126. [Google Scholar]
- Terry, N. Phytoremediation of selenium-contaminated soil and water. Glob. Adv. Selenium Res. Theory Appl. 2015, 197–198. [Google Scholar] [CrossRef]
- Yoon, J.; Cao, X.; Zhou, Q.; Ma, L.Q. Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci. Total Environ. 2006, 368, 456–464. [Google Scholar] [CrossRef]
- Smouni, A.; Ater, M.; Auguy, F.; Laplaze, L.; El Mzibri, M.; Berhada, F.; Maltouf, A.F.; Doumas, P. Évaluation de la contamination par les éléments-traces métalliques dans une zone minière du Maroc oriental. Cah. Agric. 2010, 19, 273–279. [Google Scholar] [CrossRef]
- Fennane, M.; Ibn Tattou, M.; El Oulaidi, J. Flore pratique du Maroc. In Travaux de l’Institut Scientifique; Série Botanique; Travaux de l’Institut Scientifique: Rabat, Morocco, 2014; Volume 3, p. 40. [Google Scholar]
- Fennane, M.; Ibn Tattou, M.; Mathez, J.; Ouyahya, A.; El Oualidi, J. Flore Prafique du Maroc. Manuel de determinafion des plantes vasculaires. In Travaux de L’institut Scientifique; Série Botanique; Travaux de l’Institut Scientifique: Rabat, Morocco, 1999; Volume 1, p. 36. [Google Scholar]
- Fennane, M.; Ibn Tattou, M.; Mathez, J.; Ouyahya, A.; El Oualidi, J. Flore pratique du Maroc. In Travaux Institut Scientifique, Vol. 2; Série Botanique N°38; Travaux de l’Institut Scientifique: Rabat, Morocco, 2007; Volume 38. [Google Scholar]
- Combs, S.; Nathan, M. Soil organic matter. Recommended chemical soil test procedures for the north central region. North Cent. Reg. Res. Pub. 1998, 221, 53–58. [Google Scholar]
- Temminghoff, E.E.; Houba, V.J. Plant Analysis Procedures; Springer: Berlin/Heidelberg, Germany, 2004; Volume 179. [Google Scholar]
- Marguí, E.; Queralt, I.; Carvalho, M.; Hidalgo, M. Comparison of EDXRF and ICP-OES after microwave digestion for element determination in plant specimens from an abandoned mining area. Anal. Chim. Acta 2005, 549, 197–204. [Google Scholar] [CrossRef]
- Chon, H.-T.; Ahn, J.-S.; Jung, M.C. Seasonal Variations and Chemical Forms of Heavy Metals in Soils and Dusts from the Satellite Cities of Seoul, Korea. Environ. Geochem. Health 1998, 20, 77–86. [Google Scholar] [CrossRef]
- Wu, J.; Teng, Y.; Lu, S.; Wang, Y.; Jiao, X. Evaluation of Soil Contamination Indices in a Mining Area of Jiangxi, China. PLoS ONE 2014, 9, e112917. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Pendias, H. Trace Elements in Plants and Soils; CRC Press: Boca Raton, FL, USA, 1984; pp. 233–237. [Google Scholar]
- Bern, C.R.; Walton-Day, K.; Naftz, D.L. Improved enrichment factor calculations through principal component analysis: Examples from soils near breccia pipe uranium mines, Arizona, USA. Environ. Pollut. 2019, 248, 90–100. [Google Scholar] [CrossRef]
- Blaser, P.; Zimmermann, S.; Luster, J.; Shotyk, W. Critical examination of trace element enrichments and depletions in soils: As, Cr, Cu, Ni, Pb, and Zn in Swiss forest soils. Sci. Total Environ. 2000, 249, 257–280. [Google Scholar] [CrossRef]
- Zhang, L.; Ye, X.; Feng, H.; Jing, Y.; Ouyang, T.; Yu, X.; Liang, R.; Gao, C.; Chen, W. Heavy metal contamination in western Xiamen Bay sediments and its vicinity, China. Mar. Pollut. Bull. 2007, 54, 974–982. [Google Scholar] [CrossRef]
- Liu, Y.; Li, S.; Sun, C.; Qi, M.; Yu, X.; Zhao, W.; Li, X. Pollution Level and Health Risk Assessment of PM2.5-Bound Metals in Baoding City Before and After the Heating Period. Int. J. Environ. Res. Public Health 2018, 15, 2286. [Google Scholar] [CrossRef] [Green Version]
- Ahmadpour, P.; Ahmadpour, F.; Mahmud, T.M.M.; Abdu, A.; Soleimani, M.; Tayefeh, F.H. Phytoremediation of heavy metals: A green technology. Afr. J. Biotechnol. 2012, 11, 14036–14043. [Google Scholar] [CrossRef]
- Nazir, A.; Malik, R.N.; Ajaib, M.; Khan, N.; Siddiqui, M.F. Hyperaccumulators of heavy metals of industrial areas of Islamabad and Rawalpindi. Pak. J. Bot. 2011, 43, 1925–1933. [Google Scholar]
- Lamin, H.; Alami, S.; Bouhnik, O.; Elfaik, S.; Abdelmoumen, H.; Bedmar, E.J.; Idrissi, M.M.-E. Nodulation of Retama monosperma by Ensifer aridi in an Abandonned Lead Mine Soils in Eastern Morocco. Front. Microbiol. 2019, 10, 1456. [Google Scholar] [CrossRef]
- Claveau, J.; Paulhac, J.; Pellerin, J. The lead and zinc deposits of the Bou Beker-Touissit area, eastern French Morocco. Econ. Geol. 1952, 47, 481–493. [Google Scholar] [CrossRef]
- Dupuy, J.-J.; Touray, J.-C. Multistage ore deposition at the Oued Mekta strata-bound lead deposit, Touissit-Bou Beker District, eastern Morocco. Econ. Geol. 1986, 81, 1558–1561. [Google Scholar] [CrossRef]
- Makhoukhi, S.; Marignac, C.; Pironon, J.; Schmitt, J.; Marrakchi, C.; Bouabdelli, M.; Bastoul, A. Aqueous and hydrocarbon inclusions in dolomite fromTouissit-Bou Beker district, Eastern Morocco: A Jurassic carbonate hosted PbZn (Cu) deposit. J. Geochem. Explor. 2003, 78, 545–551. [Google Scholar] [CrossRef]
- Navarro, M.; Pérez-Sirvent, C.; Martínez-Sánchez, M.; Vidal, J.; Tovar, P.; Bech, J. Abandoned mine sites as a source of contamination by heavy metals: A case study in a semi-arid zone. J. Geochem. Explor. 2008, 96, 183–193. [Google Scholar] [CrossRef]
- Ma, X.; Zuo, H.; Tian, M.; Zhang, L.; Meng, J.; Zhou, X.; Min, N.; Chang, X.; Liu, Y. Assessment of heavy metals contamination in sediments from three adjacent regions of the Yellow River using metal chemical fractions and multivariate analysis techniques. Chemosphere 2016, 144, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Bliefert, C.; Perraud, R. Chimie de l’environnement: Air, Eau, Sols, Déchets, 1st ed.; De Boeck Université: Bruxelles, Belgique, 2001. [Google Scholar]
- Yassir, B.; Sana, E.F.; Alain, P. Contamination by trace elements of agricultural soils around Sidi Bou Othmane in abandoned mine tailings in Marrakech, Morocco. Pollution Winter. 2016, 2, 93–101. [Google Scholar]
- Zhu, G.; Xiao, H.; Guo, Q.; Song, B.; Zheng, G.; Zhang, Z.; Zhao, J.; Okoli, C.P. Heavy metal contents and enrichment characteristics of dominant plants in wasteland of the downstream of a lead-zinc mining area in Guangxi, Southwest China. Ecotoxicol. Environ. Saf. 2018, 151, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, M. The Importance of Enrichment Factor (EF) and Geoaccumulation Index (Igeo) to Evaluate the Soil Contamination. J. Geol. Geophys. 2016, 5, 1–4. [Google Scholar] [CrossRef]
- El Hachimi, M.L.; Fekhaoui, M.; El Abidi, A.; Rhoujatti, A. Contamination des sols par les métaux lourds à partir de mines abandonnées: Le cas des mines Aouli-Mibladen-Zeïda au Maroc. Cah. Agric. 2014, 23, 213–219. [Google Scholar]
- Lorestani, B.; Cheraghi, M.; Yousefi, N. The Potential of Phytoremediation Using Hyperaccumulator Plants: A Case Study at a Lead-Zinc Mine Site. Int. J. Phytoremediation 2012, 14, 786–795. [Google Scholar] [CrossRef]
- Escarré, J.; Lefèbvre, C.; Raboyeau, S.; DosSantos, A.; Gruber, W.; Marel, J.C.C.; Frérot, H.; Noret, N.; Mahieu, S.; Collin, C.; et al. Heavy Metal Concentration Survey in Soils and Plants of the Les Malines Mining District (Southern France): Implications for Soil Restoration. Water Air Soil Pollut. 2010, 216, 485–504. [Google Scholar] [CrossRef] [Green Version]
- He, M. Distribution and phytoavailability of antimony at an antimony mining and smelting area, Hunan, China. Environ. Geochem. Health 2007, 29, 209–219. [Google Scholar] [CrossRef]
- Okkenhaug, G.; Zhu, Y.-G.; Luo, L.; Lei, M.; Li, X.; Mulder, J. Distribution, speciation and availability of antimony (Sb) in soils and terrestrial plants from an active Sb mining area. Environ. Pollut. 2011, 159, 2427–2434. [Google Scholar] [CrossRef]
- Li, J.; Wei, Y.; Zhao, L.; Zhang, J.; Shangguan, Y.; Li, F.; Hou, H. Bioaccessibility of antimony and arsenic in highly polluted soils of the mine area and health risk assessment associated with oral ingestion exposure. Ecotoxicol. Environ. Saf. 2014, 110, 308–315. [Google Scholar] [CrossRef]
- Takaoka, M.; Fukutani, S.; Yamamoto, T.; Horiuchi, M.; Satta, N.; Takeda, N.; Oshita, K.; Yoneda, M.; Morisawa, S.; Tanaka, T. Determination of Chemical Form of Antimony in Contaminated Soil around a Smelter Using X-ray Absorption Fine Structure. Anal. Sci. 2005, 21, 769–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Shehbaz, I.A. A generic and tribal synopsis of the Brassicaceae (Cruciferae). TAXON 2012, 61, 931–954. [Google Scholar] [CrossRef]
- Thanos, C.A.; Georghiou, K.; Kadis, C.; Pantazi, C. Cistaceae: A plant family with hard seeds. Isr. J. Plant Sci. 1992, 41, 251–263. [Google Scholar]
- Al-Sghair, F.G.; Mahklouf, M.H.; Abudaya, E.A. Species Diversity and Floristic Analysis of the Family Poaceae in Libya Depending on the Flora of Libya. Adv. Biosci. Bioeng. 2019, 7, 13. [Google Scholar] [CrossRef] [Green Version]
- Martín-Bravo, S.; Escudero, M. Biogeography of fiowering plants: A case study in mignonettes (Resedaceae) and sedges (Carex, Cyperaceae). In Global Advances in Biogeography; Intech: Rijeka, Croatia, 2012; pp. 257–290. [Google Scholar]
- Hassan, N.S.; Hartmann, H.E.K.; Liede-Schumann, S. Conspectus of Aizoaceae, Gisekiaceae and Molluginaceae of Egypt and the Sudan. Feddes Repert. 2005, 116, 1–42. [Google Scholar] [CrossRef]
- Aykurt, C.; Suembuel, H. Varieties and chorology of Convolvulus oleifolius Desr.(Convolvulaceae) in Turkey. Biol. Divers. Conserv. 2010, 3, 155–162. [Google Scholar]
- Brullo, S.; Minissale, P.; Salmeri, C.; Del Galdo, G.G. Taxonomic investigations on Psoralea palaestina (Fabaceae), a critical species of Mediterranean flora. Phytotaxa 2016, 266, 61. [Google Scholar] [CrossRef]
- Bengtson, A.; Anderberg, A.A. Species diversification in the Mediterranean genus Chiliadenus (Inuleae-Asteraceae). Plant. Syst. Evol. 2018, 304, 853–860. [Google Scholar] [CrossRef] [Green Version]
- Gisbert, C.; Clemente, R.; Navarro-Aviñó, J.; Baixauli, C.; Ginér, A.; Serrano, R.; Walker, D.J.; Bernal, M.P. Tolerance and accumulation of heavy metals by Brassicaceae species grown in contaminated soils from Mediterranean regions of Spain. Environ. Exp. Bot. 2006, 56, 19–27. [Google Scholar] [CrossRef]
- Casado, M.; Anawar, H.M.; Garcia-Sanchez, A.; Santa-Regina, I. Antimony and Arsenic Uptake by Plants in an Abandoned Mining Area. Commun. Soil Sci. Plant. Anal. 2007, 38, 1255–1275. [Google Scholar] [CrossRef]
- Wenzel, W.; Jockwer, F. Accumulation of heavy metals in plants grown on mineralised soils of the Austrian Alps. Environ. Pollut. 1999, 104, 145–155. [Google Scholar] [CrossRef]
- Nouri, J.; Lorestani, B.; Yousefi, N.; Khorasani, N.; Hasani, A.H.; Seif, F.; Cheraghi, M. Phytoremediation potential of native plants grown in the vicinity of Ahangaran lead–zinc mine (Hamedan, Iran). Environ. Earth Sci. 2010, 62, 639–644. [Google Scholar] [CrossRef]
- Mahdavian, K.; Ghaderian, S.M.; Torkzadeh-Mahani, M. Accumulation and phytoremediation of Pb, Zn, and Ag by plants growing on Koshk lead–zinc mining area, Iran. J. Soils Sediments 2015, 17, 1310–1320. [Google Scholar] [CrossRef]
- Marrugo-Negrete, J.; Marrugo-Madrid, S.; Pinedo-Hernández, J.; Durango-Hernández, J.; Díez, S. Screening of native plant species for phytoremediation potential at a Hg-contaminated mining site. Sci. Total Environ. 2016, 542, 809–816. [Google Scholar] [CrossRef]
- Ha, N.T.H.; Sakakibara, M.; Sano, S.; Nhuan, M.T. Uptake of metals and metalloids by plants growing in a lead–zinc mine area, Northern Vietnam. J. Hazard. Mater. 2011, 186, 1384–1391. [Google Scholar] [CrossRef]
- Tremlová, J.; Vašíčková, I.; Száková, J.; Goessler, W.; Steiner, O.; Najmanová, J.; Horáková, T.; Tlustos, P. Arsenic compounds occurring in ruderal plant communities growing in arsenic contaminated soils. Environ. Exp. Bot. 2016, 123, 108–115. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Pendias, H. Trace Elements in Soils and Plants, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Krämer, U. Metal Hyperaccumulation in Plants. Annu. Rev. Plant. Biol. 2010, 61, 517–534. [Google Scholar] [CrossRef]
- Ghaderian, Y.S.M.; Lyon, A.J.E.; Baker, A.J.M. Seedling mortality of metal hyperaccumulator plants resulting from damping off by Pythium spp. New Phytol. 2000, 146, 219–224. [Google Scholar] [CrossRef]
- Mattina, M.I.; Lannucci-Berger, W.; Musante, C.; White, J.C. Concurrent plant uptake of heavy metals and persistent organic pollutants from soil. Environ. Pollut. 2003, 124, 375–378. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals—Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef]
- Sudmoon, R.; Neeratanaphan, L.; Thamsenanupap, P.; Tanee, T. Hyperaccumulation of Cadmium and DNA Changes in Popular Vegetable, Brassica chinensis L. Int. J. Environ. Res. 2015, 9, 433–438. [Google Scholar]
- Bui, A.T.K.; Nguyen, H.T.H.; Nguyen, M.N.; Tran, T.-H.T.; Vu, T.V.; Nguyen, C.H.; Reynolds, H.L. Accumulation and potential health risks of cadmium, lead and arsenic in vegetables grown near mining sites in Northern Vietnam. Environ. Monit. Assess. 2016, 188, 525. [Google Scholar] [CrossRef] [PubMed]
- Hesami, R.; Salimi, A.; Ghaderian, S.M. Lead, zinc, and cadmium uptake, accumulation, and phytoremediation by plants growing around Tang-e Douzan lead–zinc mine, Iran. Environ. Sci. Pollut. Res. 2018, 25, 8701–8714. [Google Scholar] [CrossRef] [PubMed]
- Fait, S.; Fakhi, S.; Elmzibri, M.; Malek, O.A.; Rachdi, B.; Faiz, Z.; Fougrach, H.; Badri, W.; Smouni, A.; Efahr, M. Behavior of As, Cd, Co, Cr, Cu, Pb, Ni, and Zn at the soil/plant interface around an uncontrolled landfill (Casablanca, Morocco). Remediat. J. 2018, 28, 65–72. [Google Scholar] [CrossRef]
- Van Der Ent, A.; Baker, A.J.M.; Reeves, R.D.; Pollard, A.J.; Schat, H. Hyperaccumulators of metal and metalloid trace elements: Facts and fiction. Plant. Soil 2012, 362, 319–334. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Reeves, R. The hyperaccumulation of nickel by serpentine plants. In The Vegetation of Ultramafic (Serpentine) Soils; Baker, A.J.M., Proctor, J., Reeves, R.D., Eds.; Intercept Ltd.: Andover, UK, 1992; pp. 253–277. [Google Scholar]
- Brunetti, G.; Soler-Rovira, P.; Farrag, K.; Senesi, N. Tolerance and accumulation of heavy metals by wild plant species grown in contaminated soils in Apulia region, Southern Italy. Plant. Soil 2008, 318, 285–298. [Google Scholar] [CrossRef]
- Pan, P.; Lei, M.; Qiao, P.; Zhou, G.; Wan, X.; Chen, T. Potential of indigenous plant species for phytoremediation of metal(loid)-contaminated soil in the Baoshan mining area, China. Environ. Sci. Pollut. Res. 2019, 26, 23583–23592. [Google Scholar] [CrossRef]
- Feng, R.; Wei, C.; Tu, S.; Ding, Y.; Wang, R.; Guo, J. The uptake and detoxification of antimony by plants: A review. Environ. Exp. Bot. 2013, 96, 28–34. [Google Scholar] [CrossRef]
- Krzesłowska, M.; Samardakiewicz, S.; Woźny, A. Trace Metals in: The Reactions of Plant Cells to Stress Factors; Woźny, A., Goździcka-Józefiak, A., Eds.; Wyd. Nauk. Uniwersytetu w Poznaniu, Poznań: Poznań, Poland, 2010; Volume 2, pp. 90–146. [Google Scholar]
- Fahr, M.; Laplaze, L.; Bendaou, N.; Hocher, V.; El Mzibri, M.; Bogusz, D.; Smouni, A. Effect of lead on root growth. Front. Plant. Sci. 2013, 4, 175. [Google Scholar] [CrossRef] [Green Version]
- Aslan, M.; Yılmaz, Y.Z.; Yilmaz, Y.Z.; Ünlü, M.Y.; Türkmen, N. Sorption of Cadmium and Effects on Growth, Protein Content, and Photosynthetic Pigment Composition of Nasturtium officinale R. Br. and Mentha aquatica L. Bull. Environ. Contam. Toxicol. 2003, 71, 323–329. [Google Scholar] [CrossRef]
- Nedjimi, B. Seasonal growth and translocation of some major and trace elements in two Mediterranean grasses (Stipa tenacissima Loefl. ex L. and Lygeum spartum Loefl. ex L.). Acta Oecologica 2018, 89, 43–50. [Google Scholar] [CrossRef]
- Baghdad, B.; Naimi, M.; Bouabdli, A.; Sonnet, P.; Lutts, S. Heavy Metals in Tailings, Soils and Vegetation of an Abandoned Lead Mine Land in Morocco. Ecol. Mediterr. 2006, 32, 85–91. [Google Scholar] [CrossRef]
- Samkaeva, L.; Revin, V.; Revin, Y.; Kulagin, A.; Novikov, O.; Pugayev, S. Studying of heavy metal accumulation by plant. Biotech 2001, 1, 54–59. [Google Scholar]
- Takeda, R.; Noriyoshi; Matsumoto, S.; Komemushi, S.; Sawabe, A. Accumulation of Heavy Metals by Japanese Weeds and Their Seasonal Movement. Contam. Soils, Sediments Water 2006, 349–359. [Google Scholar] [CrossRef]
- Alirzayeva, E.G.; Shirvani, T.S.; Alverdiyeva, S.M.; Shukurov, E.S.; Öztürk, L.; Ali-zade, V.M.; Çakmak, İ. Heavy metal accumulation in Artemisia and foliaceous lichen species from the Azerbaijan flora. For. Snow Landsc. Res. 2006, 80, 339–348. [Google Scholar]
- Ashraf, M.; Hayat, M.Q.; Mumtaz, A.S. A study on elemental contents of medicinally important species of Artemisia L. (Asteraceae) found in Pakistan. J. Med. Plants Res. 2010, 4, 2256–2263. [Google Scholar]
- Yanqun, Z.; Li, Y.; Schvartz, C.; Langlade, L.; Fan, L. Accumulation of Pb, Cd, Cu and Zn in plants and hyperaccumulator choice in Lanping lead–zinc mine area, China. Environ. Int. 2004, 30, 567–576. [Google Scholar] [CrossRef]
- Liu, X.; Gao, Y.; Khan, S.; Duan, G.; Chen, A.; Ling, L.; Zhao, L.; Liu, Z.; Wu, X. Accumulation of Pb, Cu, and Zn in native plants growing on contaminated sites and their potential accumulation capacity in Heqing, Yunnan. J. Environ. Sci. 2008, 20, 1469–1474. [Google Scholar] [CrossRef]
- Wójcik, M.; Sugier, P.; Siebielec, G. Metal accumulation strategies in plants spontaneously inhabiting Zn-Pb waste deposits. Sci. Total Environ. 2014, 487, 313–322. [Google Scholar] [CrossRef]
- Chaabani, S.; Abdelmalek-Babbou, C.; Ben Ahmed, H.; Chaabani, A.; Sebei, A. Phytoremediation assessment of native plants growing on Pb–Zn mine site in Northern Tunisia. Environ. Earth Sci. 2017, 76, 585. [Google Scholar] [CrossRef]
- El Mamoun, I.; Fahr, M.; Mohammed, A.; Najib, B.; Abidine, T.Z.-E.; Abdelkarim, G.; Didier, B.; Laurent, L.; Abdelaziz, S. Zinc, lead, and cadmium tolerance and accumulation in Cistus libanotis, Cistus albidus, and Cistus salviifolius: Perspectives on phytoremediation. Remediat. J. 2020, 30, 73–80. [Google Scholar] [CrossRef] [Green Version]
- Laplaze, L.; Doumas, P.; Brhada, F.; Ater, M.; Smouni, A. Use of Cistus Libanotis to Clean Heavy Metals Containing Soils. PATENTSCOPE International Application No. PCT/EP2010/056449, 18 November 2010. [Google Scholar]
- De La Fuente, V.; Rufo, L.; Rodríguez, N.; Amils, R.; Zuluaga, J. Metal Accumulation Screening of the Río Tinto Flora (Huelva, Spain). Biol. Trace Element Res. 2009, 134, 318–341. [Google Scholar] [CrossRef] [PubMed]
- Abreu, M.M.; Santos, E.S.; Magalhães, M.C.F.; Fernandes, E. Trace elements tolerance, accumulation and translocation in Cistus populifolius, Cistus salviifolius and their hybrid growing in polymetallic contaminated mine areas. J. Geochem. Explor. 2012, 123, 52–60. [Google Scholar] [CrossRef]
- Santos, E.S.; Abreu, M.M.; Nabais, C.; Magalhães, M.C.F. Trace element distribution in soils developed on gossan mine wastes and Cistus ladanifer L. tolerance and bioaccumulation. J. Geochem. Explor. 2012, 123, 45–51. [Google Scholar] [CrossRef]




| Site | As | Cd | Cu | Ni | Pb | Zn | Sb | PI | pH | EC (mS/cm) | % OM | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oued el Heimer | Concentration range in soils (mg kg−1) | 18.7–466 | 32–280 | 35–592 | 12–44.4 | 611–12,461 | 318–43,540 | 33.9–247.1 | 3.62–67.9 | 6.4–7.51 | 0.40–2.28 | 0.41–80% |
| Enrichment factor (EF) | 15–364.8 | 233–3656 | 5.3–98.8 | 2.5–8.2 | 131.7–2995 | 26.5–2749 | 285.7–2393 | |||||
| Touissite | Concentration range in soils (mg kg−1) | 43–82.9 | 15–36 | 328–1405 | 9–16 | 6445–18,324 | 2096–5387 | 96.7–242.8 | 14.0–38.5 | 6.9–7.7 | 0.23–0.38 | 0.28–2.07% |
| Enrichment factor (EF) | 22.2–52.5 | 133–410.4 | 29.5–166.5 | 1.7–2.3 | 833.5–2758 | 115.2–1092 | 550.3–1877 | |||||
| Site | Plants | Family | Life Span |
|---|---|---|---|
| Touissite | Reseda alba | Resedaceae | Annual |
| Convolvulus althaeoides | Convolvulaceae | Perennial | |
| Hedysarum spinosissimum | Fabaceae | Annual | |
| Phragmites communis | Poaceae | Perennial | |
| Lotus corniculatus | Fabaceae | Perennial | |
| Capsella bursa-pastoris | Brassicaceae | Annual | |
| Scolymus hispanicus | Asteraceae | Perennial | |
| Rapistrum rigosum | Brassicaceae | Annual | |
| Oued el Heimer | Cistus libanotis | Cistaceae | Perennial |
| Agathophora alopecuroides | Amaranthaceae | Perennial | |
| Hirschfeldia incana | Brassicaceae | Perennial | |
| Stipa tenacissima | Poaceae | Perennial | |
| Artemisia herba-alba | Asteraceae | Perennial | |
| Capsella bursa-pastoris | Brassicaceae | Annual |
| As | Cd | Cu | Ni | Pb | Zn | Sb | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Site | Plants | Root | Shoot | Root | Shoot | Root | Shoot | Root | Shoot | Root | Shoot | Root | Shoot | Root | Shoot |
| Touissite | R. alba | (2.6) a | (6.3) bc | (2.1) a | (4.1) ab | (45.1) ab | (46.1) de | (2.9) abc | (2.0) ab | (322.7) a | (1607.5) f | (254.3) a | (465.5) c | (6.1) ab | (21.7) abcd |
| C. althaeoides | (2.4) a | (5.1) abc | (2.9) a | (1.3) a | (59.7) ab | (35.4) bcd | (1.89) ab | (2.2) ab | (714.3) abc | (426.3) abcd | (286.1) a | (207.4) ab | (16.0) bcd | (13.6) abc | |
| H. spinosissimum | (6.0) a | (3.6) ab | (8.7) a | (3.9) ab | (58.5) ab | (32.6) bcd | (4.1) abc | (2.3) ab | (983.6) abc | (253.9) ab | (347.0) ab | (314.0) abc | (35.1) e | (33.1) cde | |
| P. communis | (3.8) a | (5.0) abc | (2.7) a | (2.0) ab | (66.7) ab | (65.8) ef | (2.2) ab | (2.6) ab | (2305.7) d | (720.4) bcde | (198.9) a | (432.5) c | (22.1) cd | (25.5) abcde | |
| L. corniculatus | (5.3) a | (3.0) ab | (3.0) a | (1.8) a | (117.3) bc | (39.6) bcd | (4.1) abc | (3.3) ab | (1493.0) bcd | (832.4) bcde | (276.6) a | (170.5) a | (27.0) de | (14.8) abcd | |
| C. bursa-pastoris | (3.8) a | (5.9) bc | (4.4) a | (4.2) ab | (65.7) ab | (49.0) def | (4.1) abc | (4.4) b | (849.6) abc | (733.8) bcde | (587.0) b | (666.9) d | (27.0) de | (55.5) e | |
| S. hispanicus L. | (3.6) a | (4.7) abc | (5.2) a | (5.4) ab | (48.8) ab | (44.3) cde | (3.6) abc | (4.2) b | (798.7) abc | (972.7) de | (307.9) a | (452.0) c | (12.3) bc | (17.2) abcd | |
| R. rigosum | (1.3) a | (0.3) a | (1.7) a | (1.8) a | (13.6) a | (10.0) a | (1.3) a | (0.5) a | (454.8) ab | (47.1) a | (239.9) a | (326.8) abc | (1.6) a | (0.5) a | |
| Oued el Heimer | C. libanotis | (3.4) a | (9.7) c | (22.7) a | (16.2) bc | (23.6) ab | (25.5) abcd | (2.4) ab | (3.1) ab | (1219.2) abcd | (1261.8) ef | (135.6) a | (161.7) a | (8.3) ab | (9.4) abc |
| A. alopecuroides | (9.1) a | (4.6) abc | (4.9) a | (18.0) bcd | (26.0) ab | (16.3) ab | (4.0) abc | (4.2) abc | (235.1) a | (293.8) ab | (142.5) a | (168.2) a | (5.2) ab | (6.2) abc | |
| H. incana | (6.0) a | (6.6) bc | (25.8) a | (32.9) d | (37.3) ab | (19.5) ab | (2.9) abc | (2.3) ab | (441.3) ab | (343.7) abc | (273.4) a | (309.7) abc | (5.3) ab | (6.4) ab | |
| S. tenacissima | (59.3) b | (19.6) d | (241.2) b | (28.9) cd | (237.9) dc | (29.7) abcd | (6.4) bc | (3.8) ab | (3785.7) e | (1146.3) ef | (637.0) b | (322.9) abc | (167.7) f | (32.1) bcde | |
| A. herba-alba | (48.7) b | (50.7) e | (50.2) a | (56.2) e | (203.1) cd | (72.3) f | (6.5) c | (8.1) c | (1748.7) cd | (4672.2) g | (577.0) b | (357.0) bc | (13.0) bc | (41.6) de | |
| C. bursa-pastoris | (4.5) a | (5.1) abc | (41.4) a | (30.0) cd | (28.7) ab | (18.2) abc | (1.7) ab | (2.1) ab | (629.7) abc | (920.9) cde | (395.3) ab | (396.1) bc | (8.7) ab | (12.9) abcd | |
| Phytotoxic concentrations of metals [66,67] | 2–80 | 0.1–3 | 20–30 | 10–50 | 0.6–28 | 100–300 | 5–10 | ||||||||
| Hyperaccumulation threshold [67] | >1000 | >100 | >1000 | >1000 | >1000 | >10,000 | >1000 | ||||||||
| Shoots | Roots | |||
|---|---|---|---|---|
| F | p | F | p | |
| As | 111.03 | <0.0001 | 46.19 | <0.0001 |
| Cd | 29.74 | <0.0001 | 19.95 | <0.0001 |
| Cu | 14.82 | <0.0001 | 10.53 | <0.0001 |
| Ni | 6.91 | <0.0001 | 3.06 | 0.0180 |
| Pb | 101.65 | <0.0001 | 14.69 | <0.0001 |
| Zn | 18.43 | <0.0001 | 8.16 | <0.0001 |
| Sb | 7.54 | <0.0001 | 250.93 | <0.0001 |
| Trace Metal Elements | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| As | Cd | Cu | Ni | Pb | Zn | Sb | ||||||||||||||||
| Site | Plants | BCF | TF | BAC | BCF | TF | BAC | BCF | TF | BAC | BCF | TF | BAC | BCF | TF | BAC | BCF | TF | BAC | BCF | TF | BAC |
| Touissite | R. alba | 0.04 | 2.37 | 0.10 | 0.08 | 1.91 | 0.15 | 0.11 | 1.02 | 0.11 | 0.32 | 0.66 | 0.21 | 0.02 | 4.98 | 0.12 | 0.04 | 1.83 | 0.08 | 0.04 | 3.55 | 0.15 |
| C. althaeoides | 0.03 | 2.12 | 0.07 | 0.08 | 0.62 | 0.04 | 0.12 | 0.59 | 0.07 | 0.13 | 1.17 | 0.15 | 0.09 | 0.59 | 0.05 | 0.05 | 0.72 | 0.03 | 0.10 | 0.85 | 0.08 | |
| H. spinosissimum | 0.13 | 0.59 | 0.08 | 0.57 | 0.44 | 0.24 | 0.17 | 0.55 | 0.09 | 0.25 | 0.57 | 0.14 | 0.15 | 0.25 | 0.03 | 0.10 | 0.90 | 0.10 | 0.36 | 0.94 | 0.34 | |
| P. communis | 0.06 | 1.31 | 0.08 | 0.07 | 0.73 | 0.05 | 0.04 | 0.99 | 0.04 | 0.18 | 1.17 | 0.20 | 0.12 | 0.31 | 0.03 | 0.04 | 2.17 | 0.08 | 0.09 | 1.15 | 0.10 | |
| L. corniculatus | 0.08 | 0.57 | 0.04 | 0.19 | 0.55 | 0.1 | 0.14 | 0.33 | 0.05 | 0.25 | 0.80 | 0.2 | 0.08 | 0.55 | 0.05 | 0.13 | 0.60 | 0.08 | 0.12 | 0.54 | 0.06 | |
| C. bursa-pastoris | 0.04 | 1.53 | 0.07 | 0.12 | 0.95 | 0.11 | 0.09 | 0.74 | 0.06 | 0.27 | 1.08 | 0.29 | 0.09 | 0.88 | 0.08 | 0.13 | 1.13 | 0.15 | 0.11 | 2.05 | 0.22 | |
| S. hispanicus L. | 0.06 | 1.32 | 0.07 | 0.20 | 1.03 | 0.21 | 0.14 | 0.90 | 0.12 | 0.27 | 1.15 | 0.32 | 0.10 | 1.21 | 0.12 | 0.1 | 1.46 | 0.14 | 0.07 | 1.39 | 0.1 | |
| R. rigosum | - | 0.2 | - | - | 0.8 | - | - | 0.7 | - | - | 0.4 | - | - | 0.1 | - | - | 1.36 | - | - | 0.3 | - | |
| Oued el Heimer | C. libanotis | 0.18 | 2.87 | 0.51 | 0.60 | 0.71 | 0.42 | 0.67 | 1.08 | 0.71 | 0.17 | 1.27 | 0.22 | 1.99 | 1.03 | 2.06 | 0.42 | 1.19 | 0.50 | 0.24 | 1.13 | 0.27 |
| A. alopecuroides | 0.48 | 0.50 | 0.24 | 0.15 | 3.67 | 0.56 | 0.74 | 0.62 | 0.05 | 0.23 | 1.05 | 0.24 | 0.38 | 1.24 | 0.48 | 0.07 | 1.18 | 0.08 | 0.03 | 1.19 | 0.04 | |
| H. incana | 0.01 | 1.10 | 0.01 | 0.09 | 1.27 | 0.11 | 0.06 | 0.52 | 0.03 | 0.06 | 0.79 | 0.05 | 0.03 | 0.77 | 0.03 | 0.01 | 1.13 | 0.01 | 0.02 | 1.2 | 0.03 | |
| S. tenacissima | 0.14 | 0.32 | 0.04 | 2.72 | 0.11 | 0.32 | 1.55 | 0.12 | 0.19 | 0.40 | 0.59 | 0.23 | 0.51 | 0.30 | 0.09 | 0.01 | 0.50 | 0.00 | 0.89 | 0.19 | 0.17 | |
| A. herba-alba | 0.86 | 1.04 | 0.90 | 0.80 | 1.11 | 1.10 | 3.63 | 0.35 | 1.29 | 0.54 | 1.24 | 0.62 | 0.48 | 2.67 | 1.30 | 1.69 | 0.61 | 1.04 | 0.38 | 3.2 | 1.22 | |
| C. bursa-pastoris | 0.01 | 1.13 | 0.01 | 0.86 | 0.72 | 0.16 | 0.05 | 0.63 | 0.03 | 0.07 | 1.24 | 0.09 | 0.05 | 1.46 | 0.07 | 0.03 | 1.00 | 0.03 | 0.03 | 1.48 | 0.05 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasnaoui, S.E.; Fahr, M.; Keller, C.; Levard, C.; Angeletti, B.; Chaurand, P.; Triqui, Z.E.A.; Guedira, A.; Rhazi, L.; Colin, F.; et al. Screening of Native Plants Growing on a Pb/Zn Mining Area in Eastern Morocco: Perspectives for Phytoremediation. Plants 2020, 9, 1458. https://doi.org/10.3390/plants9111458
Hasnaoui SE, Fahr M, Keller C, Levard C, Angeletti B, Chaurand P, Triqui ZEA, Guedira A, Rhazi L, Colin F, et al. Screening of Native Plants Growing on a Pb/Zn Mining Area in Eastern Morocco: Perspectives for Phytoremediation. Plants. 2020; 9(11):1458. https://doi.org/10.3390/plants9111458
Chicago/Turabian StyleHasnaoui, Said El, Mouna Fahr, Catherine Keller, Clément Levard, Bernard Angeletti, Perrine Chaurand, Zine El Abidine Triqui, Abdelkarim Guedira, Laila Rhazi, Fabrice Colin, and et al. 2020. "Screening of Native Plants Growing on a Pb/Zn Mining Area in Eastern Morocco: Perspectives for Phytoremediation" Plants 9, no. 11: 1458. https://doi.org/10.3390/plants9111458