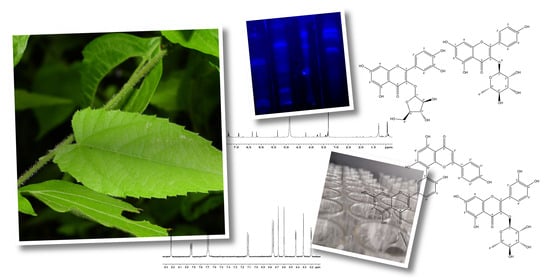
Flavonoid Composition and Antioxidant Activity of Tragia volubilis L. Methanolic Extract
Abstract
:1. Introduction
The Genus Tragia (Euphorbiaceae)
2. Results and Discussion
2.1. Extract
2.2. Phytochemical Screening
2.3. Antioxidant Activity
| TPC mg GAE/ g Extract | ABTS μmol TE/ g Extract | FRAP μmol TE/ g Extract | DPPH μmol TE/ g Extract | IC50 mg Extract/ mg DPPH | AAI [DPPH] (μg mL−1)/IC50 |
|---|---|---|---|---|---|
| 127 ± 2 | 2004 ± 36 | 1250 ± 15 | 585 ± 5 | 1.30 ± 0.06 | 1.14 ± 0.01 |
2.4. Compounds
3. Materials and Methods
3.1. Plant Material
3.2. Preparation of the Extract
3.3. Phytochemical Screening
3.4. Antioxidant Activity
3.5. Isolation of Secondary Metabolites
3.6. Characterization and Identification of Secondary Metabolites
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shukla, S.S.; Jain, S.K.; Kalyani, G.; Gidwani, B.; Pandey, R.K.; Pandey, R.; Vyas, A. Zoopharmacognosy (Plant-Animal Interaction). In Evidence Based Validation of Traditional Medicines: A comprehensive Approach; Mandal, S.C., Chakraborty, R., Sen, S., Eds.; Springer: Singapore, 2021; pp. 727–741. ISBN 9789811581274. [Google Scholar]
- Solecki, R.S. Shanidar IV, a Neanderthal Flower Burial in Northern Iraq. Science 1975, 190, 880–881. [Google Scholar] [CrossRef]
- Ekor, M. The Growing Use of Herbal Medicines: Issues Relating to Adverse Reactions and Challenges in Monitoring Safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef]
- Vyas, S.; Kothari, S.L.; Kachhwaha, S. Nootropic Medicinal Plants: Therapeutic Alternatives for Alzheimer’s Disease. J. Herb. Med. 2019, 17–18, 100291. [Google Scholar] [CrossRef]
- Mwine, J.T.; Damme, P.V. Why Do Euphorbiaceae Tick as Medicinal Plants? A Review of Euphorbiaceae Family and Its Medicinal Features. J. Med. Plants Res. 2011, 5, 652–662. [Google Scholar]
- Kar, A.; Choudhary, B.K.; Bandyopadhyay, N.G. Comparative Evaluation of Hypoglycaemic Activity of Some Indian Medicinal Plants in Alloxan Diabetic Rats. J. Ethnopharmacol. 2003, 84, 105–108. [Google Scholar] [CrossRef]
- Narasimhan, S. Pharmacological Potential of the Stinging Plant Tragia Species: A Review. Pharmacogn. J. 2021, 13, 278–284. [Google Scholar] [CrossRef]
- Ruffo, C.K.; Birnie, A.; Tengnäs, B. Edible Wild Plants of Tanzania; Regional Land Management Unit/Sida: Nairobi, Kenya, 2002; ISBN 978-9966-896-62-9. [Google Scholar]
- Nath, T.M.; Das, S.; Zothanpuia; Chattopadhyay, P. Investigating the Effects of Dermal Exposure to In-Vivo Animal Models on the Riot-Control Properties of a Powder Formulation of Tragia Involucrata Leaf Hair Lining. Cutan. Ocul. Toxicol. 2023, 42, 151–161. [Google Scholar] [CrossRef]
- Duarte-Casar, R.; Romero-Benavides, J.C.; Tragia, L. Genus: Ethnopharmacological Use, Phytochemical Composition and Biological Activity. Plants 2021, 10, 2717. [Google Scholar] [CrossRef]
- Elmore, F.H. The Ethnobotany of the Kayenta Navaho. An Analysis of the John and Louisa Wetherill Ethnobotanical Collections. Leland C. Wyman and Stuart K. Harris. University of New Mexico Publications in Biology, Number Five. The University of New Mexico Press, Albuquerque, 1951. 66pp. Am. Antiq. 1952, 17, 276. [Google Scholar] [CrossRef]
- Hernández, F. Historia de las Plantas de Nueva España; Imprenta Universitaria: Mexico City, Mexico, 1943; Volume 2. [Google Scholar]
- Moges, A.; Moges, Y. Ethiopian Common Medicinal Plants: Their Parts and Uses in Traditional Medicine—Ecology and Quality Control. In Plant Science—Structure, Anatomy and Physiology in Plants Cultured In Vivo and In Vitro; Gonzalez, A., Rodriguez, M., Gören Sağlam, N., Eds.; IntechOpen: London, UK, 2020; ISBN 978-1-78984-746-8. [Google Scholar]
- Novara, L. Flora Del Valle de Lerma: Euphorbiaceae. Aportes Bot. Salta Ser. Flora 2013, 11, 1–188. [Google Scholar]
- Dalfonso, C.; Scaramuzzino, R. Flora Medicinal de Las Sierras de Azul (Sistema de Tandilia): Catálogo, Importancia y Su Relación Con Las Actividades Antrópicas; UNCPBA: Tandil, Buenos Aires, Argentina, 2012. [Google Scholar]
- González-Rodríguez, H.; Maiti, R.; Kumari, A. Nutrient Profile of Native Woody Species and Medicinal Plants in Northeastern Mexico: A Synthesis. J. Bioprocess. Biotech. 2016, 6, 283. [Google Scholar] [CrossRef]
- Swank, G. The Ethnobotany of the Acoma and Laguna Indians. Master’s Thesis, University of New Mexico, Albuquerque, NM, USA, 1932. Biology ETDs. [Google Scholar]
- Silberbauer-Gottsberger, I. O Cerrado Como Potencial de Plantas Medicinais e Tóxicas. Oréades 1982, 8, 15–30. [Google Scholar]
- Arnason, T.; Uck, F.; Lambert, J.; Hebda, R. Maya Medicinal Plants of San Jose Succotz, Belize. J. Ethnopharmacol. 1980, 2, 345–364. [Google Scholar] [CrossRef] [PubMed]
- Kapere, K.N.; Mugisha, M.K.; Tweheyo, O.A.; Waisindye, N. Community Perceptions on the Use of Traditional Medicine among People Experiencing Sexual Dysfunctions in Greater Mbarara, Western Uganda. J. Pharmacogn. Phytochem. 2022, 11, 46–59. [Google Scholar]
- Carlomagno, A.; Pardini, A.; Esquijerosa, Y.C. Medicinal Plants in Ethnobotanical and Religious Traditions in Cuba: A First Review and Updating; Associazione Scienze Agrarie Tropicali: Ragusa, Italy, 2015; p. 21. [Google Scholar]
- Miller, K.I.; Webster, G.L. A Preliminary Revision of Tragia (Euphorbiaceae) in the United States. Rhodora 1967, 69, 241–305. [Google Scholar]
- Tropicos.org Missouri Botanical Garden. !Tragia Volubilis L. Available online: http://legacy.tropicos.org/Name/12801898?projectid=56 (accessed on 30 June 2023).
- Gillespie, L.J.; Armbruster, W.S. A Contribution to the Guianan Flora: Dalechampia, Haematostemon, Omphalea, Pera, Plukenetia, and Tragia (Euphorbiaceae) with Notes on Subfamily Acalyphoideae. Smithson. Contrib. Bot. 1997, 86, 77507. [Google Scholar] [CrossRef]
- POWO Tragia Plum. Ex L.|Plants of the World Online|Kew Science. Available online: http://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:327688-2 (accessed on 25 October 2021).
- Pardo-Castello, V. Dermatitis Venenata: A Study of the Tropical Plants Producing Dermatitis. JAMA Dermatol. 1923, 7, 81–90. [Google Scholar] [CrossRef]
- Thurston, E.L.; Lersten, N.R. The Morphology and Toxicology of Plant Stinging Hairs. Bot. Rev. 1969, 35, 393–412. [Google Scholar] [CrossRef]
- Marineros, L.; Vega, H. Contribución al Conocimiento de Algunas Plantas Urticantes de Importancia Médica En Honduras. Sci. Hondurensis 2021, 4, 8–19. [Google Scholar]
- Mulgura de Romero, M.E.; de Sanguinetti, M.M.G. Actualizacion Taxonómica de Tragia (Euphorbiaceae) Para Argentina y Regiones Limítrofes. Darwiniana 1989, 29, 77–138. [Google Scholar]
- Reko, B. La Hierba de Quetzalcoatl. Bot. Sci. 1946, 4, 13–14. [Google Scholar] [CrossRef]
- Jiofack, T.; Fokunang, C.; Guedje, N.M.; Victor, K.; Evariste, F.; Nkongmeneck, B.; Mapongmetsem, P.; Tsabang, N. Ethnobotanical Uses of Some Plants of Two Ethnoecological Regions of Cameroon. Int. J. Med. Sci. 2010, 2, 60–79. [Google Scholar]
- Fonnegra-Gómez, R.; Villa-Londoño, J.; Fonnegra, Z.I.M. Plantas Usadas Como Medicinales En El Altiplano Del Oriente Antioqueño—Colombia; Herbario Universidad de Antioquia: Antioquia, Colombia, 2012; ISBN 978-958-8790-36-7. [Google Scholar]
- Corrêa, M.P. Dicionário das Plantas Úteis do BRASIL e das Exóticas Cultivadas; Imprensa Nacional: Rio de Janeiro, Brazil, 1926; Volume 2.
- Cunha Lima, S.; Rodrigues, E.; Melo, T.; Nascimento, A.; Guedes, M.; Cruz, T.; Alves, C.; Meyer, R. Levantamento da flora medicinal usada no tratamento de doenças metabólicas em Salvador, BA Brasil. Rev. Bras. Plantas Med. 2008, 10, 83–89. [Google Scholar]
- Posthouwer, C.; Verheijden, T.M.S.; van Andel, T.R. A Rapid Sustainability Assessment of Wild Plant Extraction on the Dutch Caribbean Island of St. Eustatius. Econ. Bot. 2016, 70, 320–331. [Google Scholar] [CrossRef]
- Barboza, G.E.; Cantero, J.J.; Núñez, C.; Pacciaroni, A.; Espinar, L.A. Medicinal Plants: A General Review and a Phytochemical and Ethnopharmacological Screening of the Native Argentine Flora. Kurtziana 2009, 34, 7–365. [Google Scholar]
- Mesa, R.Y.; Tomás, J. Plantas Medicinales, Aromáticas o Venenosas de Cuba, 2nd ed.; Ciencia y Técnica: Havana, Cuba, 1974. [Google Scholar]
- Morton, J.F. Atlas of Medicinal Plants of Middle America: Bahamas to Yucatan; C. C. Thomas: Springfield, IL, USA, 1980; ISBN 978-0-398-04036-9. [Google Scholar]
- Pájaro-González, Y.; Oliveros-Díaz, A.F.; Cabrera-Barraza, J.; Cerra-Dominguez, J.; Díaz-Castillo, F. Chapter 1—A Review of Medicinal Plants Used as Antimicrobials in Colombia. In Medicinal Plants as Anti-Infectives; Chassagne, F., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 3–57. ISBN 978-0-323-90999-0. [Google Scholar]
- Digital Science Dimensions. Available online: https://app.dimensions.ai/ (accessed on 25 May 2022).
- De Souza, P.; Mariano, L.N.B.; Cechinel-Zanchett, C.C.; Cechinel-Filho, V. Promising Medicinal Plants with Diuretic Potential Used in Brazil: State of the Art, Challenges, and Prospects. Planta Medica 2021, 87, 24–37. [Google Scholar] [CrossRef]
- Lima, S.T.C.; Merrigan, T.L.; Rodrigues, E.D. Synthetic and Plant Derived Thyroid Hormone Analogs. In Thyroid and Parathyroid Diseases—New Insights into Some Old and Some New Issues; Ward, L., Ed.; InTech: Houston, TX, USA, 2012; ISBN 978-953-51-0221-2. [Google Scholar]
- Nawaz, H.; Aslam, M.; Shahzad, H. Mehboob Comparative Evaluation of Phytochemical Composition and Antioxidant Potential of Some Medicinally-Important Euphorbiaceous Plants. J. Anim. Plant Sci. 2022, 32, 1703–1712. [Google Scholar] [CrossRef]
- Varma, G.G.; Mathai, B.K.; Das, K.; Gowda, G.; Rammohan, S.; Einstein, J.W. Evaluation of Antiepileptic Activity of Methanolic Leaves Extract of Tragia Involucrata Linn. in Mice. Int. Lett. Nat. Sci. 2014, 12, 167–179. [Google Scholar] [CrossRef]
- Samy, R.P.; Gopalakrishnakone, P.; Sarumathi, M.; Ignacimuthu, S. Wound Healing Potential of Tragia Involucrata Extract in Rats. Fitoterapia 2006, 77, 300–302. [Google Scholar] [CrossRef]
- Pallie, M.S.; Perera, P.K.; Kumarasinghe, N.; Arawwawala, M.; Goonasekara, C.L. Ethnopharmacological Use and Biological Activities of Tragia Involucrata L. Evid. Based Complement. Altern. Med. 2020, 2020, 8848676. [Google Scholar] [CrossRef]
- Menon, M.; Varghese, L. Evaluation of the Phytochemical Constituents and Tumor Reduction Potentials of Tragia Involucrata Linn. J. App Biol. Biotech. 2023, 11, 84–91. [Google Scholar] [CrossRef]
- Mothana, R.A.A.; Abdo, S.A.A.; Hasson, S.; Althawab, F.M.N.; Alaghbari, S.A.Z.; Lindequist, U. Antimicrobial, Antioxidant and Cytotoxic Activities and Phytochemical Screening of Some Yemeni Medicinal Plants. Evid. Based Complement. Altern. Med. 2010, 7, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Oladosu, I.A.; Balogun, S.O.; Ademowo, G.O. Phytochemical Screening, Antimalarial and Histopathological Studies of Allophylus Africanus and Tragia Benthamii. Chin. J. Nat. Med. 2013, 11, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Antibacterial Plant Compounds, Extracts and Essential Oils: An Updated Review on Their Effects and Putative Mechanisms of Action. Phytomedicine 2021, 90, 153626. [Google Scholar] [CrossRef]
- Maaliki, D.; Shaito, A.A.; Pintus, G.; El-Yazbi, A.; Eid, A.H. Flavonoids in Hypertension: A Brief Review of the Underlying Mechanisms. Curr. Opin. Pharmacol. 2019, 45, 57–65. [Google Scholar] [CrossRef]
- Perveen, S.; Al-Taweel, A.M. Terpenes and Terpenoids: Recent Advances; IntechOpen: London, UK, 2021; ISBN 978-1-83881-916-3. [Google Scholar]
- Velu, V.; Banerjee, S.; Radhakrishnan, V.; Gupta, G.; Chellappan, D.K.; Fuloria, N.K.; Fuloria, S.; Mehta, M.; Dua, K.; Malipeddi, H. Identification of Phytoconstituents of Tragia Involucrata Leaf Extracts and Evaluate Their Correlation with Anti-Inflammatory & Antioxidant Properties. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2021, 20, 308–315. [Google Scholar] [CrossRef]
- Scherer, R.; Teixeira Godoy, H. Antioxidant Activity Index (AAI) by the 2,2-Diphenyl-1-Picrylhydrazyl Method. Food Chem. 2009, 112, 654–658. [Google Scholar] [CrossRef]
- Mendoza-Meneses, C.J.; Burgos-Araiza, A.K.; Feregrino-Pérez, A.A. Chapter 18—Antidiabetic Herbal Biomolecules. In Herbal Biomolecules in Healthcare Applications; Mandal, S.C., Nayak, A.K., Dhara, A.K., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 407–434. ISBN 978-0-323-85852-6. [Google Scholar]
- Saddiqe, Z.; Naeem, I.; Maimoona, A. A Review of the Antibacterial Activity of Hypericum Perforatum L. J. Ethnopharmacol. 2010, 131, 511–521. [Google Scholar] [CrossRef]
- Chen, J.; Li, G.; Sun, C.; Peng, F.; Yu, L.; Chen, Y.; Tan, Y.; Cao, X.; Tang, Y.; Xie, X.; et al. Chemistry, Pharmacokinetics, Pharmacological Activities, and Toxicity of Quercitrin. Phytother. Res. 2022, 36, 1545–1575. [Google Scholar] [CrossRef]
- Zhang, Z.; ElSohly, H.N.; Li, X.-C.; Khan, S.I.; Broedel, S.E.; Raulli, R.E.; Cihlar, R.L.; Burandt, C.; Walker, L.A. Phenolic Compounds from Nymphaea Odorata. J. Nat. Prod. 2003, 66, 548–550. [Google Scholar] [CrossRef]
- Xiong, X.; Tang, N.; Lai, X.; Zhang, J.; Wen, W.; Li, X.; Li, A.; Wu, Y.; Liu, Z. Insights into Amentoflavone: A Natural Multifunctional Biflavonoid. Front. Pharmacol. 2021, 12, 768708. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yang, W.; Liu, J.; Liu, H.; Lv, Z.; Zhang, C.; Chen, D.; Jiao, Z. Identification of Six Flavonoids as Novel Cellular Antioxidants and Their Structure-Activity Relationship. Oxidative Med. Cell. Longev. 2020, 2020, e4150897. [Google Scholar] [CrossRef] [PubMed]
- Tatsimo, S.J.N.; Tamokou, J.d.D.; Havyarimana, L.; Csupor, D.; Forgo, P.; Hohmann, J.; Kuiate, J.-R.; Tane, P. Antimicrobial and Antioxidant Activity of Kaempferol Rhamnoside Derivatives from Bryophyllum Pinnatum. BMC Res. Notes 2012, 5, 158. [Google Scholar] [CrossRef]
- Frota, L.S.; Alves, D.R.; Marinho, M.M.; Da Silva, L.P.; de Queiroz Almeida Neto, F.W.; Marinho, E.S.; De Morais, S.M. Antioxidant and Anticholinesterase Activities of Amentoflavone Isolated from Ouratea Fieldingiana (Gardner) Engl. through In Vitro and Chemical-Quantum Studies. J. Biomol. Struct. Dyn. 2023, 41, 1206–1216. [Google Scholar] [CrossRef] [PubMed]
- Heim, K.C.; Tagliaferro, A.R.; Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid Antioxidants: Chemistry, Metabolism and Structure-Activity Relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Li, X.; Jiang, Q.; Wang, T.; Liu, J.; Chen, D. Comparison of the Antioxidant Effects of Quercitrin and Isoquercitrin: Understanding the Role of the 6″-OH Group. Molecules 2016, 21, 1246. [Google Scholar] [CrossRef]
- Farhadi, F.; Khameneh, B.; Iranshahi, M.; Iranshahy, M. Antibacterial Activity of Flavonoids and Their Structure–Activity Relationship: An Update Review. Phytother. Res. 2019, 33, 13–40. [Google Scholar] [CrossRef]
- da Silva Sa, F.A.; de Paula, J.A.M.; dos Santos, P.A.; de Almeida Ribeiro Oliveira, L.; de Almeida Ribeiro Oliveira, G.; Liao, L.M.; de Paula, J.R.; do Rosario Rodrigues Silva, M. Phytochemical Analysis and Antimicrobial Activity of Myrcia Tomentosa (Aubl.) DC. Leaves. Molecules 2017, 22, 1100. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, M.; Chen, S.; Wu, Q. Avicularin Inhibits Cell Proliferation and Induces Cell Apoptosis in Cutaneous Squamous Cell Carcinoma. Exp. Ther. Med. 2019, 19, 1065–1071. [Google Scholar] [CrossRef]
- Wang, W.; Zheng, H.; Zheng, M.; Liu, X.; Yu, J. Protective Effect of Avicularin on Rheumatoid Arthritis and Its Associated Mechanisms. Exp. Ther. Med. 2018, 16, 5343–5349. [Google Scholar] [CrossRef]
- Babujanarthanam, R.; Kavitha, P.; Pandian, M.R. Quercitrin, a Bioflavonoid Improves Glucose Homeostasis in Streptozotocin-Induced Diabetic Tissues by Altering Glycolytic and Gluconeogenic Enzymes. Fundam. Clin. Pharmacol. 2010, 24, 357–364. [Google Scholar] [CrossRef]
- de Barros, M.; da Silva, L.M.; Boeing, T.; Somensi, L.B.; Cury, B.J.; de Moura Burci, L.; Santin, J.R.; de Andrade, S.F.; Monache, F.D.; Cechinel-Filho, V. Pharmacological Reports about Gastroprotective Effects of Methanolic Extract from Leaves of Solidago Chilensis (Brazilian Arnica) and Its Components Quercitrin and Afzelin in Rodents. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2016, 389, 403–417. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; So, Y.-J.; Shin, M.S.; Cho, J.Y.; Lee, J. Antibacterial Effects of Afzelin Isolated from Cornus Macrophylla on Pseudomonas Aeruginosa, A Leading Cause of Illness in Immunocompromised Individuals. Molecules 2014, 19, 3173–3180. [Google Scholar] [CrossRef] [PubMed]
- Cechinel-Zanchett, C.C.; Mariano, L.N.B.; Boeing, T.; da Costa, J.d.C.; Da Silva, L.M.; Bastos, J.K.; Cechinel-Filho, V.; de Souza, P. Diuretic and Renal Protective Effect of Kaempferol 3-O-Alpha-l-Rhamnoside (Afzelin) in Normotensive and Hypertensive Rats. J. Nat. Prod. 2020, 83, 1980–1989. [Google Scholar] [CrossRef] [PubMed]
- Ndongo, J.T.; Issa, M.E.; Messi, A.N.; Mbing, J.N.; Cuendet, M.; Pegnyemb, D.E.; Bochet, C.G. Cytotoxic Flavonoids and Other Constituents from the Stem Bark of Ochna Schweinfurthiana. Nat. Prod. Res. 2015, 29, 1684–1687. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, C.C.; Lopes, A.J.O.; Sousa, E.L.F.; Camelo, D.S.; Lima, F.C.V.M.; da Rocha, C.Q.; Silva, G.E.B.; Garcia, J.B.S.; de Sousa Cartágenes, M.D.S. Effects of Extract of Arrabidaea Chica Verlot on an Experimental Model of Osteoarthritis. Int. J. Mol. Sci. 2019, 20, 4717. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Fan, B.; Zhen, T.; Wang, J. A Pre-Clinical Trial Study on Afzelin: Anti-Human Lung Cancer, Anti-Cholinesterase, and Anti-Glucosidase Properties. Arch. Med. Sci. 2021. [Google Scholar] [CrossRef]
- Bonam, S.; Manoharan, S.; Pandy, V.; Raya, A.; Nadendla, R.; Jagadeesan, M.; Babu, A. Phytochemical, in Vitro Antioxidant and In Vivo Safety Evaluation of Leaf Extracts of Tragia Plukenetii. Pharmacogn. J. 2019, 11, 338–345. [Google Scholar] [CrossRef]
- Alanazi, A.; Anwar, M.J.; Ahmad, M.A. Hepatoprotective and Antioxidant Activity of Tragia Involucrata Root Extracts against CCl4 Induced Hepatotoxicity in Rats. Pharm. Lett. 2015, 7, 146–152. [Google Scholar]
- Pallie, M.; Perera, P.; Goonasekara, C.; Kumarasinghe, N.; Arawwawala, M. Efficacy and Safety of Freeze-Dried Form of Tragia Involucrata L. Decoction in Treating Diabetes: A Randomized Controlled Clinical Trial. Clin. Trials Degener. Dis. 2020, 5, 31–36. [Google Scholar] [CrossRef]
- Sulaiman, T.; Balachandran, I. LC/MS Characterization of Antioxidant Flavonoids from Tragia Involucrata L. Beni-Suef Univ. J. Basic Appl. Sci. 2016, 5, 231–235. [Google Scholar] [CrossRef]
- Jan, R.; Khan, M.; Asaf, S.; Lubna; Asif, S.; Kim, K.-M. Bioactivity and Therapeutic Potential of Kaempferol and Quercetin: New Insights for Plant and Human Health. Plants 2022, 11, 2623. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.; Kebede, A. In Vitro Antibacterial Activities of Methanol and Aqueous Leave Extracts of Selected Medicinal Plants against Human Pathogenic Bacteria. Saudi J. Biol. Sci. 2020, 27, 2261–2268. [Google Scholar] [CrossRef]
- Mandal, S.C.; Mandal, V.; Das, A.K. Qualitative Phytochemical Screening. In Essentials of Botanical Extraction; Elsevier: Amsterdam, The Netherlands, 2015; pp. 173–185. ISBN 978-0-12-802325-9. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.-O.; Chung, S.-J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH Assays to Measure Antioxidant Capacity in Popular Antioxidant-Rich US Foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Figueroa, J.G.; Borrás-Linares, I.; Del Pino-García, R.; Curiel, J.A.; Lozano-Sánchez, J.; Segura-Carretero, A. Functional Ingredient from Avocado Peel: Microwave-Assisted Extraction, Characterization and Potential Applications for the Food Industry. Food Chem. 2021, 352, 129300. [Google Scholar] [CrossRef]
- Ahn, S.; Jung, H.; Jung, Y.; Lee, J.; Shin, S.Y.; Lim, Y.; Lee, Y.H. Identification of the Active Components Inhibiting the Expression of Matrix Metallopeptidase-9 by TNFα in Ethyl Acetate Extract of Euphorbia Humifusa Willd. J. Appl. Biol. Chem. 2019, 62, 367–374. [Google Scholar] [CrossRef]
- Hardiyanti, R.; Marpaung, L.; Adnyana, I.K.; Simanjuntak, P. Isolation of Quercitrin from Dendrophthoe Pentandra (L.) Miq Leaves Ans Its Antioxidant and Antibacterial Activities. RJC 2019, 12, 1822–1827. [Google Scholar] [CrossRef]
- Hanrahan, J.R.; Chebib, M.; Davucheron, N.L.M.; Hall, B.J.; Johnston, G.A.R. Semisynthetic Preparation of Amentoflavone: A Negative Modulator at GABA(A) Receptors. Bioorg Med. Chem. Lett. 2003, 13, 2281–2284. [Google Scholar] [CrossRef]
- Le, T.-K.-D.; Hao, B.; Nguyen, T.; Pham, T.; Duong, H. Chemical Constituents of Euphorbia Tirucalli L. Sci. Technol. Dev. J.—Nat. Sci. 2019, 2, 76–82. [Google Scholar] [CrossRef]




| Species | Region | Uses | Refs. |
|---|---|---|---|
| Tragia cordata Michx. | USA | Urinary tract conditions | [13] |
| Tragia geraniifolia Klotzsch ex Müll. Arg. | Bolivia, Paraguay, Uruguay, Argentina | Emollient | [14] |
| Tragia nepetifolia Cav. | USA, Mexico | Snakebite | [11] |
| Tragia pinnata (Poir.) A. Juss. | Brazil, Argentina | Emollient | [15] |
| Tragia ramosa Torr. | USA, Mexico | Ant bite | [16,17] |
| Tragia uberabana Müll. Arg. | Brazil | NS | [18] |
| Tragia volubilis L. | Mexico to Argentina | See below | [10] |
| Tragia yucatanensis Millsp. | Mexico, Belize, Honduras | Burns, rheumatism | [19] |
| Use | Plant Organ | Country | Preparation/Administration | Refs. |
|---|---|---|---|---|
| Analgesic | Stem, leaves | Cameroon | Decoction | [31] |
| Antirheumatic | Leaves, branches | Colombia | Lightly whip affected joints | [32] |
| Anti-ulcer | NS | Brazil | NS | [33] |
| Blood pressure | Leaf | Brazil | Infusion; oral | [34] |
| Cancer prevention | Leaf | DutchCaribbean | Infusion, oral | [35] |
| Diuretic | NS | Argentina | NS | [36] |
| Fertility | Stem, leaves | Cameroon | Decoction | [31] |
| Skin ulcers | Aerial parts | Cuba | Plant juice mixed with salt, topical | [37] |
| Sudorific | Root | Cuba | Decoction, oral | [37,38] |
| Venereal diseases | Leaves | Mexico | Decoction, NS | [30] |
| Venereal diseases | Root | Cuba | Decoction, NS | [37] |
| Wound anti-infective | Branches with leaves | Colombia | NS, oral | [39] |
| Wound anti-infective | Branches with leaves | Colombia | Decoction, topical | [32] |
| Compound Family | Presence | Test |
|---|---|---|
| Protein | − | Biuret |
| Carbohydrates | ++ | Fehling |
| Fats | − | Sudan |
| Alkaloids | +++ | Dragendorff |
| Terpenoids | + | Lieberman Burchard |
| Flavonoids | ++ | Shinoda |
| Saponins | − | Foam |
| Quinones | − | Bornträger |
| Tannins | +++ | Ferric Chloride Assay |
| Compound | DPPH IC50 (µM) | Refs. |
|---|---|---|
| 1 | 71.68 ± 0.06 | [60] |
| 2 | 68.26 ± 1.37 | [60] |
| 3 | 14.89 ± 1.71 | [61] |
| 4 | 10.64 ± 0.15 | [62] |
| Compound | Activity | Biological Model | Effect | Refs. |
|---|---|---|---|---|
| Avicularin (1) | Anti-fungal | Candida albicans. | MIC: 4 μg/mL | [66] |
| Antiproliferative | SCC13 cells | Dose and time-dependent apoptosis induction | [67] | |
| Antirheumatic | Human synovial Rheumatoid arthritis cells | Dose-dependent viability inhibition and apoptosis induction | [68] | |
| Quercitrin (2) | Antidiabetic | Male albino Wistar rats, streptomycin-induced diabetes | Glucose homeostasis improvement (p < 0.05) effect at 30 mg/kg dose. | [69] |
| Anti-ulcer | Female Swiss mice | 1.38 mg/kg reduces MPO activity | [70] | |
| Afzelin (3) | Antibacterial | Pseudomonas aeruginosa | MIC: 31 µg/mL | [71] |
| Diuretic | Female Wistar rats | Calcium-sparing diuretic activity. Nephroprotective | [72] | |
| Anti-ulcer | Female Swiss mice | 0.078 mg/kg reduces MPO activity | [70] | |
| Amentoflavone (4) | Cytotoxic | HeLa cells | IC50 20.7 μM | [73] |
| Antirheumatic | Osteoarthritis-induced Wistar rats | Improvements in incapacitation, motor activity, allodynia, and hyperalgesia parameters | [74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero-Benavides, J.C.; Atiencie-Valarezo, N.C.; Duarte-Casar, R. Flavonoid Composition and Antioxidant Activity of Tragia volubilis L. Methanolic Extract. Plants 2023, 12, 3139. https://doi.org/10.3390/plants12173139
Romero-Benavides JC, Atiencie-Valarezo NC, Duarte-Casar R. Flavonoid Composition and Antioxidant Activity of Tragia volubilis L. Methanolic Extract. Plants. 2023; 12(17):3139. https://doi.org/10.3390/plants12173139
Chicago/Turabian StyleRomero-Benavides, Juan Carlos, Nora Cecilia Atiencie-Valarezo, and Rodrigo Duarte-Casar. 2023. "Flavonoid Composition and Antioxidant Activity of Tragia volubilis L. Methanolic Extract" Plants 12, no. 17: 3139. https://doi.org/10.3390/plants12173139