The Synergistic Effects of Curcumin and Chemotherapeutic Drugs in Inhibiting Metastatic, Invasive and Proliferative Pathways
Abstract
:1. Introduction
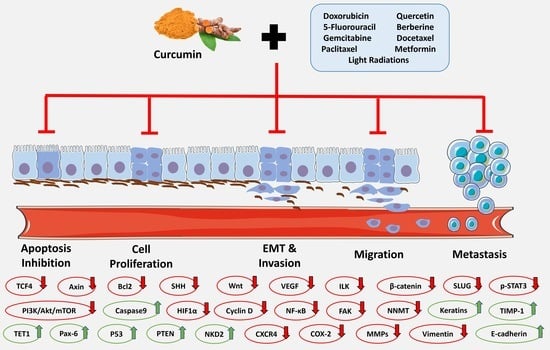
2. Results
2.1. The Hindrance of Metastatic/Proliferative/Invasive Pathways Using Curcumin and Light Radiation
2.2. The Hindrance of Metastatic/Proliferative/Invasive Pathways by Curcumin and Doxorubicin
2.3. The Hindrance of Metastatic/Proliferative/Invasive Pathways by Curcumin and 5-Fluorouracil
2.4. The Hindrance of Metastatic/Proliferative/Invasive Pathways by Curcumin and Paclitaxel
2.5. The Hindrance of Metastatic/Proliferative/Invasive Pathways by Curcumin and Berberine
2.6. The Hindrance of Metastatic/Proliferative/Invasive Pathways by Curcumin and Docetaxel
2.7. The Hindrance of Metastatic/Proliferative/Invasive Pathways by Curcumin and Metformin
2.8. The Hindrance of Metastatic/Proliferative/Invasive Pathways by Curcumin and Gemcitabine
2.9. The Hindrance of Metastatic/Proliferative/Invasive Pathways by Curcumin and Carboplatin
2.10. The Hindrance of Metastatic/Proliferative/Invasive Pathways by Curcumin and Other Drugs
3. Discussion
4. Methods
4.1. Search Strategy
4.2. Selection of Studies
4.2.1. Inclusion Criteria
4.2.2. Exclusion Criteria
4.2.3. Data Extraction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Wang, Y.; Wang, Y.; Cai, N.; Xu, T.; He, F. Anti-Inflammatory Effects of Curcumin in Acute Lung Injury: In Vivo and In Vitro Experimental Model Studies. Int. Immunopharmacol. 2021, 96, 107600. [Google Scholar] [CrossRef] [PubMed]
- Tavaf, Z.; Dangolani, S.K.; Yousefi, R.; Panahi, F.; Shahsavani, M.B.; Khalafi-Nezhad, A. Synthesis of New Curcumin Derivatives as Influential Antidiabetic α-Glucosidase and α-Amylase Inhibitors with Anti-Oxidant Activity. Carbohydr. Res. 2020, 494, 108069. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, M.; Babaei, E.; Neri, F.; Feizi, M.A.H. Anti-Proliferative and Apoptotic Effect of Gemini Curcumin in P53-Wild Type and P53-Mutant Colorectal Cancer Cell Lines. Int. J. Pharm. 2021, 601, 120592. [Google Scholar] [CrossRef] [PubMed]
- Namwan, N.; Senawong, G.; Phaosiri, C.; Kumboonma, P.; Somsakeesit, L.; Samankul, A.; Leerat, C.; Senawong, T. HDAC Inhibitory and Anti-Cancer Activities of Curcumin and Curcumin Derivative CU17 against Human Lung Cancer A549 Cells. Molecules 2022, 27, 4014. [Google Scholar] [CrossRef]
- Fan, X.; Zhu, M.; Qiu, F.; Li, W.; Wang, M.; Guo, Y.; Xi, X.; Du, B. Curcumin May Be a Potential Adjuvant Treatment Drug for Colon Cancer by Targeting CD44. Int. Immunopharmacol. 2020, 88, 106991. [Google Scholar] [CrossRef]
- Li, M.; Guo, T.; Lin, J.; Huang, X.; Ke, Q.; Wu, Y.; Fang, C.; Hu, C. Curcumin Inhibits the Invasion and Metastasis of Triple Negative Breast Cancer via Hedgehog/Gli1 Signaling Pathway. J. Ethnopharmacol. 2022, 283, 114689. [Google Scholar] [CrossRef]
- Seo, J.; Kim, B.; Dhanasekaran, D.N.; Tsang, B.K.; Song, Y.S. Curcumin Induces Apoptosis by Inhibiting Sarco/Endoplasmic Reticulum Ca2+ ATPase Activity in Ovarian Cancer Cells. Cancer Lett. 2016, 371, 30–37. [Google Scholar] [CrossRef]
- Kharat, M.; Du, Z.; Zhang, G.; McClements, D.J. Physical and Chemical Stability of Curcumin in Aqueous Solutions and Emulsions: Impact of PH, Temperature, and Molecular Environment. J. Agric. Food Chem. 2017, 65, 1525–1532. [Google Scholar] [CrossRef]
- Costantino, M.; Corno, C.; Colombo, D.; Perego, P. Curcumin and Related Compounds in Cancer Cells: New Avenues for Old Molecules. Front. Pharmacol. 2022, 13, 889816. [Google Scholar] [CrossRef]
- Imran, M.; Ullah, A.; Saeed, F.; Nadeem, M.; Arshad, M.U.; Suleria, H.A.R. Cucurmin, Anticancer, & Antitumor Perspectives: A Comprehensive Review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1271–1293. [Google Scholar] [CrossRef]
- Ungurianu, A.; Zanfirescu, A.; Margină, D. Regulation of Gene Expression through Food—Curcumin as a Sirtuin Activity Modulator. Plants 2022, 11, 1741. [Google Scholar] [CrossRef]
- Tang, Y.; Cao, Y. Curcumin Inhibits the Growth and Metastasis of Melanoma via MiR-222-3p/SOX10/Notch Axis. Dis. Markers 2022, 2022, e3129781. [Google Scholar] [CrossRef]
- Deng, Y.; Verron, E.; Rohanizadeh, R. Molecular Mechanisms of Anti-Metastatic Activity of Curcumin. Anticancer Res. 2016, 36, 5639–5648. [Google Scholar] [CrossRef]
- Xia, L.; Tan, S.; Zhou, Y.; Lin, J.; Wang, H.; Oyang, L.; Tian, Y.; Liu, L.; Su, M.; Wang, H.; et al. Role of the NFκB-Signaling Pathway in Cancer. Onco Targets Ther. 2018, 11, 2063–2073. [Google Scholar] [CrossRef]
- Bachmeier, B.E.; Killian, P.H.; Melchart, D. The Role of Curcumin in Prevention and Management of Metastatic Disease. Int. J. Mol. Sci. 2018, 19, 1716. [Google Scholar] [CrossRef]
- Bharti, A.C.; Donato, N.; Singh, S.; Aggarwal, B.B. Curcumin (Diferuloylmethane) down-Regulates the Constitutive Activation of Nuclear Factor–ΚB and IκBα Kinase in Human Multiple Myeloma Cells, Leading to Suppression of Proliferation and Induction of Apoptosis. Blood 2003, 101, 1053–1062. [Google Scholar] [CrossRef]
- Tong, W.; Wang, Q.; Sun, D.; Suo, J. Curcumin Suppresses Colon Cancer Cell Invasion via AMPK-Induced Inhibition of NF–ΚB, UPA Activator and MMP9. Oncol. Lett. 2016, 12, 4139–4146. [Google Scholar] [CrossRef]
- Roomi, M.W.; Kalinovsky, T.; Niedzwiecki, A.; Rath, M. Modulation of UPA, MMPs and Their Inhibitors by a Novel Nutrient Mixture in Human Colorectal, Pancreatic and Hepatic Carcinoma Cell Lines. Int. J. Oncol. 2015, 47, 370–376. [Google Scholar] [CrossRef]
- Zong, H.; Wang, F.; Fan, Q.; Wang, L. Curcumin Inhibits Metastatic Progression of Breast Cancer Cell through Suppression of Urokinase-Type Plasminogen Activator by NF-Kappa B Signaling Pathways. Mol. Biol. Rep. 2012, 39, 4803–4808. [Google Scholar] [CrossRef]
- Corvinus, F.M.; Orth, C.; Moriggl, R.; Tsareva, S.A.; Wagner, S.; Pfitzner, E.B.; Baus, D.; Kaufman, R.; Huber, L.A.; Zatloukal, K.; et al. Persistent STAT3 Activation in Colon Cancer Is Associated with Enhanced Cell Proliferation and Tumor Growth. Neoplasia 2005, 7, 545–555. [Google Scholar] [CrossRef]
- Thomas, S.J.; Snowden, J.A.; Zeidler, M.P.; Danson, S.J. The Role of JAK/STAT Signalling in the Pathogenesis, Prognosis and Treatment of Solid Tumours. Br. J. Cancer 2015, 113, 365–371. [Google Scholar] [CrossRef]
- Glienke, W.; Maute, L.; Wicht, J.; Bergmann, L. Curcumin Inhibits Constitutive STAT3 Phosphorylation in Human Pancreatic Cancer Cell Lines and Downregulation of Survivin/BIRC5 Gene Expression. Cancer Investig. 2009, 28, 166–171. [Google Scholar] [CrossRef]
- Xu, X.; Zhu, Y. Curcumin Inhibits Human Non-Small Cell Lung Cancer Xenografts by Targeting STAT3 Pathway. Am. J. Transl. Res. 2017, 9, 3633–3641. [Google Scholar]
- Mohammadi Kian, M.; Salemi, M.; Bahadoran, M.; Haghi, A.; Dashti, N.; Mohammadi, S.; Rostami, S.; Chahardouli, B.; Babakhani, D.; Nikbakht, M. Curcumin Combined with Thalidomide Reduces Expression of STAT3 and Bcl-XL, Leading to Apoptosis in Acute Myeloid Leukemia Cell Lines. Drug Des. Dev. Ther. 2020, 14, 185–194. [Google Scholar] [CrossRef]
- Mahata, S.; Behera, S.K.; Kumar, S.; Sahoo, P.K.; Sarkar, S.; Fazil, M.H.U.T.; Nasare, V.D. In-Silico and in-Vitro Investigation of STAT3-PIM1 Heterodimeric Complex: Its Mechanism and Inhibition by Curcumin for Cancer Therapeutics. Int. J. Biol. Macromol. 2022, 208, 356–366. [Google Scholar] [CrossRef]
- Tseng, Y.-H.; Yang, R.-C.; Chiou, S.-S.; Shieh, T.-M.; Shih, Y.-H.; Lin, P.-C. Curcumin Induces Apoptosis by Inhibiting BCAT1 Expression and MTOR Signaling in Cytarabine-resistant Myeloid Leukemia Cells. Mol. Med. Rep. 2021, 24, 565. [Google Scholar] [CrossRef]
- Farghadani, R.; Naidu, R. Curcumin as an Enhancer of Therapeutic Efficiency of Chemotherapy Drugs in Breast Cancer. IJMS 2022, 23, 2144. [Google Scholar] [CrossRef]
- Wang, L.; Hu, R.; Dai, A. Curcumin Increased the Sensitivity of Non-Small-Cell Lung Cancer to Cisplatin through the Endoplasmic Reticulum Stress Pathway. Evid. Based Complement. Altern. Med. 2022, 2022, 6886366. [Google Scholar] [CrossRef] [PubMed]
- Muhanmode, Y.; Wen, M.K.; Maitinuri, A.; Shen, G. Curcumin and Resveratrol Inhibit Chemoresistance in Cisplatin-Resistant Epithelial Ovarian Cancer Cells via Targeting P13K Pathway. Hum. Exp. Toxicol. 2022, 41, 09603271221095929. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Ganapathy, S.; Chen, Q.; Srivastava, R.K. Curcumin Sensitizes TRAIL-Resistant Xenografts: Molecular Mechanisms of Apoptosis, Metastasis and Angiogenesis. Mol. Cancer 2008, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Huang, P.; Law, S.; Tian, H.; Leung, W.; Xu, C. Preventive Effect of Curcumin Against Chemotherapy-Induced Side-Effects. Front. Pharmacol. 2018, 9, 1374. [Google Scholar] [CrossRef]
- Deng, X.; Chen, C.; Wu, F.; Qiu, L.; Ke, Q.; Sun, R.; Duan, Q.; Luo, M.; Luo, Z. Curcumin Inhibits the Migration and Invasion of Non-Small-Cell Lung Cancer Cells Through Radiation-Induced Suppression of Epithelial-Mesenchymal Transition and Soluble E-Cadherin Expression. Technol. Cancer Res. Treat. 2020, 19, 153303382094748. [Google Scholar] [CrossRef]
- Rutz, J.; Maxeiner, S.; Justin, S.; Bachmeier, B.; Bernd, A.; Kippenberger, S.; Zöller, N.; Chun, F.K.-H.; Blaheta, R.A. Low Dosed Curcumin Combined with Visible Light Exposure Inhibits Renal Cell Carcinoma Metastatic Behavior in Vitros. Cancers 2020, 12, 302. [Google Scholar] [CrossRef]
- Rutz, J.; Benchellal, A.; Kassabra, W.; Maxeiner, S.; Bernd, A.; Kippenberger, S.; Zöller, N.; Chun, F.K.-H.; Juengel, E.; Blaheta, R.A. Growth, Proliferation and Metastasis of Prostate Cancer Cells Is Blocked by Low-Dose Curcumin in Combination with Light Irradiation. IJMS 2021, 22, 9966. [Google Scholar] [CrossRef]
- Mani, J.; Fleger, J.; Rutz, J.; Maxeiner, S.; Bernd, A.; Kippenberger, S.; Zöller, N.; Chun, F.K.-H.; Relja, B.; Juengel, E.; et al. Curcumin Combined with Exposure to Visible Light Blocks Bladder Cancer Cell Adhesion and Migration by an Integrin Dependent Mechanism. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 10564–10574. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Jung, C.-W.; Lee, W.S.; Kim, H.-J.; Jeong, H.-J.; Park, M.-J.; Jang, W.I.; Kim, E.H. Interaction of Curcumin with Glioblastoma Cells via High and Low Linear Energy Transfer Radiation Therapy Inducing Radiosensitization Effects. J. Radiat. Res. 2022, 63, 342–353. [Google Scholar] [CrossRef]
- Al-malky, H.S.; Al Harthi, S.E.; Osman, A.-M.M. Major Obstacles to Doxorubicin Therapy: Cardiotoxicity and Drug Resistance. J. Oncol. Pharm. Pract. 2020, 26, 434–444. [Google Scholar] [CrossRef]
- Christowitz, C.; Davis, T.; Isaacs, A.; van Niekerk, G.; Hattingh, S.; Engelbrecht, A.-M. Mechanisms of Doxorubicin-Induced Drug Resistance and Drug Resistant Tumour Growth in a Murine Breast Tumour Model. BMC Cancer 2019, 19, 757. [Google Scholar] [CrossRef]
- Gote, V.; Nookala, A.R.; Bolla, P.K.; Pal, D. Drug Resistance in Metastatic Breast Cancer: Tumor Targeted Nanomedicine to the Rescue. IJMS 2021, 22, 4673. [Google Scholar] [CrossRef]
- Lin, X.; Xiang, X.; Hao, L.; Wang, T.; Lai, Y.; Abudoureyimu, M.; Zhou, H.; Feng, B.; Chu, X.; Wang, R. The Role of Aurora-A in Human Cancers and Future Therapeutics. Am. J. Cancer Res. 2020, 10, 2705. [Google Scholar]
- Biswas, S.; Mahapatra, E.; Ghosh, A.; Das, S.; Roy, M.; Mukherjee, S. Curcumin Rescues Doxorubicin Responsiveness via Regulating Aurora a Signaling Network in Breast Cancer Cells. Asian Pac. J. Cancer Prev. 2021, 22, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Firouzi Amoodizaj, F.; Baghaeifar, S.; Taheri, E.; Farhoudi Sefidan Jadid, M.; Safi, M.; Seyyed Sani, N.; Hajazimian, S.; Isazadeh, A.; Shanehbandi, D. Enhanced Anticancer Potency of Doxorubicin in Combination with Curcumin in Gastric Adenocarcinoma. J. Biochem. Mol. Toxicol. 2020, 34, e22486. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, C.; Zou, Y.; Jin, Y.; Han, S.; Liu, Q.; Hu, X.; Wang, L.; Ma, Y.; Liu, Y. Multi PH-Sensitive Polymer–Drug Conjugate Mixed Micelles for Efficient Co-Delivery of Doxorubicin and Curcumin to Synergistically Suppress Tumor Metastasis. Biomater. Sci. 2020, 8, 5029–5046. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Fang, S.; Shao, X.; Li, Y.; Tong, Q.; Kong, B.; Chen, L.; Wang, Y.; Yang, J.; Yu, H.; et al. Curcumin Reverses NNMT-Induced 5-Fluorouracil Resistance via Increasing ROS and Cell Cycle Arrest in Colorectal Cancer Cells. Biomolecules 2021, 11, 1295. [Google Scholar] [CrossRef]
- Xu, T.; Guo, P.; Pi, C.; He, Y.; Yang, H.; Hou, Y.; Feng, X.; Jiang, Q.; Wei, Y.; Zhao, L. Synergistic Effects of Curcumin and 5-Fluorouracil on the Hepatocellular Carcinoma In Vivo and Vitro through Regulating the Expression of COX-2 and NF-ΚB. J. Cancer 2020, 11, 3955–3964. [Google Scholar] [CrossRef]
- Ham, I.-H.; Wang, L.; Lee, D.; Woo, J.; Kim, T.H.; Jeong, H.Y.; Oh, H.J.; Choi, K.S.; Kim, T.-M.; Hur, H. Curcumin Inhibits the Cancer-Associated Fibroblast-Derived Chemoresistance of Gastric Cancer through the Suppression of the JAK/STAT3 Signaling Pathway. Int. J. Oncol. 2022, 61, 85. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, R.; Zhang, X.; Zhang, B.; Yao, Q. Curcumin May Reverse 5-Fluorouracil Resistance on Colonic Cancer Cells by Regulating TET1-NKD-Wnt Signal Pathway to Inhibit the EMT Progress. Biomed. Pharmacother. 2020, 129, 110381. [Google Scholar] [CrossRef]
- Swanton, C.; Marani, M.; Pardo, O.; Warne, P.H.; Kelly, G.; Sahai, E.; Elustondo, F.; Chang, J.; Temple, J.; Ahmed, A.A.; et al. Regulators of Mitotic Arrest and Ceramide Metabolism Are Determinants of Sensitivity to Paclitaxel and Other Chemotherapeutic Drugs. Cancer Cell 2007, 11, 498–512. [Google Scholar] [CrossRef]
- Zhan, Y.; Chen, Y.; Liu, R.; Zhang, H.; Zhang, Y. Potentiation of Paclitaxel Activity by Curcumin in Human Breast Cancer Cell by Modulating Apoptosis and Inhibiting EGFR Signaling. Arch. Pharmacal Res. 2014, 37, 1086–1095. [Google Scholar] [CrossRef]
- Zhao, M.-D.; Li, J.-Q.; Chen, F.-Y.; Dong, W.; Wen, L.-J.; Fei, W.-D.; Zhang, X.; Yang, P.-L.; Zhang, X.-M.; Zheng, C.-H. Co-Delivery of Curcumin and Paclitaxel by “Core-Shell” Targeting Amphiphilic Copolymer to Reverse Resistance in the Treatment of Ovarian Cancer. IJN 2019, 14, 9453–9467. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Shishodia, S.; Takada, Y.; Banerjee, S.; Newman, R.A.; Bueso-Ramos, C.E.; Price, J.E. Curcumin Suppresses the Paclitaxel-Induced Nuclear Factor-ΚB Pathway in Breast Cancer Cells and Inhibits Lung Metastasis of Human Breast Cancer in Nude Mice. Clin. Cancer Res. 2005, 11, 7490–7498. [Google Scholar] [CrossRef]
- Vemuri, S.K.; Halder, S.; Banala, R.R.; Rachamalla, H.K.; Devraj, V.M.; Mallarpu, C.S.; Neerudu, U.K.; Bodlapati, R.; Mukherjee, S.; Venkata, S.G.P.; et al. Modulatory Effects of Biosynthesized Gold Nanoparticles Conjugated with Curcumin and Paclitaxel on Tumorigenesis and Metastatic Pathways-In Vitro and In Vivo Studies. Int. J. Mol. Sci. 2022, 23, 2150. [Google Scholar] [CrossRef]
- Kashyap, A.; Umar, S.M.; Dev J.R., A.; Mendiratta, M.; Prasad, C.P. In Vitro Anticancer Efficacy of a Polyphenolic Combination of Quercetin, Curcumin, and Berberine in Triple Negative Breast Cancer (TNBC) Cells. Phytomed. Plus 2022, 2, 100265. [Google Scholar] [CrossRef]
- Ziasarabi, P.; Sahebkar, A.; Ghasemi, F. Evaluation of the Effects of Nanomicellar Curcumin, Berberine, and Their Combination with 5-Fluorouracil on Breast Cancer Cells. In Natural Products and Human Diseases: Pharmacology, Molecular Targets, and Therapeutic Benefits; Sahebkar, A., Sathyapalan, T., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 21–35. ISBN 978-3-030-73234-9. [Google Scholar]
- Liu, P.; Ying, Q.; Liu, H.; Yu, S.; Bu, L.; Shao, L.; Li, X. Curcumin Enhances Anti-cancer Efficacy of Either Gemcitabine or Docetaxel on Pancreatic Cancer Cells. Oncol. Rep. 2020, 44, 1393–1402. [Google Scholar] [CrossRef]
- Deng, L.; Wu, X.; Zhu, X.; Yu, Z.; Liu, Z.; Wang, J.; Zheng, Y. Combination Effect of Curcumin with Docetaxel on the PI3K/AKT/MTOR Pathway to Induce Autophagy and Apoptosis in Esophageal Squamous Cell Carcinoma. Am. J. Transl. Res. 2021, 13, 57–72. [Google Scholar]
- Yan, J.; Wang, Y.; Jia, Y.; Liu, S.; Tian, C.; Pan, W.; Liu, X.; Wang, H. Co-Delivery of Docetaxel and Curcumin Prodrug via Dual-Targeted Nanoparticles with Synergistic Antitumor Activity against Prostate Cancer. Biomed. Pharmacother. 2017, 88, 374–383. [Google Scholar] [CrossRef]
- Seko, I.; Tonbul, H.; Tavukçuoğlu, E.; Şahin, A.; Akbas, S.; Yanık, H.; Öztürk, S.C.; Esendagli, G.; Khan, M.; Capan, Y. Development of Curcumin and Docetaxel Co-Loaded Actively Targeted PLGA Nanoparticles to Overcome Blood Brain Barrier. J. Drug Deliv. Sci. Technol. 2021, 66, 102867. [Google Scholar] [CrossRef]
- Zhang, H.-H.; Zhang, Y.; Cheng, Y.-N.; Gong, F.-L.; Cao, Z.-Q.; Yu, L.-G.; Guo, X.-L. Metformin Incombination with Curcumin Inhibits the Growth, Metastasis, and Angiogenesis of Hepatocellular Carcinoma in Vitro and In Vivo. Mol. Carcinog. 2018, 57, 44–56. [Google Scholar] [CrossRef]
- Zarei, E.; Sefidi-Heris, Y.; Saadat, I. Synergistic Effects of Metformin and Curcumin on Cytotoxicity of Chemotherapy Drugs Using a Gastric Cancer Cell Line Model. EXCLI J. 2021, 20, 1488. [Google Scholar] [CrossRef]
- Pyrimidine Analogues. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012. [Google Scholar]
- Pastorelli, D.; Fabricio, A.S.C.; Giovanis, P.; D’Ippolito, S.; Fiduccia, P.; Soldà, C.; Buda, A.; Sperti, C.; Bardini, R.; Da Dalt, G.; et al. Phytosome Complex of Curcumin as Complementary Therapy of Advanced Pancreatic Cancer Improves Safety and Efficacy of Gemcitabine: Results of a Prospective Phase II Trial. Pharmacol. Res. 2018, 132, 72–79. [Google Scholar] [CrossRef]
- Khan, S.; Setua, S.; Kumari, S.; Dan, N.; Massey, A.; Hafeez, B.B.; Yallapu, M.M.; Stiles, Z.E.; Alabkaa, A.; Yue, J.; et al. Superparamagnetic Iron Oxide Nanoparticles of Curcumin Enhance Gemcitabine Therapeutic Response in Pancreatic Cancer. Biomaterials 2019, 208, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Feng, Q.; Zhang, H.; Liu, Q.; Gong, J. Curcumin Enhances Drug Sensitivity of Gemcitabine-Resistant Lung Cancer Cells and Inhibits Metastasis. Pharmazie 2021, 76, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, J.; Fu, Z.; Zhu, B.; Wang, J.; Guan, S.; Hua, Z. Curcumin Activates DNA Repair Pathway in Bone Marrow to Improve Carboplatin-Induced Myelosuppression. Sci. Rep. 2017, 7, 17724. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Kang, H.S.; Kim, I.K.; Lee, H.Y.; Ha, J.H.; Yeo, C.D.; Kang, H.H.; Moon, H.S.; Lee, S.H. Curcumin Sensitizes Human Lung Cancer Cells to Apoptosis and Metastasis Synergistically Combined with Carboplatin. Exp. Biol. Med. 2015, 240, 1416–1425. [Google Scholar] [CrossRef]
- Wang, G.; Duan, P.; Wei, Z.; Liu, F. Curcumin Sensitizes Carboplatin Treatment in Triple Negative Breast Cancer through Reactive Oxygen Species Induced DNA Repair Pathway. Mol. Biol. Rep. 2022, 49, 3259–3270. [Google Scholar] [CrossRef]
- Wiegmans, A.P.; Al-Ejeh, F.; Chee, N.; Yap, P.-Y.; Gorski, J.J.; Silva, L.D.; Bolderson, E.; Chenevix-Trench, G.; Anderson, R.; Simpson, P.T.; et al. Rad51 Supports Triple Negative Breast Cancer Metastasis. Oncotarget 2014, 5, 3261–3272. [Google Scholar] [CrossRef]
- Moreno-Q, G.; Herrera-R, A.; Yepes, A.F.; Naranjo, T.W.; Cardona-G, W. Proapoptotic Effect and Molecular Docking Analysis of Curcumin–Resveratrol Hybrids in Colorectal Cancer Chemoprevention. Molecules 2022, 27, 3486. [Google Scholar] [CrossRef]
- Panda, S.S.; Tran, Q.L.; Rajpurohit, P.; Pillai, G.G.; Thomas, S.J.; Bridges, A.E.; Capito, J.E.; Thangaraju, M.; Lokeshwar, B.L. Design, Synthesis, and Molecular Docking Studies of Curcumin Hybrid Conjugates as Potential Therapeutics for Breast Cancer. Pharmaceuticals 2022, 15, 451. [Google Scholar] [CrossRef]
- Aromokeye, R.; Si, H. Combined Curcumin and Luteolin Synergistically Inhibit Colon Cancer Associated with Notch1 and TGF-β Signaling Pathways in Cultured Cells and Xenograft Mice. Cancers 2022, 14, 3001. [Google Scholar] [CrossRef]
- Muñoz, M.; Coveñas, R. The Neurokinin-1 Receptor Antagonist Aprepitant: An Intelligent Bullet against Cancer? Cancers 2020, 12, 2682. [Google Scholar] [CrossRef]
- Li, Y.; Wu, J.; Lu, Q.; Liu, X.; Wen, J.; Qi, X.; Liu, J.; Lian, B.; Zhang, B.; Sun, H.; et al. GA & HA-Modified Liposomes for Co-Delivery of Aprepitant and Curcumin to Inhibit Drug-Resistance and Metastasis of Hepatocellular Carcinoma. IJN 2022, 17, 2559–2575. [Google Scholar] [CrossRef]
- Saddiq, A.A.; El-Far, A.H.; Mohamed Abdullah, S.A.; Godugu, K.; Almaghrabi, O.A.; Mousa, S.A. Curcumin, Thymoquinone, and 3, 3′-Diindolylmethane Combinations Attenuate Lung and Liver Cancers Progression. Front. Pharmacol. 2022, 13, 2563. [Google Scholar] [CrossRef]
- Shao, M.; Lou, D.; Yang, J.; Lin, M.; Deng, X.; Fan, Q. Curcumin and Wikstroflavone B, a New Biflavonoid Isolated from Wikstroemia Indica, Synergistically Suppress the Proliferation and Metastasis of Nasopharyngeal Carcinoma Cells via Blocking FAK/STAT3 Signaling Pathway. Phytomedicine 2020, 79, 153341. [Google Scholar] [CrossRef]
- Liu, S.; Liu, J.; He, L.; Liu, L.; Cheng, B.; Zhou, F.; Cao, D.; He, Y. A Comprehensive Review on the Benefits and Problems of Curcumin with Respect to Human Health. Molecules 2022, 27, 4400. [Google Scholar] [CrossRef]
- Alven, S.; Aderibigbe, B.A. Efficacy of Polymer-Based Nanocarriers for Co-Delivery of Curcumin and Selected Anticancer Drugs. Nanomaterials 2020, 10, 1556. [Google Scholar] [CrossRef]
- Mahmoudi, A.; Kesharwani, P.; Majeed, M.; Teng, Y.; Sahebkar, A. Recent Advances in Nanogold as a Promising Nanocarrier for Curcumin Delivery. Colloids Surf. B Biointerfaces 2022, 215, 112481. [Google Scholar] [CrossRef]






| Reference | Cancer Type (Cell Lines) | Type of Intervention | Methods | Molecular Outcome | Study Conclusion |
|---|---|---|---|---|---|
| [69] | Colorectal cancer cells | Pharmacological: curcumin + resveratrol | In vitro: staining for mitochondrial membrane potential and plasma membrane integrity Molecular docking |
| Combination of curcumin and resveratrol has synergistic antiproliferative, antimetastatic and pro-apoptotic effects in colorectal cancer cells |
| [70] | Breast cancer cells | Curcumin + dichloroacetate | In vitro MTT assay, colony formation assay, molecular docking |
| Curcumin and dichloroacetate inhibit breast cancer cells’ survival, proliferation and metastasis |
| [71] | Colon cancer cells | Curcumin + luteolin | In vitro: cell proliferation assay, wound-healing assay, Western blot |
| Curcumin and luteolin synergistically inhibit the proliferation as well as invasion and metastasis of colon cancer cells via the suppression of the Notch-1 and TGF-β signaling pathway |
| [73] | Hepatocellular carcinoma | Curcumin + aprepitant | In vitro: cytotoxicity assay, cell migration assay In vivo: lung metastasis |
| Co-delivery of curcumin and aprepitant via modified liposome conjugates, effective in suppressing tumor cell proliferation, invasion and metastasis as well as inhibiting lung metastasis in vivo |
| [74] | Lung and liver cancer cells | Curcumin + thymoquinone + 3,3′-diindolymethane (DIM) | In vitro: cell proliferation assay, migration assay, colony formation assay and Western blot analysis |
| Combination of curcumin with thymoquinone and DIM exhibits anti-proliferative as well as anti-metastatic activity on lung and liver cancer cells |
| [75] | Nasopharyngeal carcinoma cells (NCC) | Curcumin + wikstroflavone B (WFB) | In vitro: cytotoxicity assay, colony formation assay, wound healing assay and transwell migration assay |
| Curcumin when combined with WFB inhibits nasopharyngeal carcinoma cell proliferation, invasion and metastasis via the suppression of the FAK/STAT3 signaling pathway |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Younes, M.; Mardirossian, R.; Rizk, L.; Fazlian, T.; Khairallah, J.P.; Sleiman, C.; Naim, H.Y.; Rizk, S. The Synergistic Effects of Curcumin and Chemotherapeutic Drugs in Inhibiting Metastatic, Invasive and Proliferative Pathways. Plants 2022, 11, 2137. https://doi.org/10.3390/plants11162137
Younes M, Mardirossian R, Rizk L, Fazlian T, Khairallah JP, Sleiman C, Naim HY, Rizk S. The Synergistic Effects of Curcumin and Chemotherapeutic Drugs in Inhibiting Metastatic, Invasive and Proliferative Pathways. Plants. 2022; 11(16):2137. https://doi.org/10.3390/plants11162137
Chicago/Turabian StyleYounes, Maria, Rita Mardirossian, Liza Rizk, Tia Fazlian, Jean Paul Khairallah, Christopher Sleiman, Hassan Y. Naim, and Sandra Rizk. 2022. "The Synergistic Effects of Curcumin and Chemotherapeutic Drugs in Inhibiting Metastatic, Invasive and Proliferative Pathways" Plants 11, no. 16: 2137. https://doi.org/10.3390/plants11162137