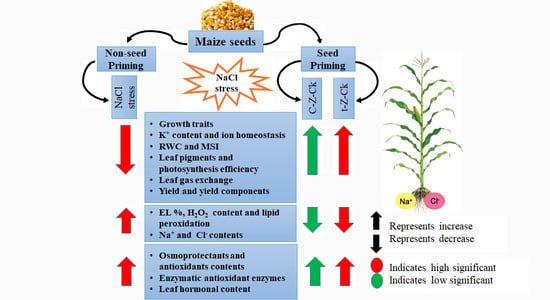
Soaking Maize Seeds in Zeatin-Type Cytokinin Biostimulators Improves Salt Tolerance by Enhancing the Antioxidant System and Photosynthetic Efficiency
Abstract
:1. Introduction
2. Materials and Methods
2.1. Growth Conditions, Treatments, and Layout of Experiments
2.2. Growth, Yield Components, and Photosynthesis Efficiency Determinations
2.3. Relative Water Content (RWC), Membrane Stability Index (MSI), Malondialdehyde (MDA), Electrolyte Leakage (EL), Soluble Sugars, Proline, Ascorbic Acid (AsA), and Glutathione (GSH) Determinations
2.4. Assaying Antioxidant Enzyme Activities and Hormones Assessment
2.5. Assessment of K+, Na+, and Cl− Contents
2.6. Determination of Yield and Yield Components
2.7. Data Analysis
3. Results
3.1. Components of Maize Growth and Yield
3.2. Leaf Photosynthetic Pigments and Photosynthetic Efficiency
3.3. Leaf Gas Exchange
3.4. Relative Water Content (RWC), Membrane Stability Index (MSI), and Ion Leakage (EL)
3.5. Lipid Peroxidation, Osmoprotectants, and Antioxidants Contents of Maize
3.6. Enzymatic Antioxidant Activities of Maize
3.7. Leaf Hormonal Content of Maize
3.8. Leaf Content of Na+, Cl− and K+, and K+/Na+ Ratio of Maize
3.9. Estimation of Yield and Yield Components
3.10. Relationship between Different Treatments and the Parameters Studied
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khrueasan, N.; Siangliw, M.; Toojinda, T.; Imyim, A.; Buaboocha, T.; Chadchawan, S. Physiological mechanisms of the seedling stage salt tolerance of near isogenic rice lines with the ‘KDML105’ genetic background. Int. J. Agric. Biol. 2020, 23, 927–934. [Google Scholar]
- Tester, M.; Bacic, A. Abiotic stress tolerance in grasses. From model plants to crop plants. Plant Physiol. 2005, 137, 791–793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamal, Y.; Shafi1, M.; Bakht, J. Effect of seed priming on growth and biochemical traits of wheat under saline conditions. Afr. J. Biotechnol. 2011, 10, 17127–17133. [Google Scholar]
- Yokoi, S.; Bressan, R.A.; Hasegawa, P.M. Salt stress tolerance of plants. JIRCAS Work. Rep. 2002, 2002, 25–33. [Google Scholar]
- El-Menshawi, M.M.; Ashry, N.A.; Azzam, C.R. Evaluation of some grain sorghum hybrids under saline conditions and identification of salinity tolerant genotypes using some biochemical genetic markers. Egypt. J. Plant Breed. 2003, 7, 183–203. [Google Scholar]
- Yusuf, M.; Hasan, S.A.; Ali, B.; Hayat, S.; Fariduddin, Q.; Ahmad, A. Effect of salicylic acid on salinity-induced changes in Brassica Juncea. J. Integr. Plant Biol. 2008, 50, 1096–1102. [Google Scholar] [CrossRef]
- Keshta, M.M.A.; Hassan, N.M.; Azzam, C.R.; Hassanin, O.S. Embryogenic callus induction of some sunflower (Helianthus annuus L.) genotypes under in vitro salt stress. J. Plant Prod. Mansoura Univ. 2011, 2, 327–333. [Google Scholar] [CrossRef]
- Azzam, C.R.; Abd El Naby, Z.M.; Mohamed, N.A. Salt tolerance associated with molecular markers in alfalfa. J. Biosci. Appl. Res. 2019, 5, 416–428. [Google Scholar] [CrossRef]
- Hamada, M.S.I.; Azzam, C.R.; Abdel-Moneam, M.A.; Abd El-Aziz, M.H.; Hamed, R.M.I. Molecular and Phenotypic evaluation of some maize genotypes. Egypt. J. Plant Breed. 2019, 23, 875–903. [Google Scholar]
- Desoky, E.M.; Elrys, A.S.; Rady, M.M. Integrative Moringa and Licorice extracts application improves performance and reduces fruit contamination content of pepper plants grown on heavy metals-contaminated saline soil. Ecotoxicol. Environ. Saf. 2019, 169, 50–60. [Google Scholar] [CrossRef]
- Bekhiet, A.M.A.; Helmy, A.M.; Fouda, S.E.; Azzam, C.R. Evaluation of Salinity Tolerance of Some Egyptian Faba Bean Varieties During the Germination Stage. CIACR 2022, 10, 1316–1328. [Google Scholar]
- Rady, M.M.; Talaat, N.B.; Abdelhamid, M.T.; Shawky, B.T.; Desoky, E.M. Maize (Zea mays L.) grains extract mitigates the deleterious effects of salt stress on common bean (Phaseolus vulgaris L.) growth and physiology. J. Hortic. Sci. Biotechnol. 2019, 94, 777–789. [Google Scholar] [CrossRef]
- Rady, M.M.; Desoky, E.M.; Elrys, A.S.; Boghdady, M.S. Can licorice root extract be used as effective natural biostimulant for salt-stressed common bean plants? South Afr. J. Bot. 2019, 121, 294–305. [Google Scholar] [CrossRef]
- Abd El-Mageed, T.A.; Rady, M.M.; Taha, R.S.; Abdelaziz, S.A.; Simpson, C.R.; Semida, W.M. Effects of integrated use of residual sulfur-enhanced biochar with effective microorganisms on soil properties, plant growth and short-term productivity of Capsicum annuum under salt stress. Sci. Hortic. 2020, 261, 108930. [Google Scholar] [CrossRef]
- IPCC. Intergovernmental panel on climate change. In Proceedings of the 5th Assessment Report, WGII, Climate Change 2014: Impacts, Adaptation, and Vulnerability; Cambridge University Press: Cambridge, UK, 2014; Available online: http://www.ipcc.ch/report/ar5/wg2/ (accessed on 20 April 2021).
- Turan, M.A.; Elkarim, A.H.; Taban, N.; Taban, S. Effect of salt stress on, stomatal resistance, proline and chlorophyll concentrations on maize plant. Afr. J. Agric. Res. 2009, 4, 893–897. [Google Scholar]
- Farooq, M.; Usman, M.; Nadeem, F.; Rehman, H.; Wahid, A.; Basra, S.M.A.; Siddique, K.H.M. Seed priming in field crops–potential benefits, adoption and challenges. Crop Past. Sci. 2019, 70, 731–771. [Google Scholar] [CrossRef]
- Singh, M.; Kumar, J.; Singh, S.; Singh, V.P.; Prasad, S.M. Roles of osmoprotectants in improving salinity and drought tolerance in plants. Rev. Environ. Sci. Biotechnol. 2015, 14, 407–426. [Google Scholar] [CrossRef]
- Azzam, C.R.; Omran, S.E.H. The promotive effect of PDB biofertilizer on growth, enzymatic activity and biochemical changes of sunflower (Helianthus annuus L.) plants sprayed with micronutrients. In Proceedings of the 3rd Conference of Recent Technologies in Agriculture, Giza, Egypt, 14–16 November 2005; Volume 2, pp. 255–267. [Google Scholar]
- Dobeie, A.M.; Abbas, M.S.; Soliman, A.S.; Azzam, C.R. In vitro screening of some Egyptian and Nigerian peanut genotypes for salt tolerance. Egypt. J. Plant Breed. 2017, 21, 1035–1050. [Google Scholar] [CrossRef]
- Alharby, H.F.; Alzahrani, Y.M.; Rady, M.M. Seeds Pretreatment with Zeatins or Maize Grain-Derived Organic Biostimulant Improved Hormonal Contents, Polyamine Gene Expression, and Salinity and Drought Tolerance of Wheat. Int. J. Agric. Biol. 2020, 24, 714–724. [Google Scholar]
- Schäfer, M.; Brütting, C.; Meza-Canales, I.D.; Groβkinsky, D.K.; Vankova, R.; Baldwin, I.T.; Meldau, S. The role of cis-zeatin-type cytokinins in plant growth regulation and mediating responses to environmental interactions—A review. J. Exp. Bot. 2015, 66, 4873–4884. [Google Scholar] [CrossRef] [Green Version]
- Barciszewski, J.; Siboska, G.; Rattan, S.I.S.; Clark, B.F.C. Occurrence, biosynthesis and properties of kinetin (N6-furfuryladenine). Plant Growth Regul. 2000, 32, 257–265. [Google Scholar] [CrossRef]
- Rehman, H.U.; Basra, S.M.A.; Rady, M.M.; Ghoneim, A.M.; Wang, Q. Moringa oleifera leaf extract improves wheat growth and productivity by delaying senescence and source-sink relationship. Int. J. Agric. Biol. 2017, 19, 479–484. [Google Scholar] [CrossRef]
- Abou-Sreea, A.I.B.; Azzam, C.R.; Al-Taweel, S.; Abdel-Aziz, R.M.; Belal, H.E.E.; Rady, M.M.; Ali, E.F.; Majrashi, A.; Khaled, K.A.M. Natural Biostimulant Attenuates Salinity Stress Effects in Chili Pepper by Remodeling Antioxidant, Ion, and Phytohormone Balances, and Augments Gene Expression. Plants 2021, 10, 2316. [Google Scholar] [CrossRef] [PubMed]
- Alharby, H.F.; Alzahrani, H.S.; Alzahrani, Y.; Alsamadany, H.; Hakeem, K.R.; Rady, M.M. Maize grain extract enriched with polyamines alleviates drought stress in Triticum aestivum through up-regulation of the ascorbate-glutathione cycle, glyoxalase system, and polyamine gene expression. Agronomy 2021, 11, 949. [Google Scholar] [CrossRef]
- Desoky, E.-S.M.; Selem, E.; Abo El-Maati, M.F.; Hassn, A.A.S.A.; Belal, H.E.E.; Rady, M.M.; AL-Harbi, M.S.; Ali, E.F. Foliar supplementation of clove fruit extract and salicylic acid maintains the performance and antioxidant defense system of Solanum tuberosum L. under deficient irrigation regimes. Horticulturae 2021, 7, 435. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The Water Culture Method for Growing Plants without Soil; University of California, College of Agriculture, Agricultural Experiment Station: Baltimore, MD, USA, 1950. [Google Scholar]
- Großkinsky, D.; Edelsbrunner, K.; Pfeifhofer, H.; Van der Graaff, E.; Roitsch, T. Cis- and trans-zeatin differentially modulate plant immunity. Plant Sign. Behav. 2013, 8, e24798. [Google Scholar] [CrossRef]
- Sundstrom, F.J.; Reader, R.B.; Edwards, R.L. Effect of seed treatment and planting method on Tabasco pepper. J. Amer. Soc. Hortic. Sci. 1987, 112, 641–644. [Google Scholar]
- Arnon, D.I. Copper enzymes in isolated chloroplasts, polyphenoxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Li, P.M.; Cai, R.G.; Gao, H.Y.; Peng, T.; Wang, Z.L. Partitioning of excitation energy in two wheat cultivars with different grain protein contents grown under three nitrogen applications in the field. Physiol. Plant. 2007, 129, 822–829. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Jagendorf, A.T. Oxidation and reduction of pyridine nucleotides by purified chloroplasts. Biochem. Biophys. Acta 1956, 40, 257–272. [Google Scholar] [CrossRef]
- Avron, M. Photophosphorylation by swisschard chloroplasts. Biochim. Biophys. Acta 1960, 40, 257–272. [Google Scholar] [CrossRef]
- Osman, A.S.; Rady, M.M. Ameliorative effects of sulphur and humic acid on the growth, antioxidant levels, and yields of pea (Pisum sativum L.) plants grown in reclaimed saline soil. J. Hortic. Sci. Biotechnol. 2014, 87, 626–632. [Google Scholar] [CrossRef]
- Rady, M.M. Effect of 24-epibrassinolide on growth, yield, antioxidant system and cadmium content of bean (Phaseolus vulgaris L.) plants under salinity and cadmium stress. Sci. Hortic. 2011, 129, 232–237. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photo peroxidation isolated chloroplasts: Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Irigoyen, J.J.; Emerich, D.W.; Sanchez-Diaz, M. Water stress induced changes in the concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativa) plants. Plant Physiol. 1992, 8, 455–460. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Kampfenkel, K.; Van Montagu, M. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal. Biochem. 1995, 225, 165–167. [Google Scholar] [CrossRef]
- Griffith, O.W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef]
- Havir, E.A.; McHale, N.A. Biochemical and developmental characterization of multiple forms of catalase in tobacco leaves. Plant Physiol. 1987, 84, 450–455. [Google Scholar] [CrossRef] [Green Version]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Martinez, V.; Nieves-Cordones, M.; Lopez-Delacalle, M.; Rodenas, R.; Mestre, T.C.; Garcia-Sanchez, F.; Rubio, F.; Nortes, P.A.; Mittler, R.; Rivero, R.M. Tolerance to Stress Combination in Tomato Plants: New Insights in the Protective Role of Melatonin. Molecules 2018, 23, 535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Novák, O.; Hauserová, E.; Amakorová, P.; Doležal, K.; Strnad, M. Cytokinin profiling in plant tissues using ultra-performance liquid chromatography-electrospray tandem mass spectrometry. Phytochem 2008, 69, 2214–2224. [Google Scholar] [CrossRef]
- Chapman, H.D.; Pratt, P.F. Methods of Analysis for Soil, Plants and Water; University of California, Division of Agricultural Science: Berkeley, CA, USA, 1961; pp. 56–63. [Google Scholar]
- Lachica, M.; Aguilar, A.; Yanez, J. Analisis foliar. Métodos Utilizados enla estaciln experimental del zaidin. Anales de Edafologia y Agrobiologia 1973, 32, 1033–1047. [Google Scholar]
- Steel, R.G.D.; Torrie, J.H.; Dicky, D.A. Principles and Procedures of Statistics: A Biometrical Approach, 3rd ed.; McGraw-Hill, Book International Co.: Singapore, 1997. [Google Scholar]
- Aina, O.; Quesenberry, K.; Gallo, M. Thidiazuron-induced tissue culture regeneration from quartered-seed explants of Arachis paraguariensis. Crop Sci. 2012, 52, 1076–1083. [Google Scholar]
- Mok, D.W.; Mok, M.C. Cytokinin metabolism and action. Ann. Rev. Plant Biol. 2001, 52, 89–118. [Google Scholar] [CrossRef] [PubMed]
- Sakakibara, H. Cytokinins: Activity, biosynthesis, and translocation. Ann. Rev. Plant Biol. 2006, 57, 431–449. [Google Scholar] [CrossRef] [Green Version]
- Stirk, W.A.; Gold, J.D.; Novák, O.; Strnad, M.; Van Staden, J. Changes in endogenous cytokinins during germination and seedling establishment of Tagetes minuta L. Plant Growth Regul. 2005, 47, 1–7. [Google Scholar] [CrossRef]
- Kiba, T.; Takei, K.; Kojima, M.; Sakakibara, H. Side-chain modification of cytokinins controls shoot growth in Arabidopsis. Dev. Cell 2013, 27, 452–461. [Google Scholar] [CrossRef] [Green Version]
- Li, S.-M.; Zheng, H.-X.; Zhang, X.-S.; Sui, N. Cytokinins as central regulators during plant growth and stress response. Plant Cell Rep. 2021, 40, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Hyoung, S.; Cho, S.H.; Chung, J.H.; So, W.M.; Cui, M.H.; Shin, J.S. Cytokinin oxidase PpCKX1 plays regulatory roles in development and enhances dehydration and salt tolerance in Physcomitrella patens. Plant Cell Rep. 2020, 39, 419–430. [Google Scholar] [CrossRef]
- Cortleven, A.; Leuendorf, J.E.; Frank, M.; Pezzetta, D.; Bolt, S.; Schmülling, T. Cytokinin action in response to abiotic and biotic stresses in plants. Plant Cell Environ. 2019, 42, 998–1018. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shen, W.; Chan, Z.; Wu, Y. Endogenous cytokinin overproduction modulates ROS homeostasis and decreases salt stress resistance in Arabidopsis thaliana. Front. Plant Sci. 2015, 6, 1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gadallah, M. Effects of kinetin on growth, grain yield and some mineral elements in wheat plants growing under excess salinity and oxygen deficiency. Plant Growth Regul. 1999, 27, 63–74. [Google Scholar] [CrossRef]
- Zaki, S.S.; Rady, M.M. Moringa oleifera leaf extract improves growth, physio-chemical attributes, antioxidant defence system and yields of salt-stressed Phaseolus vulgaris L. plants. Int. J. Chem. Technol. Res. 2015, 8, 120–134. [Google Scholar]
- Ismael, R.R.; Omar, M.N.A.; Azzam, C.R.; Ahmad, E.S.; Abdel-Fattah, M.; Zahran, H.H. Characterization and evaluation of rhizobium isolates from Vicia faba for some plant growth promoting traits. Biosci. Res. 2018, 15, 2971–2982. [Google Scholar]
- Ismael, R.R.; Ahmad, E.S.; Abdel-Fattah, M.; Omar, M.N.A.; Azzam, C.R.; Zahran, H.H. Effect of plant growth-promoting rhizobacteria (PGPR) on growth and symbiotic nitrogen fixation of Vicia faba plants under salt stress. Plant Sci. J. 2018, 7, 01–19. [Google Scholar]
- Abo-Doma, A.; Azzam, C.R. Hunting of some differentially expressed genes under salt stress in wheat. Egypt. J. Plant Breed. 2007, 11, 233–244. [Google Scholar]
- Azzam, C.R.; Edris, S.; Mansour, A.A. Changes in wheat P5CS gene expression in response to salt stress in wheat. Egypt. J. Genet. Cytol. 2009, 38, 375–386. [Google Scholar]
- Azzam, C.R.; Abd-Elnaby, Z.M.; Salem, A.K. Influence of Agro-Ecological Conditions on Gene Expression, Yield and Yield Components of the Mono-Cut (Fahl) Type of Berseem. Egypt. J. Plant Breed. 2012, 16, 135–159. [Google Scholar] [CrossRef]
- Azzam, C.R.; Al-Taweel, S.; Abdel-Aziz, R.M.; Rabe, K.M.; Abou-Sreea, A.I.B.; Rady, M.M.; Ali, E.F. Salinity Effects on Gene Expression, Morphological, and Physio-Biochemical Responses of Stevia rebaudiana Bertoni In Vitro. Plants 2021, 10, 820. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.S.; Dobeie, A.M.; Azzam, C.R.; Soliman, A.S. Identification of salt tolerant genotypes among Egyptian and Nigerian peanut (Arachis hypogaea L.) using biochemical and molecular tools. In Mitigating Environmental Stresses for Agricultural Sustainability in Egypt, Part IV: Advanced Procedures in Improving Crop Productivity; Springer Nature Switzerland AG: Cham, Switzerland, 2021; pp. 437–469. [Google Scholar]
- Žižková, E.; Dobrev, P.I.; Muhovski, Y.; Hošek, P.; Hoyerová, K.; Haisel, D.; Procházková, D.; Lutts, S.; Motyka, V.; Hichri, I. Tomato (Solanum lycopersicum L.) SlIPT3 and SlIPT4 isopentenyltransferases mediate salt stress response in tomato. BMC Plant Biol. 2015, 5, 85. [Google Scholar] [CrossRef] [PubMed]
- Assaha, D.V.M.; Ueda, A.; Saneoka, H.; Al-Yahyai, R.; Yaishm, M.W. The role of Na+ and K+ transporters in salt stress adaptation in glycophytes. Front. Physiol. 2017, 8, 509–528. [Google Scholar] [CrossRef] [PubMed]
- Robert-Seilaniantz, A.; Grant, M.R.; Jones, J.D.G. Hormone crosstalk in plant disease and defense: More than just jasmonate-salicylate antagonism. Ann. Rev. Phytopathol. 2011, 49, 317–343. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.J.; Shimono, M.; Sugano, S.; Kojima, M.; Liu, X.; Inoue, H.; Sakakibara, H.; Takatsuji, H. Cytokinins act synergistically with salicylic acid to activate defense gene expression in rice. Mol. Plant Microb. Interact. 2013, 26, 287–296. [Google Scholar] [CrossRef] [Green Version]
- Kudo, T.; Makita, N.; Kojima, M.; Tokunaga, H.; Sakakibara, H. Cytokinin activity of cis-zeatin and phenotypic alterations induced by overexpression of putative cis-Zeatin-O-glucosyltransferase in rice. Plant Physiol. 2012, 160, 319–331. [Google Scholar] [CrossRef] [Green Version]
- Gajdosová, S.; Spichal, L.; Kaminek, M.; Hoyerová, K.; Novák, O.; Dobrev, P.I.; Galuszka, P.; Klıma, P.; Gaudinova, A.; Zizkova, E.; et al. Distribution, biological activities, metabolism, and the conceivable function of cis-zeatin-type cytokinins in plants. J. Exp. Bot. 2011, 62, 2827–2840. [Google Scholar] [CrossRef] [Green Version]
- Osugi, A.; Sakakibara, H. Q&A: How do plants respond to cytokinins and what is their importance? BMC Biol. 2015, 13, 102. [Google Scholar]
- Kakimoto, T. CKI1, a histidine kinase homolog implicated in cytokinin signal transduction. Science 1996, 274, 982–985. [Google Scholar] [CrossRef] [PubMed]
- Zwack, P.J.; De Clercq, I.; Howton, T.C.; Hallmark, H.T.; Hurny, A.; Keshishian, E.A.; Parish, A.M.; Benkova, E.; Mukhtar, M.S.; Breusegem, F.V.; et al. Cytokinin response factor 6 represses cytokinin-associated genes during oxidative stress. Plant Physiol. 2016, 172, 1249–1258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jabłonski, B.; Ogonowska, H.; Szala, K.; Bajguz, A.; Orczyk, W.; Nadolska-Orczyk, A. Silencing of TaCKX1 mediates expression of other TaCKX genes to increase grain yield in wheat. Int. J. Mol. Sci. 2020, 21, 4809. [Google Scholar] [CrossRef] [PubMed]
- Mason, M.G.; Jha, D.; Salt, D.E.; Tester, M.; Hill, K.; Kieber, J.J.; Schaller, G.E. Type-B response regulators ARR1 and ARR12 regulate expression of AtHKT1; 1 and accumulation of sodium in Arabidopsis shoots. Plant J. 2010, 64, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Rashotte, A.M.; Mason, M.G.; Hutchison, C.E.; Ferreira, F.J.; Schaller, G.E.; Kieber, J.J. A subset of Arabidopsis AP2 transcription factors mediates cytokinin responses in concert with a two-component pathway. Proc. Natl. Acad. Sci. USA 2006, 103, 11081–11085. [Google Scholar] [CrossRef] [Green Version]
- Rashotte, A.M.; Goertzen, L.R. The CRF domain defines cytokinin response factor proteins in plants. BMC Plant Biol. 2010, 10, 74. [Google Scholar] [CrossRef] [Green Version]
- Azzam, C.R.; Saba, M.F.; El-Menshawy, M.M. Prospects for improving grain sorghum protein quality. Egypt. J. Plant Breed. 2010, 14, 23–36. [Google Scholar]
- Sairam, R.K.; Rao, K.V.; Srivastava, G.C. Differential response of wheat genotypes to long term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration. Plant Sci. 2002, 163, 1037–1046. [Google Scholar] [CrossRef]
- Iqbal, M.; Ashraf, M.; Jamil, A. Seed enhancement with cytokinins: Changes in growth and grain yield in salt stressed wheat plants. Plant Growth Regul. 2006, 50, 29–39. [Google Scholar] [CrossRef]
- Alzahrani, Y.; Rady, M.M. Compared to antioxidants and polyamines, the role of maize grain-derived organic biostimulants in improving cadmium tolerance in wheat plants. Ecotoxicol. Environ. Saf. 2019, 182, 109378. [Google Scholar] [CrossRef]
- Semida, W.M.; Rady, M.M. Presoaking application of propolis and maize grain extracts alleviates salinity stress in common bean (Phaseolus vulgaris L.). Sci. Hortic. 2014, 168, 210–217. [Google Scholar] [CrossRef]
- Semida, W.M.; Rady, M.M. Pre-soaking in 24-epibrassinolide or salicylic acid improves seed germination, seedling growth, and anti-oxidant capacity in Phaseolus vulgaris L. grown under NaCl stress. J. Hortic. Sci. Biotechnol. 2014, 89, 338–344. [Google Scholar] [CrossRef]





| Salt Treatment (NaCl) | Zeatins Application | Shoot Fresh Weight (g) | Shoot Dry Weight (g) | Root Fresh Weight (g) | Root Dry Weight (g) | Number of Leaves Plant−1 | Leaves Area (Dm2 Plant−1) |
|---|---|---|---|---|---|---|---|
| 0 mM | Control | 86.4 ± 8.0 a | 38.9 ± 3.7 a | 51.2 ± 4.8 a | 20.5 ± 1.8 a | 8.32 ± 0.76 a | 34.8 ± 3.2 a |
| c-Z-Ck | 86.6 ± 8.3 a | 39.0 ± 3.8 a | 50.9 ± 4.6 a | 20.3 ± 1.8 a | 8.15 ± 0.74 a | 35.1 ± 3.2 a | |
| t-Z-Ck | 86.7 ± 7.9 a | 39.5 ± 4.0 a | 51.9 ± 4.8 a | 20.5 ± 1.9 a | 8.28 ± 0.73 a | 35.2 ± 3.3 a | |
| 75 mM | Control | 65.0 ± 6.7 c | 30.8 ± 3.1 c | 38.5 ± 3.2 c | 15.3 ± 1.3 c | 6.08 ± 0.55 c | 24.4 ± 2.2 c |
| c-Z-Ck | 76.7 ± 7.2 b | 35.0 ± 3.2 b | 43.7 ± 4.5 b | 18.2 ± 1.8 b | 7.43 ± 0.72 b | 27.7 ± 2.5 b | |
| t-Z-Ck | 86.1 ± 8.4 a | 39.6 ± 3.8 a | 50.5 ± 4.8 a | 20.7 ± 1.8 a | 8.36 ± 0.83 a | 34.9 ± 3.2 a | |
| 150 mM | Control | 41.5 ± 4.3 f | 20.2 ± 2.1 f | 27.1 ± 2.9 e | 10.0 ± 0.9 e | 4.31 ± 0.34 e | 16.1 ± 1.4 e |
| c-Z-Ck | 49.9 ± 4.7 e | 22.8 ± 2.1 e | 32.0 ± 3.1 d | 11.6 ± 1.0 d | 5.11 ± 0.40 d | 19.9 ± 1.5 d | |
| t-Z-Ck | 59.6 ± 6.3 d | 25.4 ± 2.4 d | 38.8 ± 3.3 c | 14.5 ± 1.3 c | 5.93 ± 0.52 c | 23.7 ± 2.1 c | |
| LSD at p ≤ 0.05 | 1.6 | 0.32 | 1.1 | 0.29 | 0.31 | 4.2 | |
| Salt Treatment (NaCl) | Zeatins Application | TChls | TCars | Fv/Fm | Photochemical Activity |
|---|---|---|---|---|---|
| (mg g−1 FW) | |||||
| 0 mM | Control | 2.68 ± 0.04 a | 0.86 ± 0.01 c | 0.83 ± 0.01 a | 45.2 ± 1.1 a |
| c-Z-Ck | 2.70 ± 0.05 a | 0.85 ± 0.01 c | 0.83 ± 0.01 a | 44.9 ± 1.2 a | |
| t-Z-Ck | 2.66 ± 0.04 a | 0.88 ± 0.01 c | 0.83 ± 0.02 a | 45.5 ± 1.2 a | |
| 75 mM | Control | 1.89 ± 0.03 c | 0.97 ± 0.02 b | 0.69 ± 0.01 c | 32.4 ± 0.9 c |
| c-Z-Ck | 2.29 ± 0.04 b | 0.98 ± 0.02 b | 0.75 ± 0.01 b | 38.8 ± 1.0 b | |
| t-Z-Ck | 2.72 ± 0.05 a | 1.07 ± 0.02 a | 0.82 ± 0.01 a | 45.3 ± 1.3 a | |
| 150 mM | Control | 0.94 ± 0.02 f | 0.60 ± 0.01 f | 0.51 ± 0.00 e | 24.7 ± 0.7 f |
| c-Z-Ck | 1.22 ± 0.03 e | 0.70 ± 0.01 e | 0.57 ± 0.01 d | 28.7 ± 0.7 e | |
| t-Z-Ck | 1.56 ± 0.03 d | 0.78 ± 0.01 d | 0.65 ± 0.01 c | 36.2 ± 1.0 d | |
| LSD at p ≤ 0.05 | 0.18 | 0.07 | 0.06 | 3.4 | |
| Salt Treatment (NaCl) | Zeatins Application | Net Photosynthetic Rate | Transpiration Rate | Stomatal Conductance |
|---|---|---|---|---|
| 0 mM | Control | 8.98 ± 0.16 a | 7.07 ± 0.15 a | 0.62 ± 0.02 a |
| c-Z-Ck | 8.91 ± 0.15 a | 7.11 ± 0.14 a | 0.64 ± 0.02 a | |
| t-Z-Ck | 9.02 ± 0.16 a | 7.10 ± 0.13 a | 0.62 ± 0.02 a | |
| 75 mM | Control | 6.12 ± 0.14 c | 5.26 ± 0.10 c | 0.44 ± 0.01 c |
| c-Z-Ck | 6.84 ± 0.12 b | 5.92 ± 0.12 b | 0.49 ± 0.02 b | |
| t-Z-Ck | 8.94 ± 0.15 a | 7.12 ± 0.15 a | 0.61 ± 0.02 a | |
| 150 mM | Control | 5.14 ± 0.11 d | 4.22 ± 0.08 d | 0.34 ± 0.01 e |
| c-Z-Ck | 5.61 ± 0.12 d | 4.56 ± 0.09 d | 0.39 ± 0.01 d | |
| t-Z-Ck | 6.69 ± 0.13 b | 5.08 ± 0.11 c | 0.45 ± 0.02 c | |
| LSD at p ≤ 0.05 | 0.56 | 0.50 | 0.04 | |
| Salt Treatment (NaCl) | Zeatins Application | RWC (%) | MSI (%) | EL (%) |
|---|---|---|---|---|
| 0 mM | Control | 81.4 ± 5.3 a | 77.8 ± 4.4 a | 17.4 ± 2.2 e |
| c-Z-Ck | 80.9 ± 4.5 a | 77.7 ± 4.6 a | 17.3 ± 2.1 e | |
| t-Z-Ck | 81.5 ± 5.1 a | 78.0 ± 4.8 a | 17.0 ± 2.2 e | |
| 75 mM | Control | 62.2 ± 4.0 c | 52.4 ± 3.5 c | 27.6 ± 3.5 c |
| c-Z-Ck | 69.8 ± 4.6 b | 61.3 ± 4.1 b | 22.4 ± 3.2 d | |
| t-Z-Ck | 81.3 ± 5.2 a | 78.1 ± 4.7 a | 17.2 ± 2.1 e | |
| 150 mM | Control | 43.5 ± 3.1 e | 40.1 ± 3.2 e | 35.8 ± 4.1 a |
| c-Z-Ck | 49.8 ± 3.4 d | 47.6 ± 3.8 d | 30.2 ± 3.8 b | |
| t-Z-Ck | 58.7 ± 4.3 c | 55.2 ± 4.4 c | 27.4 ± 3.2 c | |
| LSD at p ≤ 0.05 | 6.2 | 5.8 | 2.1 | |
| Salt Treatment (NaCl) | Zeatins Application | MDA | H2O2 | Proline (µmol g−1 DW) | Soluble Sugars (mg g− DW) | Ascorbic Acid | Glutathione |
|---|---|---|---|---|---|---|---|
| µmol g−1 FW | µmol g−1 FW | ||||||
| 0 mM | Control | 22.8 ± 0.3 e | 11.4 ± 0.2 f | 71.2 ± 1.2 f | 10.4 ± 0.2 f | 1.62 ± 0.02 g | 0.91 ± 0.01 g |
| c-Z-Ck | 22.4 ± 0.4 e | 11.5 ± 0.2 f | 71.4 ± 1.4 f | 10.4 ± 0.2 f | 1.59 ± 0.02 g | 0.90 ± 0.01 g | |
| t-Z-Ck | 22.4 ± 0.3 e | 11.2 ± 0.2 f | 71.5 ± 1.4 f | 10.6 ± 0.3 f | 1.64 ± 0.02 g | 0.90 ± 0.01 g | |
| 75 mM | Control | 34.2 ± 0.5 c | 18.8 ± 0.3 d | 114.8 ± 1.9 e | 15.2 ± 0.4 e | 1.97 ± 0.03 f | 0.99 ± 0.01 f |
| c-Z-Ck | 27.4 ± 0.4 d | 15.5 ± 0.2 e | 131.2 ± 2.2 d | 18.7 ± 0.4 d | 2.24 ± 0.04 e | 1.08 ± 0.02 e | |
| t-Z-Ck | 22.5 ± 0.3 e | 11.2 ± 0.2 f | 184.6 ± 2.8 c | 22.8 ± 0.4 c | 2.46 ± 0.04 d | 1.21 ± 0.02 d | |
| 150 mM | Control | 41.6 ± 0.7 a | 30.4 ± 0.4 a | 182.8 ± 3.0 c | 23.0 ± 0.5 c | 2.68 ± 0.05 c | 1.34 ± 0.02 c |
| c-Z-Ck | 38.1 ± 0.7 b | 26.1 ± 0.4 b | 216.5 ± 3.4 b | 27.8 ± 0.5 b | 2.94 ± 0.05 b | 1.45 ± 0.02 b | |
| t-Z-Ck | 35.1 ± 0.6 c | 21.4 ± 0.3 c | 254.2 ± 3.8 a | 34.2 ± 0.6 a | 3.32 ± 0.06 a | 1.68 ± 0.03 a | |
| LSD at p ≤ 0.05 | 2.2 | 1.4 | 10.4 | 1.8 | 0.21 | 0.09 | |
| Salt Treatment (NaCl) | Zeatins Application | Na+ | Cl− | K+ | K+/Na+ |
|---|---|---|---|---|---|
| mg g−1 DW | |||||
| 0 mM | Control | 1.23 ± 0.03 f | 1.88 ± 0.05 f | 2.08 ± 0.07 a | 1.69 ± 0.04 a |
| c-Z-Ck | 1.24 ± 0.03 f | 1.91 ± 0.06 f | 2.11 ± 0.08 a | 1.70 ± 0.04 a | |
| t-Z-Ck | 1.23 ± 0.02 f | 1.89 ± 0.06 f | 2.12 ± 0.08 a | 1.72 ± 0.04 a | |
| 75 mM | Control | 8.56 ± 0.22 c | 13.27 ± 0.40 c | 1.62 ± 0.05 c | 0.19 ± 0.00 d |
| c-Z-Ck | 5.21 ± 0.16 d | 7.62 ± 0.21 d | 1.84 ± 0.06 b | 0.35 ± 0.01 c | |
| t-Z-Ck | 2.67 ± 0.07 e | 3.82 ± 0.17 e | 2.10 ± 0.07 a | 0.79 ± 0.02 b | |
| 150 mM | Control | 13.45 ± 0.36 a | 19.84 ± 0.58 a | 1.08 ± 0.04 e | 0.08 ± 0.00 f |
| c-Z-Ck | 10.57 ± 0.28 b | 16.21 ± 0.46 b | 1.31 ± 0.04 d | 0.12 ± 0.00 e | |
| t-Z-Ck | 8.92 ± 0.24 c | 14.12 ± 0.42 c | 1.58 ± 0.05 c | 0.18 ± 0.00 d | |
| LSD at p ≤ 0.05 | 1.12 | 1.38 | 0.19 | 0.04 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azzam, C.R.; Zaki, S.-n.S.; Bamagoos, A.A.; Rady, M.M.; Alharby, H.F. Soaking Maize Seeds in Zeatin-Type Cytokinin Biostimulators Improves Salt Tolerance by Enhancing the Antioxidant System and Photosynthetic Efficiency. Plants 2022, 11, 1004. https://doi.org/10.3390/plants11081004
Azzam CR, Zaki S-nS, Bamagoos AA, Rady MM, Alharby HF. Soaking Maize Seeds in Zeatin-Type Cytokinin Biostimulators Improves Salt Tolerance by Enhancing the Antioxidant System and Photosynthetic Efficiency. Plants. 2022; 11(8):1004. https://doi.org/10.3390/plants11081004
Chicago/Turabian StyleAzzam, Clara R., Safi-naz S. Zaki, Atif A. Bamagoos, Mostafa M. Rady, and Hesham F. Alharby. 2022. "Soaking Maize Seeds in Zeatin-Type Cytokinin Biostimulators Improves Salt Tolerance by Enhancing the Antioxidant System and Photosynthetic Efficiency" Plants 11, no. 8: 1004. https://doi.org/10.3390/plants11081004