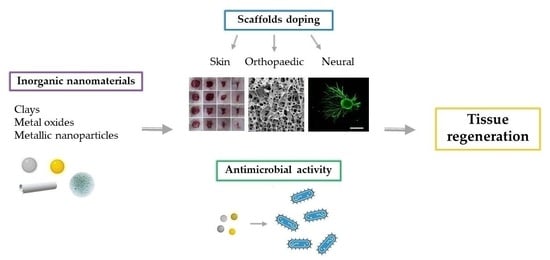
Inorganic Nanomaterials in Tissue Engineering
Abstract
:1. Introduction
2. Clays
2.1. Tissue Engineering Applications
2.1.1. Skin Applications
2.1.2. Orthopaedic Applications
3. Carbon-Based Nanomaterials
3.1. Carbon Nanotubes (CNTs)
3.2. Carbon Nano-Onions (CNOs)
- (a)
- small-sized, which are below 10 nm, or big-sized, which are above 10 nm;
- (b)
- spherical or polygonal;
- (c)
- dense, which are characterized by a core filled with different metals, or hollow, which have an empty core.
3.3. Tissue Engineering Applications
3.3.1. Skin Applications
3.3.2. Orthopaedic Applications
3.3.3. Neural Applications
4. Metal Oxides
4.1. Bioceramics and Bioglasses
4.2. Magnetic Nanoparticles
4.3. Tissue Engineering Applications
4.3.1. Skin Applications
4.3.2. Orthopaedic Applications
4.3.3. Neural Applications
5. Metallic Nanoparticles
5.1. Au Nanoparticles (Au NPs)
5.2. Ag Nanoparticles (Ag NPs)
5.3. Tissue Engineering Applications
5.3.1. Skin Applications
5.3.2. Orthopaedic Applications
5.3.3. Neural Applications
6. Antimicrobial Properties
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, S.-W.; Im, G.-B.; Kim, Y.-J.; Kim, Y.H.; Lee, T.-J.; Bhang, S.H. Bio-application of Inorganic Nanomaterials in Tissue Engineering. In Bioinspired Biomaterials. Advances in Experimental Medicine and Biology; Chun, H.J., Reis, L.R., Motta, A., Khang, G., Eds.; Springer: Singapore, 2020; Volume 1, pp. 115–130. [Google Scholar] [CrossRef]
- Pina, S.; Ribeiro, V.P.; Marques, C.F.; Maia, F.R.; Silva, T.H.; Reis, R.L.; Oliveira, M. Scaffolding Strategies for tissue engineering and regenerative medicine applications. Materials 2019, 12, 1824. [Google Scholar] [CrossRef] [Green Version]
- SCENIHR (Scientific Committee on Emerging and Newly Identified Health Risks) by European Commission. The Existing and Proposed Definitions Relating to Products of Nanotechnologies; European Commission: Brussels, Belgium, 2008. [Google Scholar]
- Krpetić, Ž.; Anguissola, S.; Garry, D.; Kelly, P.M.; Dawson, K.A. Nanomaterials: Impact on Cells and Cell Organelles. In Nanomaterial. Advances in Experimental Medicine and Biology; Capco, D., Chen, Y., Eds.; Springer: Dordrecht, Germany, 2014; Volume 811, pp. 135–156. [Google Scholar] [CrossRef]
- Nikolova, M.P.; Chavali, M.S. Recent advances in biomaterials for 3D scaffolds: A review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef]
- Daculsi, G.; Laboux, O.; Malard, O.; Weiss, P. Current state of the art of biphasic calcium phosphate bioceramics. J. Mater. Sci.-Mater. Med. 2003, 14, 195–200. [Google Scholar] [CrossRef]
- García-Villén, F.; Carazo, E.; Borrego-Sánchez, A.; Sánchez-Espejo, R.; Cerezo, P.; Viseras, C.; Aguzzi, C. Clay minerals in drug delivery systems. Modified Clay and Zeolite Nanocomposite Materials: Environmental and Pharmaceutical Applications; Mercurio, M., Sarkar, B., Langella, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 1, pp. 129–166. [Google Scholar] [CrossRef]
- Dawson, J.I.; Oreffo, R.O.C. Clay: New opportunities for tissue regeneration and biomaterial design. Avd. Mater. 2013, 25, 4069–4086. [Google Scholar] [CrossRef]
- Garcìa-Villén, F.; Viseras, C. Clay-based pharmaceutical formulations and drug delivery systems. Pharmaceutics 2020, 12, 1142. [Google Scholar] [CrossRef]
- Sandri, G.; Bonferoni, M.C.; Rossi, S.; Ferrari, F.; Aguzzi, C.; Viseras, C.; Caramella, C. Clay minerals for tissue regeneration, repair, and engineering. In Wound Healing Biomaterials; Ågren, M.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 2, pp. 385–402. [Google Scholar] [CrossRef]
- Sandri, G.; Faccendini, A.; Longo, M.; Ruggeri, M.; Rossi, S.; Bonferoni, M.C.; Miele, D.; Prina-Mello, A.; Aguzzi, C.; Viseras, C.; et al. Halloysite- and Montmorillonite- loaded scaffolds as enhancers of chronic wound healing. Pharmaceutics 2020, 12, 179. [Google Scholar] [CrossRef] [Green Version]
- Council of Europe & Convention on the Elaboration of a European Pharmacopoeia. European Pharmacopoeia, 9th ed.; Maisonneuve: Sainte-Ruffine, France, 2017. [Google Scholar]
- Garcìa-Villen, F.; Ruiz-Alonso, S.; Lafuente-Merchan, M.; Gallego, I.; Sainz-Ramos, M.; Saenz-del-Burgo, L.; Pedraz, J.L. Clay Minerals as Bioink Ingredients for 3D Printing and 3D Bioprinting: Application in Tissue Engineering and Regenerative Medicine. Pharmaceutics 2021, 13, 1806. [Google Scholar] [CrossRef]
- Ruggeri, M.; Bianchi, E.; Rossi, S.; Vigani, B.; Bonferoni, M.C.; Caramella, C.; Sandri, G.; Ferrari, F. Nanotechnology-based medical devices for the treatment of chronic skin lesions: From research to the clinic. Pharmaceutics 2020, 12, 815. [Google Scholar] [CrossRef]
- Naumenko, E.A.; Guryanov, I.D.; Yendluri, R.; Lvov, Y.M.; Fakhrullin, R.F. Clay nanotube-biopolymer composite scaffolds for tissue engineering. Nanoscale 2016, 8, 7257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, K.; Zhu, T.; Wu, T.; Zhou, X.; Yang, X.; Wang, J.; Fang, J.; El-Hamshary, H.; Al-Deyab, S.S.; Mo, X. Incorporation of amoxicillin-loaded organic montmorillonite intopoly(ester-urethane) urea nanofibers as a functional tissueengineering scaffold. Colloids Surf. B 2017, 154, 314–323. [Google Scholar] [CrossRef]
- Massaro, M.; Borrego-Sánchez, A.; Sánchez-Espejo, R.; Viseras Iborra, C.; Cavallaro, G.; García-Villén, F.; Guernelli, S.; Lazzara, G.; Miele, D.; Sainz-Díaz, C.I.; et al. Ciprofloxacin carrier systems based on hectorite/halloysite hybrid hydrogels for potential wound healing applications. Appl. Clay Sci. 2021, 215, 106310. [Google Scholar] [CrossRef]
- Tenci, M.; Rossi, S.; Aguzzi, C.; Carazo, E.; Sandri, G.; Bonferoni, M.C.; Grisoli, P.; Viseras, C.; Caramella, C.M.; Ferrari, F. Carvacrol/clay hybrids loaded into in situ gel-ling films. Int. J. Pharm. 2017, 531, 676–688. [Google Scholar] [CrossRef]
- Bianchi, E.; Ruggeri, M.; Rossi, S.; Vigani, B.; Miele, D.; Bonferoni, M.C.; Sandri, G.; Ferrari, F. Innovative strategies in tendon tissue engineering. Pharmaceutics 2021, 13, 89. [Google Scholar] [CrossRef] [PubMed]
- Brahmill, J.; Ross, S.; Ross, G. Bioactive nanocomposites for tissue repair and regeneration: A review. Int. J. Environ. Res. Public Health 2017, 14, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaharwar, A.K.; Mukundan, S.; Karaca, E.; Dolatshahi-Pirouz, A.; Patel, A.; Rangarajan, K.; Mihaila, S.M.; Iviglia, G.; Zhang, H.; Khademhosseini, A. Nanoclay-enriched poly(ɛ-Caprolactone) electrospun scaffolds for osteogenic differentiation of human mesenchymal stem cells. Tissue Eng. Part A 2014, 20, 2088–2101. [Google Scholar] [CrossRef] [Green Version]
- Kundu, K.; Afshar, A.; Katti, D.R.; Edirisinghe, M.; Katti, K.S. Composite nanoclay-hydroxyapatite-polymer fiber scaffolds for bone tissue engineering manufactured using pressurized gyration. Compos. Sci. Technol. 2021, 202, 108598. [Google Scholar] [CrossRef]
- Kazemi-Aghdam, F.; Jahed, V.; Dehghan-Niri, M.; Ganji, F.; Vasheghani-Farahani, E. Injectable chitosan hydrogel embedding modified halloysite nanotubes for bone tissue engineering. Carbohydr. Polym. 2021, 269, 118311. [Google Scholar] [CrossRef]
- Huang, Z.; Ou, Q.; Xie, Y.; Chen, X.; Fang, Y.; Huang, C.; Wang, Y.; Gu, Z.; Wu, J. Halloysite Nanotube Based Scaffold for Enhanced Bone Regeneration. ACS Biomater. Sci. Eng. 2019, 5, 4037–4047. [Google Scholar] [CrossRef]
- Bonifacio, M.A.; Cochis, A.; Cometa, S.; Scalzone, A.; Gentile, P.; Procino, G.; Milano, S.; Scalia, A.C.; Rimondini, L.; De Giglio, E. Advances in cartilage repair: The influence of inorganic clays to improve mechanical and healing properties of antibacterial Gellan gum-Manuka honey hydrogels. Mater. Sci. Eng. 2020, 108, 110444. [Google Scholar] [CrossRef]
- Ahlawat, J.; Masoudi Asil, S.; Guillama Barroso, G.; Nurunnabi, M.; Narayan, M. Application of carbon nano onions in the biomedical field: Recent advances and challenges. Biomater. Sci. 2021, 9, 626. [Google Scholar] [CrossRef]
- Sok, V.; Fragoso, A. Preparation and characterization of alkaline phosphatase, horseradish peroxidase, and glucose oxidase conjugates with carboxylated carbon nano-onions. Prep. Biochem. Biotechnol. 2018, 48, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Madanagopal, T.T.; Shruti, V.; Agarwalla, S.V.; Rosa, V. Carbon nanocomposites for implant dentistry and bone tissue engineering. In Applications of Nanocomposite Materials in Dentistry; Asiri, A.M., Inamuddin, D., Mohammad, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 1, pp. 47–63. [Google Scholar] [CrossRef]
- Mamidi, N.; Velasco Delgadillo, R.M.; Villela Castrejòn, J. Unconventional and facile production of a stimuli-responsive multifunctional system for simultaneous drug delivery and environmental remediation. Environ. Sci. Nano 2021, 8, 2081–2097. [Google Scholar] [CrossRef]
- Prasek, J.; Drbohlavova, J.; Chomoucka, J.; Hubalek, J.; Jasek, O.; Adam, V.; Kizek, R. Methods for carbon nanotubes synthesis—review. J. Mater. Chem. 2011, 21, 15872. [Google Scholar] [CrossRef]
- Martins-Junior, P.A.; Alcantara, C.E.; Resende, R.R.; Ferreira, A.J. Carbon nanotubes: Directions and perspectives in oral regenerative medicine. J. Dent. Res. 2013, 92, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Pei, X.; He, R.; Wan, Q.; Wang, J. Multiwall carbon nanotubes/polycaprolactone composites for bone tissue engineering application. Colloids Surf. B Biointerfaces 2012, 93, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Jell, G.; Verdejo, R.; Safinia, L.; Shaffer, M.S.P.; Stevens, M.M.; Bismarck, A. Carbon nanotube-enhanced polyurethane scaffolds fabricated by thermally induced phase separation. J. Mater. Chem. 2008, 18, 1865. [Google Scholar] [CrossRef]
- Mwenifumbo, S.; Shaffer, M.S.; Stevens, M.M. Exploring cellular behaviour with multiwalled carbon nanotube constructs. J. Mater. Chem. 2007, 17, 1894. [Google Scholar] [CrossRef]
- Zanello, L.P.; Zhao, B.; Hu, H.; Haddon, R.C. Bone cell proliferation on carbon nanotubes. Nano Lett. 2006, 6, 562–567. [Google Scholar] [CrossRef]
- Cui, L.; Liang, J.; Liu, H.; Zhang, K.; Li, J. Nanomaterials for Angiogenesis in Skin Tissue Engineering. Tissue Eng. Part B Rev. 2020, 26, 203–216. [Google Scholar] [CrossRef]
- Azizi-Lalabadi, M.; Hashemi, H.; Feng, J.; Mahdi Jafari, S. Carbon nanomaterials against pathogens; the antimicrobial activity of carbon nanotubes, graphene/graphene oxide, fullerenes, and their nanocomposites. Adv. Colloid Interface Sci. 2020, 284, 102250. [Google Scholar] [CrossRef]
- Plonska-Brzezinska, M.E. Carbon Nano-Onions: A Review of Recent Progress in Synthesis and Applications. Chem. Nano Mat. 2019, 5, 568–580. [Google Scholar] [CrossRef]
- Camisasca, A.; Giordani, S. Carbon nano-onions in biomedical applications: Promising theranostic agents. Inorg. Chim. Acta 2017, 468, 67–76. [Google Scholar] [CrossRef]
- Mykhailiv, O.; Zubyk, H.; Plonska-Brzezinska, M.E. Carbon nano-onions: Unique carbon nanostructures with fascinating properties and their potential applications. Inorg. Chim. Acta 2017, 468, 49–66. [Google Scholar] [CrossRef]
- Grande Tovar, C.D.; Castro, J.I.; Valencia, C.H.; Navia Porras, D.P.; Mina Hernandez, J.H.; Valencia, M.E.; Velásquez, J.D.; Chaur, M.N. Preparation of Chitosan/Poly(Vinyl Alcohol) Nanocomposite Films Incorporated with Oxidized Carbon Nano-Onions (Multi-Layer Fullerenes) for Tissue-Engineering Applications. Biomolecules 2019, 9, 684. [Google Scholar] [CrossRef] [Green Version]
- Mamidi, N.; Velasco Delgadillo, R.M. Design, fabrication and drug release potential of dual stimuli-responsive composite hydrogel nanoparticle interfaces. Colloids Surf. B 2021, 204, 111819. [Google Scholar] [CrossRef] [PubMed]
- Diabb Zavala, J.M.; Leija Gutiérrez, H.M.L.; Segura-Càrdenas, E.; Mamidi, N.; Morales-Avalos, R.; Villela-Castrejòn, J.; Elías-Zúniga, A. Manufacture and mechanical properties of knee implants using SWCNTs/UHMWPE composites. J. Mech. Behav. Biomed. Mater. 2021, 120, 104554. [Google Scholar] [CrossRef]
- Mamidi, N.; Velasco Delgadillo, R.M.; Barrera, E.V. Covalently Functionalized Carbon Nano-Onions Integrated Gelatin Methacryloyl Nanocomposite Hydrogel Containing γ-Cyclodextrin as Drug Carrier for High-Performance pH-Triggered Drug Release. Pharmaceuticals 2021, 14, 291. [Google Scholar] [CrossRef]
- Mamidi, N.; Velasco Delgadillo, R.M.; Gonzàles Ortiz, A.; Barrera, E.V. Carbon Nano-Onions Reinforced Multilayered Thin Film System for Stimuli-Responsive Drug Release. Pharmaceutics 2020, 12, 1208. [Google Scholar] [CrossRef]
- Mamidi, N.; Martìnez Gamero, M.R.; Velasco Delgadillo, R.M.; Villela Castrejòn, J.; Zùniga, A.E. Engineering of functionalized carbon nano-onions reinforced nanocomposites: Fabrication, biocompatibility, and mechanical properties. J. Mater. Res. 2020, 35, 8. [Google Scholar] [CrossRef] [Green Version]
- Patra, H.K.; Sharma, Y.; Islam, M.M.; Jafari, M.J.; Murugan, N.A.; Kobayashi, H.; Turner, A.P.F.; Tiwari, A. Inflammation-sensitive in situ smart scaffolding for regenerative medicine. Nanoscale 2016, 8, 17213–17222. [Google Scholar] [CrossRef] [Green Version]
- Hirata, E.; Uo, M.; Takita, H.; Akasaka, T.; Watari, F.; Yokoyama, A. Multiwalled carbon nanotube-coating of 3D collagen scaffolds for bone tissue engineering. Carbon 2011, 49, 3284–3291. [Google Scholar] [CrossRef]
- Duan, S.; Yang, X.; Mei, F.; Tang, Y.; Li, X.; Shi, Y.; Mao, J.; Zhang, H.; Cai, Q. Enhanced osteogenic differentiation of mesenchymal stem cells on poly(L-lactide) nanofibrous scaffolds containing carbon nanomaterials. J. Biomed. Mater. Res. A 2014, 103, 1424–1435. [Google Scholar] [CrossRef] [PubMed]
- Scapin, G.; Salice, P.; Tescari, S.; Menna, E.; De Filippis, V.; Filippini, F. Enhanced neuronal cell differentiation combining biomimetic peptides and a carbon nanotube-polymer scaffold. Nanomedicine 2015, 11, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Sharan, S.; Roy, P.; Lahiri, D. Aligned carbon nanotube reinforced polymeric scaffolds with electrical cues for neural tissue regeneration. Carbon 2015, 95, 715–724. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, J.-Y.; Yang, S.H.; Lee, E.-J.; Kim, H.-W. Carbon nanotube-collagen three-dimensional culture of mesenchymal stem cells promotes expression of neural phenotypes and secretion of neurotrophic factors. Acta Biomater. 2014, 10, 4425–4436. [Google Scholar] [CrossRef]
- Goloboy, J.E.; Klemperer, W.G.; Marquart, T.A.; Westwood, G.; Yaghi, O.M. Complex oxides as molecular materials: Structure and bonding in IDGH-valent early transition metal compounds. In Polyoxometalate Molecular Science; Borras-Almenar, J., Coronado, E., Muller, A., Pope, M., Eds.; Kluwer Academic Publisjers: Amsterdam, The Netherlands, 2003; Volume 98, pp. 79–174. [Google Scholar] [CrossRef]
- Murthy, S.; Effiong, P.; Fei, C.C. Metal-oxide nanoparticles in biomedical applications. In Metal Oxide Powder Technologies, Fundamentals, Processing Methods and Applications; Al-Douri, Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 1, pp. 233–248. [Google Scholar] [CrossRef]
- Laurent, S.; Bountry, S.; Muller, R.N. Metal oxide particles and their prospects for applications. In Iron Oxide Nanoparticles for Biomedical Applications, Synthesis, Functionalization and Application; Laurent, S., Mahmoudi, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 1, pp. 3–42. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkiab, K. Antimicrobial activity of the metal oxide nanoparticles. Mater. Sci. Eng. C 2014, 44, 278–284. [Google Scholar] [CrossRef]
- Shivaramakrishnan, B.; Gurumurthy, B.; Balasubramanian, A. Potential biomedical applications of metallic nanobiomaterials: A review. Int. J. Pharm. Sci. Res. 2017, 8, 985–1000. [Google Scholar] [CrossRef]
- Kumar, P.; Dehiya, B.S.; Sindhu, A. Bioceramics for hard tissue engineering applications: A review. Int. J. Appl. Eng. Res. 2018, 13, 2744–2752. [Google Scholar]
- Thamaraisletvi, T.V.; Rajeswari, S. Biological evaluation of bioceramics materials. Trends Biomater. Artif. Organs 2004, 18, 9–17. [Google Scholar]
- Yamamuro, T. Bioceramics. In Biomechanics and Biomaterials in Orthopedics; Poitut, D.G., Ed.; Springer: London, UK, 2004; pp. 22–33. [Google Scholar] [CrossRef]
- Anjaneyulu, U.; Zhang, V.; Ren, P.-G. Bioinert Ceramics for Biomedical Applications; Biomedical Science and Technology Series; Ramalingam, M., Ed.; Wiley/Scrivener: Hoboken, NJ, USA, 2019. [Google Scholar]
- De Aza, A.H.; Chevalier, J.; Fantozzi, G.; Schehl, M.; Torrecillas, R. Crack growth resistance of alumina, zirconia and zirconia toughened alumina ceramics for joint prostheses. Biomaterials 2002, 23, 937–945. [Google Scholar] [CrossRef]
- Fabris, S.; Paxton, A.T.; Finnis, M.W. A stabilization mechanism of zirconia based on oxygen vacancies only. Acta Mater. 2002, 50, 5171–5178. [Google Scholar] [CrossRef] [Green Version]
- Hench, L.L. The future of bioactive ceramics. J. Mater. Sci. Mater. Med. 2015, 26, 86. [Google Scholar] [CrossRef] [PubMed]
- Day, R.M.; Boccaccini, A.R.; Shurey, S.; Roether, J.A.; Forbes, A.; Hench, L.L.; Gabe, S.M. Assessment of poly(glycolic acid) mesh and bioactive glass for soft tissue engineering scaffolds. Biomaterials 2004, 25, 5857–5866. [Google Scholar] [CrossRef] [PubMed]
- Keshaw, H.; Forbes, A.; Day, R.M. Release of angiogenic growth factors from cells encapsulated in alginate beads with bioactive glass. Biomaterials 2005, 26, 4171–4179. [Google Scholar] [CrossRef]
- Rahaman, M.N.; Day, D.E.; Bal, B.S.; Fu, Q.; Jung, S.B.; Bonewald, L.F.; Tomsia, A.P. Bioactive glass in tissue engineering. Acta Biomater. 2011, 7, 2355–2373. [Google Scholar] [CrossRef] [Green Version]
- Fathi-Achachelouei, M.; Knopf-Marques, H.; Ribeiro da Silva, C.E.; Barthès, J.; Bat, E.; Tezcaner, A.; Vrana, N.E. Use of nanoparticles in tissue engineering and regenerative medicine. Front. Bioeng. Biotechnol. 2019, 7, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, C.; Zhu, J.; Xie, A.; Shen, Y.; Li, H.; Zheng, B.; Wei, Y. Graphene oxide and creatine phosphate disodium dual template-directed synthesis of GO/hydroxyapatite and its application in drug delivery. Mater. Sci. Eng. C 2017, 73, 709–715. [Google Scholar] [CrossRef]
- Li, X.; Wei, J.; Aifantis, K.E.; Fan, Y.; Feng, Q.; Cui, F.Z.; Watari, F. Current investigations into magnetic nanoparticles for biomedical applications. J. Biomed. Mater. Res. A 2016, 104, 1285–1296. [Google Scholar] [CrossRef]
- Srivastava, P.; Sharma, P.K.; Muheem, A.; Warsi, M.H. Magnetic Nanoparticles: A Review on Stratagems of Fabrication an d its Biomedical Applications. Recent Pat. Drug Deliv. Formul. 2017, 11, 101–113. [Google Scholar] [CrossRef]
- Medeiros, S.F.; Santos, A.M.; Fessi, H.; Elaissari, A. Stimuli-responsive magnetic particles for biomedical applications. Int. J. Pharm. 2011, 403, 139–161. [Google Scholar] [CrossRef]
- Matos, A.M.; Gonçalves, A.I.; El Hajd, A.J.; Gomes, M.E. Magnetic biomaterials and nano-instructive tools as mediators of tendon mechanotransduction. Nanoscale Adv. 2020, 2, 140–148. [Google Scholar] [CrossRef] [Green Version]
- Lu, D.; Wu, X.; Wang, W.; Ma, C.; Pei, B.; Wu, S. Synthesis and application of Iron Oxide nanoparticles in bone tissue repair. J. Nanomat. 2021, 7, 3762490. [Google Scholar] [CrossRef]
- Hasan, A.; Morshed, M.; Memic, A.; Hassan, S.; Webster, T.J.; El-Sayed Marei, H. Nanoparticles in tissue engineering: Applications, challenges and prospects. Int. J. Nanomed. 2018, 13, 5637–5655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorustovich, A.A.; Roether, J.A.; Boccaccini, A.R. Effect of bioactive glasses on angiogenesis: A review of in vitro and in vivo evidences. Tissue Eng. Part B Rev. 2010, 16, 199–207. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, F.; Cui, Y.; Ren, H.; Xie, Y.; Li, A.; Ji, L.; Qu, X.; Qiu, D.; Yang, Z. An easy-to-use wound dressing gelatin-bioactive nanoparticle gel and its preliminary in vivo study. J. Mater. Sci. Mater. Med. 2017, 28, 10. [Google Scholar] [CrossRef] [PubMed]
- Samadian, H.; Salehi, M.; Farzamfar, S.; Vaez, A.; Ehterami, A.; Sahrapeyma, H.; Goodarzi, A.; Ghorbani, S. In vitro and in vivo evaluation of electrospun cellulose acetate/gelatin/hydroxyapatite nanocomposite mats for wound dressing applications. Artif. Cells Nanomed. Biotechnol. 2018, 46, 964–974. [Google Scholar] [CrossRef] [Green Version]
- Babitha, S.; Korrapati, P.S. Biodegradable zein–polydopamine polymeric scaffold impregnated with TiO2 nanoparticles for skin tissue engineering. Biomed. Mater. 2017, 12, 055008. [Google Scholar] [CrossRef]
- Jones, J.R. Review of bioactive glass: From Hench to hybrids. Acta Biomater. 2012, 9, 4457–4486. [Google Scholar] [CrossRef]
- Covarrubias, C.; Cádiz, M.; Maureira, M.; Celhay, I.; Cuadra, F.; Von Marttens, A. Bionanocomposite scaffolds based on chitosan-gelatin and nanodimensional bioactive glass particles: In vitro properties and in vivo bone regeneration. J. Biomater. Appl. 2018, 32, 1155–1163. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Y.; Niu, W.; Chen, M.; Xue, Y.; Ge, J.; Ma, P.X.; Lei, B. Biodegradable Multifunctional Bioactive Glass-Based Nanocomposite Elastomers with Controlled Biomineralization Activity, Real-Time Bioimaging Tracking, and Decreased Inflammatory Response. ACS Appl. Mater. Interfaces 2018, 10, 17722–17731. [Google Scholar] [CrossRef]
- Guo, M.; Dong, Y.; Xiao, J.; Gu, R.; Ding, M.; Huang, T.; Li, J.; Zhao, N.; Liao, H. In vivo immuno-reactivity analysis of the porous three-dimensional chitosan/SiO2 and chitosan/SiO2/hydroxyapatite hybrids. J. Biomed. Mater. Res. A 2018, 106, 1223–1235. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; ten Hoopen, S.; Zhang, W.; Yi, C.; Yang, W.; Yang, F.; Jansen, J.A.; Walboomers, X.F.; Yelick, P.C. Influence of highly porous electrospun PLGA/PCL/nHA fibrous scaffolds on the differentiation of tooth bud cells in vitro. J. Biomed. Mater. Res. A 2017, 105, 2597–2607. [Google Scholar] [CrossRef] [PubMed]
- Faccendini, A.; Bianchi, E.; Ruggeri, M.; Vigani, B.; Perotti, C.; Pavesi, F.C.; Caliogna, L.; Natali, F.; Del Favero, E.; Cantù, E.; et al. Smart device for biologically enhanced functional regeneration of osteo-tendon interface. Pharmaceutics 2021, 13, 1996. [Google Scholar] [CrossRef]
- Ghosh, M.; Helperin-Sternfeld, M.; Grigoriants, I.; Lee, J.; Nam, K.T.; Adler-Abramovich, L. Arginine-Presenting Peptide Hydrogels Decorated with Hydroxyapatite as Biomimetic Scaffolds for Bone Regeneration. Biomacromolecules 2017, 18, 3541–3550. [Google Scholar] [CrossRef] [PubMed]
- Alon, N.; Havdala, T.; Skaat, H.; Baranes, K.; Marcus, M.; Levy, I.; Margel, S.; Sharoni, A.; Shefi, O. Magnetic micro-device for manipulating PC12 cell migration and organization. Lab Chip 2015, 15, 2030–2036. [Google Scholar] [CrossRef] [PubMed]
- Marcus, M.; Skaat, H.; Alon, N.; Margel, S.; Shefi, O. NGF-conjugated iron oxide nanoparticles promote differentiation and outgrowth of PC12 cells. Nanoscale 2015, 7, 1058–1066. [Google Scholar] [CrossRef]
- Zuidema, J.M.; Provenza, C.; Caliendo, T.; Dutz, S.; Gilbert, R.J. Magnetic NGF-releasing PLLA/iron oxide nanoparticles direct extending neurites and preferentially guide neurites along aligned electrospun microfibers. ACS Chem. Neurosci. 2015, 6, 1781–1788. [Google Scholar] [CrossRef]
- Yuan, M.; Wang, Y.; Qin, Y.-X. Promoting neuroregeneration by applying dynamic magnetic fields to a novel nanomedicine: Superparamagnetic iron oxide (SPIO)-gold nanoparticles bounded with nerve growth factor (NGF). Nanomedicine 2018, 14, 1337–1347. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Chen, M.-H.; Liao, S.-Y.; Wu, H.-C.; Kuan, C.-H.; Sun, J.-S.; Wang, T.-W. Multichanneled Nerve Guidance Conduit with Spatial Gradients of Neurotrophic Factors and Oriented Nanotopography for Repairing the Peripheral Nervous System. ACS Appl. Mater. Interfaces 2017, 9, 37623–37636. [Google Scholar] [CrossRef]
- Vinzant, N.; Scholl, J.L.; Wu, C.-M.; Kindle, T.; Koodali, R.; Forster, G.L. Iron Oxide Nanoparticle Delivery of Peptides to the Brain: Reversal of Anxiety during Drug Withdrawal. Front. Neurosci. 2017, 11, 608. [Google Scholar] [CrossRef] [Green Version]
- Naseri-Nosar, M.; Farzamfar, S.; Sahrapeyma, H.; Ghorbani, S.; Bastami, F.; Vaez, A.; Salehi, M. Cerium oxide nanoparticle containing poly (ε-caprolactone)/gelatin electrospun film as a potential wound dressing material: In vitro and in vivo evaluation. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 81, 366–372. [Google Scholar] [CrossRef]
- Marino, A.; Tonda-Turo, C.; De Pasquale, D.; Ruini, F.; Genchi, G.; Nitti, S.; Cappello, V.; Gemmi, M.; Mattoli, V.; Ciardelli, G.; et al. Gelatin/nanoceria nanocomposite fibers as antioxidant scaffolds for neuronal regeneration. Biochim. Biophys. Acta Gen. Subj. 2016, 1861, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Salata, O. Applications of nanoparticles in biology and medicine. J. Nanobiotechnol. 2004, 2, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singla, R.; Guliani, A.; Kumari, A.; Yadav, S.K. Metallic nanoparicles, toxicity issues and applications in medicine. In Nanoscale Materials in Targeted Drug Delivery, Theragnosis and Tissue Regeneration; Yadav, S.K., Ed.; Springer: Singapore, 2016; pp. 41–80. [Google Scholar] [CrossRef]
- Abdal Dayem, A.; Lee, S.B.; Cho, S.-G. The Impact of Metallic Nanoparticles on Stem Cell Proliferation and Differentiation. Nanomaterials 2018, 8, 761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Sayed, M.A. Some interesting properties of metals confined in time and nanometer space of different shapes. Acc. Chem. Res. 2001, 34, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Ramos, M.A.D.S.; Da Silva, P.B.; Spósito, L.; De Toledo, L.G.; Bonifácio, B.V.; Rodero, C.F.; Dos Santos, K.C.; Chorilli, M.; Bauab, T.M. Nanotechnology-based drug delivery systems for control of microbial biofilms: A review. Int. J. Nanomed. 2018, 13, 1179. [Google Scholar] [CrossRef] [Green Version]
- Melo, M.A., Jr.; Santos, L.S.S.; Gonçalves, M.D.C.; Nogueira, A.F. Preparation of silver and gold nanoparticles: A simple method to introduce nanotechnology into teaching laboratories. Quim. Nova 2012, 35, 1872–1878. [Google Scholar] [CrossRef] [Green Version]
- Nikzamir, M.; Akbarzadeh, A.; Panahi, Y. An overview on nanoparticles used in biomedicine and their cytotoxicity. J. Drug Deliv. Sci. Technol. 2021, 61, 102316. [Google Scholar] [CrossRef]
- Islan, G.A.; Das, S.; Cacicedo, M.L.; Halder, A.; Mukherjee, A.; Cuestas, M.L.; Roy, P.; Castro, G.R.; Mukherjee, A. Silybin-conjugated gold nanoparticles for antimicrobial chemotherapy against Gram-negative bacteria. J. Drug Deliv. Sci. Technol. 2019, 53, 101181. [Google Scholar] [CrossRef]
- Mohandas, A.; Deepthi, S.; Biswas, R.; Jayakumar, R. Chitosan based metallic nanocomposite scaffolds as antimicrobial wound dressings. Bioact. Mater. 2018, 3, 267–277. [Google Scholar] [CrossRef]
- Vrcek, I.V.; Žuntar, I.; Petlevski, R.; Pavičić, I.; Sikirić, M.D.; Ćurlin, M.; Goessler, W. Comparison of in vitro toxicity of silver ions and silver nanoparticles on human hepatoma cells. Environ. Toxicol. 2016, 31, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Dobson, J. Gene therapy progress and prospects: Magnetic nanoparticle-based gene delivery. Gene Ther. 2006, 13, 283–287. [Google Scholar] [CrossRef] [Green Version]
- Mody, V.V.; Siwale, R.; Singh, A.; Mody, H.R. Introduction to metallic nanoparticles. J. Pharm. Bioall. Sci. 2010, 2, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, J.J.; Zhang, J.; Wang, X.; Kawazoe, N.; Chen, G. Gold nanoparticle size and shape influence on osteogenesis of mesenchymal stem cells. Nanoscale 2016, 8, 7992–8007. [Google Scholar] [CrossRef] [PubMed]
- Ko, W.-K.; Heo, D.N.; Moon, H.-J.; Lee, S.J.; Bae, M.S.; Lee, J.B.; Sun, I.-C.; Jeon, H.B.; Park, H.K.; Kwon, I.K. The effect of gold nanoparticle size on osteogenic differentiation of adipose-derived stem cells. J. Colloid. Interface Sci. 2015, 438, 68–76. [Google Scholar] [CrossRef]
- Zhang, R.; Lee, P.; Lui, V.C.; Chen, Y.; Liu, X.; Lok, C.N.; To, M.; Yeung, K.W.; Wong, K.K. Silver nanoparticles promote osteogenesis of mesenchymal stem cells and improve bone fracture healing in osteogenesis mechanism mouse model. Nanomedicine 2015, 11, 1949–1959. [Google Scholar] [CrossRef]
- Tateno, M.; Takase, M.; Nishinaga, T. Synthesis and conductive properties of protected by partially bicyclo[2.2.2] octane annelated and methylthio-end capped oligothiophene thiolates. Chem. Mater. 2014, 26, 3804–3810. [Google Scholar] [CrossRef]
- Borkenkov, M.; Chirico, G.; Collini, M.; Pallavicini, P. Gold nanoparticles in tissue engineering. In Environmental Nanotechnology, Environmental Chemistry for a Sustainable World; Dasgupta, N., Ed.; Springer: Cham, Switzerland, 2018; Volume 10, pp. 343–390. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, L.; Yang, C.; Liu, J. Recent advances of smart acid-responsive gold nanoparticles in tumor therapy. WIREs Nanomed. Nanobiotechnol. 2020, 12, e1619. [Google Scholar] [CrossRef]
- Yeh, Y.-C.; Creran, B.; Rotello, V.M. Gold nanoparticles: Preparation, properties, and applications in bionanotechnology. Nanoscale 2012, 4, 1871–1880. [Google Scholar] [CrossRef]
- Herizchi, R.; Abbasi, E.; Milani, M.; Akbarzadeh, A. Current methods for synthesis of gold nanoparticles. Artif. Cells Nanomed. Biotechnol. Int. J. 2016, 44, 596–602. [Google Scholar] [CrossRef]
- Wei, M.; Li, S.; Yang, Z.; Zheng, W.; Le, W. Gold nanoparticles enhance the differentiation of embryonic stem cells into dopaminergic neurons via mtor/p70s6k pathway. Nanomedicine 2017, 12, 1305–1317. [Google Scholar] [CrossRef] [PubMed]
- Baranes, K.; Shevach, M.; Shefi, O.; Dvir, T. Gold nanoparticle-decorated scaffolds promote neuronal differentiation and maturation. Nano Lett. 2016, 16, 2916–2920. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.; Song, W.; Gao, H.; Li, T.; Cao, X.; Zhong, S.; Wang, Y. Mir-29b-loaded gold nanoparticles targeting to the endoplasmic reticulum for synergistic promotion of osteogenic differentiation. ACS Appl. Mater. Interfaces 2016, 8, 19217–19227. [Google Scholar] [CrossRef]
- Prabhu, S.; Poulose, E.K. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012, 2, 32. [Google Scholar] [CrossRef] [Green Version]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef]
- Danilcauk, M.; Lund, A.; Saldo, J.; Yamada, H.; Michalik, J. Conduction electron spin resonance of small silver particles. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2006, 63, 189–191. [Google Scholar] [CrossRef]
- Kim, J.S.; Kuk, E.; Yu, K.; Kim, J.H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 95–101. [Google Scholar] [CrossRef]
- Shrivastava, S.; Bera, T.; Roy, A.; Singh, G.; Ramachandrarao, P.; Dash, D. Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology 2007, 18, 1–9. [Google Scholar] [CrossRef]
- Hatchett, D.W.; Henry, S. Electrochemistry of sulfur adlayers on low-index faces of silver. J. Phys. Chem. 1996, 100, 9854–9859. [Google Scholar] [CrossRef]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2008, 52, 662–668. [Google Scholar] [CrossRef]
- Matsumura, Y.; Yoshikata, K.; Kunisaki, S.; Tsuchido, T. Mode of bacterial action of silver zeolite and its comparison with that of silver nitrate. Appl. Environ. Microbiol. 2003, 69, 4278–4281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramirez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef] [Green Version]
- Vial, S.; Reis, R.L.; Oliveira, J.M. Recent advances using gold nanoparticles as a promising multimodal tool for tissue engineering and regenerative medicine. Curr. Opin. Solid State Mater. Sci. 2017, 21, 92–112. [Google Scholar] [CrossRef] [Green Version]
- Parani, M.; Lokhande, G.; Singh, A.; Gaharwar, A.K. Engineered nanomaterials for infection control and healing acute and chronic wounds. ACS Appl. Mater. Interfaces 2016, 8, 10049–10069. [Google Scholar] [CrossRef]
- Zi-Wei, L.; Li, C.W.; Wang, Q.; Shi, S.-J.; Hu, M.; Zhang, Q.; Cui, H.-H.; Sun, J.B.; Zhou, M.; Wu, G.-L.; et al. The cellular and molecular mechanisms underlying slver nanoparticle/chitosan oligosaccharide/poly(vinyl alcohol) nanofiber-mediated wound healing. J. Biomed. Nanotechnol. 2017, 13, 17–34. [Google Scholar] [CrossRef]
- Tian, J.; Wong, K.K.Y.; Ho, C.-M.; Lok, C.-N.; Yu, W.-Y.; Che, C.-M.; Chiu, J.-F.; Tam, P.K.H. Topical delivery of silver nanoparticles promotes wound healing. Chem. Med. Chem. 2007, 2, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dou, C.; He, G.; Ban, L.; Huang, L.; Li, Z.; Gong, J.; Zhang, J.; Yu, P. Biomedical Potential of Ultrafine Ag Nanoparticles Coated on Poly (Gamma-Glutamic Acid) Hydrogel with Special Reference to Wound Healing. Nanomateials 2018, 8, 324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Pan, S.; Xia, P.; Chang, Y.; Fu, C.; Kong, W.; Yu, Z.; Wang, K.; Yang, X.; Qi, Z. Advances in the application of gold nanoparticles in bone tissue engineering. J. Biol. Eng. 2020, 14, 14. [Google Scholar] [CrossRef]
- Heo, D.N.; Ko, W.-K.; Bae, M.S.; Lee, J.B.; Lee, D.-W.; Byun, W.; Lee, C.H.; Kim, E.C.; Jung, B.Y.; Kwon, I.K. Enhanced bone regeneration with a gold nanoparticle–hydrogel complex. J. Mater. Chem. B 2014, 2, 1584–1593. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Chen, Y.; Kawazoe, N.; Chen, G. TEMPO-conjugated gold nanoparticles for reactive oxygen species scavenging and regulation of stem cell differentiation. ACS Appl. Mater. Interfaces 2017, 9, 35683–35692. [Google Scholar] [CrossRef]
- del Mar Encabo-Berzosa, M.; Sancho-Albero, M.; Crespo, A.; Andreu, V.; Sebastian, V.; Irusta, S.; Arruebo, M.; Martìn-Duque, P.; Santamaria, J. The effect of PEGylated hollow gold nanoparticles on stem cell migration: Potential application in tissue regeneration. Nanoscale 2017, 9, 9848–9858. [Google Scholar] [CrossRef]
- Liong, M.; Lu, J.; Kovochich, M.; Xia, T.; Ruehm, S.G.; Nel, A.E.; Tamanoi, F.; Zink, J.I. Multifunctional inorganic nanoparticles for imaging, targeting, and drug delivery. ACS Nano 2008, 2, 889–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.S.; Park, K.; Moon, H.T.; Woo, D.G.; Yang, H.N.; Park, K.-H. Electrical pulsed stimulation of surfaces homogeneously coated with gold nanoparticles to induce neurite outgrowth of PC12 cells. Langmuir 2009, 25, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Alon, N.; Miroshnikov, Y.; Perkas, N.; Nissan, I.; Gedanken, A.; Shefi, O. Silver nanoparticles promote neuronal growth. Procedia Eng. 2013, 59, 25–29. [Google Scholar] [CrossRef] [Green Version]
- Nissan, I.; Schori, H.; Lipovsky, A.; Alon, N.; Gedanken, A.; Shefi, O. Effect of different densities of silver nanoparticles on neuronal growth. J. Nanopart. Res. 2016, 18, 221. [Google Scholar] [CrossRef]
- Nissan, I.; Schori, H.; Kumar, V.B.; Passig, M.A.; Shefi, O.; Gedanken, A. Topographical impact of silver nanolines on the morphology of neuronal SH-SY5Y Cells. J. Mater. Chem. B 2017, 5, 9346–9353. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Guliani, A.; Singla, R.; Yadav, R.; Yadav, S.K. Silver nanoparticles synthesised using plant extracts show strong antibacterial activity. IET Nanobiotechnol. 2014, 9, 142–152. [Google Scholar] [CrossRef]
- Krishnaraj, C.; Jagan, E.G.; Rajasekar, S.; Selvakumar, P.; Kalaichelvan, P.T.; Mohan, N. Synthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibacterial activity against water borne pathogens. Colloid. Surf. B Biointerfaces 2010, 76, 50–56. [Google Scholar] [CrossRef]
- Li, X.; Robinson, S.M.; Gupta, A.; Saha, K.; Jiang, Z.; Moyano, D.F.; Sahar, A.; Riley, M.A.; Rotello, V.M. Functional gold nanoparticles as potent antimicrobial agents against multi-drug-resistant bacteria. ACS Nano 2014, 8, 10682–10686. [Google Scholar] [CrossRef]
- Ramyadevi, J.; Jeyasubramanian, K.; Marikani, A.; Rajakumar, G.; Rahuman, A.A. Synthesis and antimicrobial activity of copper nanoparticles. Mater. Lett. 2012, 71, 114–116. [Google Scholar] [CrossRef]
- Thomas, R.; Janardhanan, A.; Varghese, R.T.; Soniya, E.V.; Mathew, J.; Radhakrishnan, E.K. Antibacterial properties of silver nanoparticles synthesized by marine Ochrobactrum sp. Braz. J. Microbiol. 2014, 45, 1221–1227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajeshkumar, S.; Malarkodi, C. In vitro antibacterial activity and mechanism of silver nanoparticles against foodborne pathogens. Bioinorg. Chem. Appl. 2014, 10. [Google Scholar] [CrossRef] [Green Version]
- Veeraapandian, S.; Sawant, S.N.; Doble, M. Antibacterial and antioxidant activity of protein capped silver and gold nanoparticles synthesized with Escherichia coli. J. Biomed. Nanotechnol. 2012, 8, 1400148. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Kong, Y.; Kundu, S.; Cirillo, J.D.; Liang, H. Antibacterial activities of gold and silver nanoparticles against Escherichia coli and Bacillus Calmette- Guérin. J. Nanobiotechnol. 2012, 10, 19. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Raouf, N.; Al-Enazi, N.M.; Ibraheem, I.B.M. Green biosynthesis of gold nanoparticles using Galaxaura elongate and characterization of their antibacterial activity. Arab. J. Chem. 2017, 10, S3029–S3039. [Google Scholar] [CrossRef] [Green Version]
- Ashajyothi, C.; Jahanara, K.; Chandrakanth, R.K. Biosynthesis and characterization of copper nanoparticles from Enterococcus faecalis. Int. J. Pharm. Bio. Sci. 2014, 5, 204–211. [Google Scholar]
- Gopinath, M.; Subbaiya, R.; Selvam, M.M.; Suresh, D. Synthesis of copper nanoparticles from nerium oleander leaf aqueous extract and its antibacterial activity. Int. J. Curr. Microbiol. App. Sci. 2014, 3, 814–818. [Google Scholar]
- Betancourt-Galindo, R.; Reyes-Rodriguez, P.Y.; Puente-Urbina, B.A.; Avila-Orta, C.A.; Rodriguez-Fernàndez, O.S.; Cadenas-Pliego, G.; Lira-Saldivar, R.H.; Garcìa-Cerda, L.A. Synthesis of copper nanoparticles by thermal decomposition and their antimicrobial properties. J. Nanomater. 2014, 2014, 980545. [Google Scholar] [CrossRef] [Green Version]
- Bala, N.; Saha, S.; Chakraborty, M.; Maiti, M.; Das, S.; Basu, R.; Nandy, P. Green synthesis of zinc oxide nanoparticles using Hibiscus subdariffa leaf extract: Effect of temperature on synthesis, anti-bacterial activity and anti-diabetic activity. RSC Adv. 2015, 5, 4993–5003. [Google Scholar] [CrossRef]
- Santhoshkumar, T.; Rahuman, A.A.; Jayaseelan, C.; Rajakumar, G.; Marimuthu, S.; Vishnu Kirthi, A.; Velayutham, K.; Thomas, J.; Venkatesan, J.; Kim, S.-K. Green synthesis of titanium dioxide nanoparticles using Psidium guajava extract and its antibacterial and antioxidant properties. Asian Pac. J. Trop. Med. 2014, 12, 968–976. [Google Scholar] [CrossRef] [Green Version]
- Kedziora, A.; Strek, W.; Kepinski, L.; Bugla-PLoSkonska, G.; Dorosziewicz, W. Synthesis and antibacterial activity of novel titanium dioxide doped with silver. J. Sol. Gel. Sci. Technol. 2012, 62, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Ismail, R.A.; Sulaiman, G.M.; Abdulrahman, S.A.; Marzoog, T.R. Antibacterial activity of magnetic iron oxide nanoparticles synthesized by laser ablation in liquid. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 53, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Naseem, T.; Farrukh, M.A. Antibacterial activity of green synthesis of iron nanoparticles using Lawsonia inermis and Gardenia jasminoides leaves extract. J. Chem. 2015, 7, 912342. [Google Scholar] [CrossRef] [Green Version]










| Material | Young’s Modulus (GPa) | Tensile Strength (MPa) | References |
|---|---|---|---|
| UHMWPE 1 | 1.51 ± 0.01 | 18.62 ± 0.16 | [43] |
| f-SWCNTs/UHMWPE 2 (0.01 wt%) | 1.69 ± 0.01 | 19.20 ± 0.11 | |
| f-SWCNTs/UHMWPE (0.1 wt%) | 1.74 ± 0.006 | 28.00 ± 0.11 | |
| GelMA 3 | 7.12 ± 3.1 | 157.81 ± 3.91 | [44] |
| CNOs/GelMA | 41.19 ± 3.78 | 356.12 ± 3.67 | |
| AN-PEEK 4 | 9.9 ± 0.3 | 195.4 ± 1.1 | [45] |
| AN-PEEK/CNOs (1.5 wt%) | 24.1 ± 0.8 | 351.1 ± 3.5 | |
| AN-PEEK/CNOs (2.5 wt%) | 31.3 ± 0.4 | 552.9 ± 6.1 | |
| AN-PEEK/CNOs (5 wt%) | 43.2 ± 1.1 | 891.4 ± 8.2 | |
| PCL | 0.041 ± 0.0009 | 41.20 ± 0.89 | [46] |
| PCL/CNOs (0.2 wt%) | 0.06 ± 0.0013 | 60.10 ± 1.27 | |
| PCL/CNOs (0.5 wt%) | 0.084 ± 0.0011 | 84.70 ± 1.14 |
| Nanoparticles | Antimicrobial Activity | References |
|---|---|---|
| Ag | Salmonella typhi, Salmonella paratyphi, V. cholera and S. aureus | [145] |
| Ag | B. subtilis, Klebsiella planticola, K. pneumonia, Serratia nematodiphila, and E. coli | [146] |
| Ag | B. subtilis, K. pneumonia, E. coli, P. aeruginosa and S. aureus | [147] |
| Au | BCG 1 and E. coli | [148] |
| Au | S. aureus, E. coli, K. Pneumonia and P. aeruginosa | [149] |
| Cu | E. coli and C. albicans | [144] |
| Cu | E. coli, K. pneumonia and S. aureus | [150] |
| Cu | S. typhi, B. subtilus, S. aureus, K. pneumoniae and E. coli | [151] |
| Cu | S. aureus and P. aeruginosa | [152] |
| Zn | E. coli and S. aureus | [153] |
| TiO2 | E. coli and S. aureus | [154] |
| TiO2 | E. coli, S. aureus and K. pneumonia | [155] |
| Fe2O3 | S. aureus, E. coli, P. aeruginosa and Serratia marcescens | [155] |
| Iron | E. coli, Salmonella enterica, Proteus mirabilis and S. aureus | [156,157] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bianchi, E.; Vigani, B.; Viseras, C.; Ferrari, F.; Rossi, S.; Sandri, G. Inorganic Nanomaterials in Tissue Engineering. Pharmaceutics 2022, 14, 1127. https://doi.org/10.3390/pharmaceutics14061127
Bianchi E, Vigani B, Viseras C, Ferrari F, Rossi S, Sandri G. Inorganic Nanomaterials in Tissue Engineering. Pharmaceutics. 2022; 14(6):1127. https://doi.org/10.3390/pharmaceutics14061127
Chicago/Turabian StyleBianchi, Eleonora, Barbara Vigani, César Viseras, Franca Ferrari, Silvia Rossi, and Giuseppina Sandri. 2022. "Inorganic Nanomaterials in Tissue Engineering" Pharmaceutics 14, no. 6: 1127. https://doi.org/10.3390/pharmaceutics14061127