Influence of the Dispersion Medium and Cryoprotectants on the Physico-Chemical Features of Gliadin- and Zein-Based Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Physico-Chemical Characterization of Protein-Based Nanoparticles
2.3. Evaluation of the Stability by Multiple Light Scattering
2.4. Freeze-Drying of Zein and Gliadin Nanoparticles
2.5. Cytocompatibility and Cell Interaction of Protein-Based Nanoparticles
2.6. Statistical Analysis
3. Results and Discussion
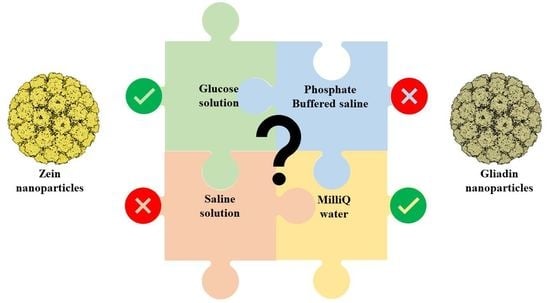
3.1. Physico-Chemical Characterization of Protein-Based Nanoparticles
3.2. Freeze-Drying of Protein-Based Nanoparticles
3.3. In Vitro Cytotoxicity and Interaction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DeFrates, K.; Markiewicz, T.; Gallo, P.; Rack, A.; Weyhmiller, A.; Jarmusik, B.; Hu, X. Protein Polymer-Based Nanoparticles: Fabrication and Medical Applications. Int. J. Mol. Sci. 2018, 19, 1717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassanin, I.A.; Elzoghby, A.O. Self-assembled non-covalent protein-drug nanoparticles: An emerging delivery platform for anti-cancer drugs. Expert Opin. Drug Deliv. 2020, 17, 1437–1458. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, A.; Voci, S.; Paolino, D.; Fresta, M.; Cosco, D. Influence of Various Model Compounds on the Rheological Properties of Zein-Based Gels. Molecules 2020, 25, 3174. [Google Scholar] [CrossRef] [PubMed]
- Voci, S.; Gagliardi, A.; Fresta, M.; Cosco, D. Antitumor Features of Vegetal Protein-Based Nanotherapeutics. Pharmaceutics 2020, 12, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voci, S.; Fresta, M.; Cosco, D. Gliadins as versatile biomaterials for drug delivery applications. J. Control. Release 2021, 329, 385–400. [Google Scholar] [CrossRef] [PubMed]
- Tseng, K.H.; Liao, C.Y.; Huang, J.C.; Tien, D.C.; Tsung, T.T. Characterization of gold nanoparticles in organic or inorganic medium (ethanol/water) fabricated by spark discharge method. Mater. Lett. 2008, 62, 3341–3344. [Google Scholar] [CrossRef]
- Allouni, Z.E.; Cimpan, M.R.; Høl, P.J.; Skodvin, T.; Gjerdet, N.R. Agglomeration and sedimentation of TiO2 nanoparticles in cell culture medium. Colloids Surf. B 2009, 68, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Lee, Y. The stability of citrate-capped silver nanoparticles in isotonic glucose solution for intravenous injection. J. Toxicol. Environ. Health Part A 2013, 76, 1236–1245. [Google Scholar] [CrossRef]
- Ross, A.M.; Kennedy, T.; McNulty, D.; Leahy, C.I.; Walsh, D.R.; Murray, P.; Grabrucker, A.; Mulvihill, J.J. Comparing nanoparticles for drug delivery: The effect of physiological dispersion media on nanoparticle properties. Mater. Sci. Eng. C 2020, 113, 110985. [Google Scholar] [CrossRef]
- Caracciolo, G.; Farokhzad, O.C.; Mahmoudi, M. Biological identity of nanoparticles in vivo: Clinical implications of the protein corona. Trends Biotechnol. 2017, 35, 257–264. [Google Scholar] [CrossRef]
- Mohammady, M.; Mohammady, Y.; Yousefi, G. Freeze-drying of pharmaceutical and nutraceutical nanoparticles: The effects of formulation and technique parameters on nanoparticles characteristics. J. Pharm. Sci. 2020, 109, 3235–3247. [Google Scholar] [CrossRef] [PubMed]
- Fonte, P.; Reis, S.; Sarmento, B. Facts and evidences on the lyophilization of polymeric nanoparticles for drug delivery. J. Control. Release 2016, 225, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Kim, M.Y.; Kim, S.; Lee, J. Cryoprotectants for freeze drying of drug nano-suspensions: Effect of freezing rate. J. Pharm. Sci. 2009, 98, 4808–4817. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, B.S.; Pikal, M.J.; Bogner, R.H. Study of the individual contributions of ice formation and freeze-concentration on isothermal stability of lactate dehydrogenase during freezing. J. Pharm. Sci. 2008, 97, 798–814. [Google Scholar] [CrossRef] [PubMed]
- Fonte, P.; Soares, S.; Sousa, F.; Costa, A.; Seabra, V.; Reis, S.; Sarmento, B. Stability study perspective of the effect of freeze-drying using cryoprotectants on the structure of insulin loaded into PLGA nanoparticles. Biomacromolecules 2014, 15, 3753–3765. [Google Scholar] [CrossRef]
- Rodrigues, S.; Cordeiro, C.; Seijo, B.; Remunan-Lopez, C.; Grenha, A. Hybrid nanosystems based on natural polymers as protein carriers for respiratory delivery: Stability and toxicological evaluation. Carbohydr. Polym. 2015, 123, 369–380. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Grainger, D.W. Lyophilized liposome-based parenteral drug development: Reviewing complex product design strategies and current regulatory environments. Adv. Drug Deliv. Rev. 2019, 151, 56–71. [Google Scholar] [CrossRef]
- Guimarães, D.; Noro, J.; Silva, C.; Cavaco-Paulo, A.; Nogueira, E. Protective effect of Saccharides on freeze-dried liposomes encapsulating drugs. Front. Bioeng. Biotechnol. 2019, 7, 424. [Google Scholar] [CrossRef] [Green Version]
- Branca, C.; Magazu, S.; Migliardo, F.; Migliardo, P. Destructuring effect of trehalose on the tetrahedral network of water: A raman and neutron diffraction comparison. Phys. A Stat. Mech. Its Appl. 2002, 304, 314–318. [Google Scholar] [CrossRef]
- Lerbret, A.; Bordat, P.; Affouard, F.; Descamps, M.; Migliardo, F. How homogeneous are the trehalose, maltose, and sucrose water solutions? An insight from molecular dynamics simulations. J. Phys. Chem. B 2005, 109, 11046–11057. [Google Scholar] [CrossRef] [Green Version]
- Shiraga, K.; Adachi, A.; Ogawa, Y. Characterization of the hydrogen-bond network of water around sucrose and trehalose: H-o-h bending analysis. Chem. Phys. Lett. 2017, 678, 59–64. [Google Scholar] [CrossRef]
- Olsson, C.; Swenson, J. Structural comparison between sucrose and trehalose in aqueous solution. J. Phys. Chem. B 2020, 124, 3074–3082. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, P.M.; Felício, M.R.; Santos, N.C.; Gonçalves, S.; Domingues, M.M. Application of light scattering techniques to nanoparticle characterization and development. Front. Chem. 2018, 6, 237. [Google Scholar] [CrossRef] [PubMed]
- Mazloumi, M.; Johnston, L.J.; Jakubek, Z.J. Dispersion, stability and size measurements for cellulose nanocrystals by static multiple light scattering. Cellulose 2018, 25, 5751–5768. [Google Scholar] [CrossRef]
- Lazzari, S.; Moscatelli, D.; Codari, F.; Salmona, M.; Morbidelli, M.; Diomede, L. Colloidal stability of polymeric nanoparticles in biological fluids. J. Nanopart. Res. 2012, 14, 920. [Google Scholar] [CrossRef] [Green Version]
- Amore, E.; Ferraro, M.; Manca, M.L.; Gjomarkaj, M.; Giammona, G.; Pace, E.; Bondì, M.L. Mucoadhesive solid lipid microparticles for controlled release of a corticosteroid in the chronic obstructive pulmonary disease treatment. Nanomedicine 2017, 12, 2287–2302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picco, A.S.; Mondo, G.B.; Ferreira, L.F.; de Souza, E.E.; Peroni, L.A.; Cardoso, M.B. Protein corona meets freeze-drying: Overcoming the challenges of colloidal stability, toxicity, and opsonin adsorption. Nanoscale 2021, 13, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Kaombe, D.D.; Lenes, M.; Toven, K.; Glomm, W.R. Turbiscan as a tool for studying the phase separation tendency of pyrolysis oil. Energy Fuels 2013, 27, 1446–1452. [Google Scholar] [CrossRef]
- Luo, M.; Qi, X.; Ren, T.; Huang, Y.; Keller, A.A.; Wang, H.; Wu, B.; Jin, H.; Li, F. Heteroaggregation of CeO2 and TiO2 engineered nanoparticles in the aqueous phase: Application of turbiscan stability index and fluorescence excitation-emission matrix (EEM) spectra. Colloids Surf. A Physicochem. Eng. Asp. 2017, 533, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Gagliardi, A.; Froiio, F.; Salvatici, M.C.; Paolino, D.; Fresta, M.; Cosco, D. Characterization and refinement of zein-based gels. Food Hydrocoll. 2020, 101, 105555. [Google Scholar] [CrossRef]
- Giuliano, E.; Fresta, M.; Cosco, D. Development and characterization of poloxamine 908-hydrogels for potential pharmaceutical applications. J. Mol. Liq. 2021, 337, 116588. [Google Scholar] [CrossRef]
- Gagliardi, A.; Paolino, D.; Costa, N.; Fresta, M.; Cosco, D. Zein-vs PLGA-based nanoparticles containing rutin: A comparative investigation. Mater. Sci. Eng. C 2021, 118, 111538. [Google Scholar] [CrossRef] [PubMed]
- Voci, S.; Gagliardi, A.; Salvatici, M.C.; Fresta, M.; Cosco, D. Development of polyoxyethylene (2) oleyl ether-gliadin nanoparticles: Characterization and in vitro cytotoxicity. Eur. J. Pharm. Sci. 2021, 162, 105849. [Google Scholar] [CrossRef]
- Gagliardi, A.; Voci, S.; Bonacci, S.; Iriti, G.; Procopio, A.; Fresta, M.; Cosco, D. SCLAREIN (SCLAREol contained in zeIN) nanoparticles: Development and characterization of an innovative natural nanoformulation. Int. J. Biol. 2021, 193, 713–720. [Google Scholar] [CrossRef]
- Gagliardi, A.; Voci, S.; Salvatici, M.C.; Fresta, M.; Cosco, D. Brij-stabilized zein nanoparticles as potential drug carriers. Colloids Surf. B 2021, 201, 111647. [Google Scholar] [CrossRef]
- Cosco, D.; Bruno, F.; Castelli, G.; Puleio, R.; Bonacci, S.; Procopio, A.; Britti, D.; Fresta, M.; Vitale, F.; Paolino, D. Meglumine Antimoniate-Loaded Aqueous-Core PLA Nanocapsules: Old Drug, New Formulation against Leishmania-Related Diseases. Macromol. Biosci. 2021, 21, 2100046. [Google Scholar] [CrossRef]
- Gagliardi, A.; Cosco, D.; Udongo, B.P.; Dini, L.; Viglietto, G.; Paolino, D. Design and Characterization of Glyceryl Monooleate-Nanostructures Containing Doxorubicin Hydrochloride. Pharmaceutics 2020, 12, 1017. [Google Scholar] [CrossRef]
- Yue, P.F.; Li, Y.; Wan, J.; Yang, M.; Zhu, W.F.; Wang, C.H. Study on formability of solid nanosuspensions during nanodispersion and solidification: I. Novel role of stabilizer/drug property. Int. J. Pharm. 2013, 454, 269–277. [Google Scholar] [CrossRef]
- Zhang, X.; Guan, J.; Ni, R.; Li, L.C.; Mao, S. Preparation and solidification of redispersible nanosuspensions. J. Pharm. Sci. 2014, 103, 2166–2176. [Google Scholar] [CrossRef]
- Gagliardi, A.; Paolino, D.; Iannone, M.; Palma, E.; Fresta, M.; Cosco, D. Sodium deoxycholate-decorated zein nanoparticles for a stable colloidal drug delivery system. Int. J. Nanomed. 2018, 13, 601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gagliardi, A.; Voci, S.; Giuliano, E.; Salvatici, M.C.; Celano, M.; Fresta, M.; Cosco, D. Phospholipid/zein hybrid nanoparticles as promising carriers for the protection and delivery of all-trans retinoic acid. Mater. Sci. Eng. C 2021, 128, 112331. [Google Scholar] [CrossRef] [PubMed]
- Sjöstrand, F.; Edsberg, L.; Hahn, R.G. Volume kinetics of glucose solutions given by intravenous infusion. Br. J. Anaesth. 2001, 87, 834–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yilmaz, B.; Pazarceviren, A.E.; Tezcaner, A.; Evis, Z. Historical development of simulated body fluids used in biomedical applications: A review. Microchem. J. 2020, 155, 104713. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, H.; Xu, C.; Gu, L. A review: Using nanoparticles to enhance absorption and bioavailability of phenolic phytochemicals. Food Hydrocoll. 2015, 43, 153–164. [Google Scholar] [CrossRef]
- Joye, I.J.; Nelis, V.A.; McClements, D.J. Gliadin-based nanoparticles: Fabrication and stability of food-grade colloidal delivery systems. Food Hydrocoll. 2015, 44, 86–93. [Google Scholar] [CrossRef]
- Joye, I.J.; Nelis, V.A.; McClements, D.J. Gliadin-based nanoparticles: Stabilization by post-production polysaccharide coating. Food Hydrocoll. 2015, 43, 236–242. [Google Scholar] [CrossRef]
- Lucky, S.S.; Muhammad Idris, N.; Li, Z.; Huang, K.; Soo, K.C.; Zhang, Y. Titania coated upconversion nanoparticles for near-infrared light triggered photodynamic therapy. ACS Nano 2015, 9, 191–205. [Google Scholar] [CrossRef]
- Lucky, S.S.; Idris, N.M.; Huang, K.; Kim, J.; Li, Z.; Thong, P.S.P.; Xu, R.; Soo, K.C.; Zhang, Y. In vivo biocompatibility, biodistribution and therapeutic efficiency of titania coated upconversion nanoparticles for photodynamic therapy of solid oral cancers. Theranostics 2016, 6, 1844. [Google Scholar] [CrossRef]
- de Chasteigner, S.; Cavé, G.; Fessi, H.; Devissaguet, J.P.; Puisieux, F. Freeze-drying of itraconazole-loaded nanosphere suspensions: A feasibility study. Drug Dev. Res. 1996, 38, 116–124. [Google Scholar] [CrossRef]
- Mitra, R.K.; Paul, B.K. Physico-chemical investigations of microemulsification of eucalyptus oil and water using mixed surfactants (AOT+ Brij-35) and butanol. J. Colloid Interface Sci. 2005, 283, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Deac, A.; Zhang, G.G. Assessing physical stability of colloidal dispersions using a turbiscan optical analyzer. Mol. Pharm. 2019, 16, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Ruiz, R.; Martínez-Rey, L.; Laca, A.; Álvarez, J.R.; Gutiérrez, G.; Matos, M. Enhancing trans-Resveratrol loading capacity by forcing W1/O/W2 emulsions up to its colloidal stability limit. Colloids Surf B Biointerfaces 2020, 193, 111130. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.Z.; Zhao, S.Y.; Ke, X.M.; Lin, J.Z.; Huang, S.S.; Xu, R.C.; Ma, H.-Y.; Zhang, Y.; Han, L.; Zhang, D.K. Study on the stability control strategy of Triphala solution based on the balance of physical stability and chemical stabilities. J. Pharm. Biomed. Anal. 2018, 158, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Su, J.; Yuan, F.; Mao, L.; Gao, Y. Preparation, characterization and stability of pea protein isolate and propylene glycol alginate soluble complexes. LWT 2019, 101, 476–482. [Google Scholar] [CrossRef]
- Alkilany, A.M.; Abulateefeh, S.R.; Mills, K.K.; Bani Yaseen, A.I.; Hamaly, M.A.; Alkhatib, H.S.; Aiedeh, K.M.; Stone, J.W. Colloidal stability of citrate and mercaptoacetic acid capped gold nanoparticles upon lyophilization: Effect of capping ligand attachment and type of cryoprotectants. Langmuir 2014, 30, 13799–13808. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, J.F.; Prestrelski, S.J.; Arakawa, T. Separation of freezing-and drying-induced denaturation of lyophilized proteins using stress-specific stabilization: I. Enzyme activity and calorimetric studies. Arch. Biochem. Biophys. 1993, 303, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, R.; Caputo, O.; Carlotti, M.E.; Trotta, M.; Scarnecchia, C.; Gasco, M.R. Sterilization and freeze-drying of drug-free and drug-loaded solid lipid nanoparticles. Int. J. Pharm. 1997, 148, 47–54. [Google Scholar] [CrossRef]
- Ball, R.L.; Bajaj, P.; Whitehead, K.A. Achieving long-term stability of lipid nanoparticles: Examining the effect of pH, temperature, and lyophilization. Int. J. Nanomed. 2017, 12, 305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saez, A.; Guzman, M.; Molpeceres, J.; Aberturas, M.R. Freeze-drying of polycaprolactone and poly (D, L-lactic-glycolic) nanoparticles induce minor particle size changes affecting the oral pharmacokinetics of loaded drugs. Eur. J. Pharm. Biopharm. 2000, 50, 379–387. [Google Scholar] [CrossRef]
- Turner, S.; Senaratna, T.; Touchell, D.; Bunn, E.; Dixon, K.; Tan, B. Stereochemical arrangement of hydroxyl groups in sugar and polyalcohol molecules as an important factor in effective cryopreservation. Plant Sci. 2001, 160, 489–497. [Google Scholar] [CrossRef]
- Hirsjärvi, S.; Peltonen, L.; Kainu, L.; Hirvonen, J. Freeze-drying of low molecular weight poly (L-lactic acid) nanoparticles: Effect of cryo-and lyoprotectants. J. Nanosci. Nanotechnol. 2006, 6, 3110–3117. [Google Scholar] [CrossRef] [PubMed]
- Almalik, A.; Alradwan, I.; Kalam, M.A.; Alshamsan, A. Effect of cryoprotection on particle size stability and preservation of chitosan nanoparticles with and without hyaluronate or alginate coating. SPJ 2017, 25, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Eliyahu, S.; Almeida, A.; Macedo, M.H.; das Neves, J.; Sarmento, B.; Bianco-Peled, H. The effect of freeze-drying on mucoadhesion and transport of acrylated chitosan nanoparticles. Int. J. Pharm. 2020, 573, 118739. [Google Scholar] [CrossRef]
- El-Say, K.M.; Ahmed, O.A.; Mohamed, A.I.; Safo, M.K.; Omar, A.M. Zein-alpha lipoic acid-loaded nanoparticles to enhance the oral bioavailability of dapoxetine: Optimization and clinical pharmacokinetic evaluation. Int. J. Nanomed. 2019, 14, 7461. [Google Scholar] [CrossRef] [Green Version]
- Nunes, R.; Baião, A.; Monteiro, D.; das Neves, J.; Sarmento, B. Zein nanoparticles as low-cost, safe, and effective carriers to improve the oral bioavailability of resveratrol. Drug Deliv. Transl. Res. 2020, 10, 826–837. [Google Scholar] [CrossRef]
- Anhorn, M.G.; Mahler, H.C.; Langer, K. Freeze drying of human serum albumin (HSA) nanoparticles with different excipients. Int. J. Pharm. 2008, 363, 162–169. [Google Scholar] [CrossRef]
- Abdelwahed, W.; Degobert, G.; Stainmesse, S.; Fessi, H. Freeze-drying of nanoparticles: Formulation, process and storage considerations. Adv. Drug Deliv. Rev. 2006, 58, 1688–1713. [Google Scholar] [CrossRef]
- Gagliardi, A.; Bonacci, S.; Paolino, D.; Celia, C.; Procopio, A.; Fresta, M.; Cosco, D. Paclitaxel-loaded sodium deoxycholate-stabilized zein nanoparticles: Characterization and in vitro cytotoxicity. Heliyon 2019, 5, e02422. [Google Scholar] [CrossRef] [Green Version]
- Hoellein, L.; Holzgrabe, U. Ficts and facts of epinephrine and norepinephrine stability in injectable solutions. Int. J. Pharm. 2012, 434, 468–480. [Google Scholar] [CrossRef]
- Rostami, I.; Alanagh, H.R.; Hu, Z.; Shahmoradian, S.H. Breakthroughs in medicine and bioimaging with up-conversion nanoparticles. Int. J. Nanomed. 2019, 14, 7759. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Composition of the Aqueous Phase | Mean Sizes (nm) | Polydispersity Index (PdI) | Z-Potential (mV) |
|---|---|---|---|
| Zein a | |||
| H2O (MilliQ) | 133 ± 1 | 0.164 ± 0.007 | −34 ± 3 |
| Saline solution 0.9% w/v | 472 ± 243 ** | 0.509 ± 0.22 ** | −7 ± 1 |
| Glucose solution 5% w/v | 127 ± 1 * | 0.137 ± 0.04 | −31 ± 1 |
| PBS 0.01 M pH 7.4 | >1000 ** | 0.870 ± 0.170 ** | −10 ± 2 |
| Gliadin b | |||
| H2O (MilliQ) | 143 ± 3 | 0.128 ± 0.01 | −28 ± 2 |
| Saline solution 0.9% w/v | 794 ± 10 ** | 0.512 ± 0.03 ** | −8 ± 1 |
| Glucose solution 5% w/v | 169 ± 2 * | 0.194 ± 0.01 * | −22 ± 2 |
| PBS 0.01 M pH 7.4 | 784 ± 35 ** | 0.680 ± 0.04 ** | −10 ± 3 |
| Nanoparticles Prepared in MilliQ Water | ||||||
| Medium of Analysis | Zein Nanoparticlesa | Gliadin Nanoparticlesb | ||||
| Mean Sizes (nm) | polydispersity Index (PdI) | Z-Potential (mV) | Mean Sizes (nm) | Polydispersity Index (PdI) | Z-Potential (mV) | |
| H2O (MilliQ) | 133 ± 1 | 0.164 ± 0.010 | −34 ± 2 | 143 ± 3 | 0.128 ± 0.021 | −28 ± 2 |
| Saline solution 0.9% w/v | 232 ± 27 ** | 0.187 ± 0.010 | −13 ± 1 | 200 ± 12 ** | 0.258 ± 0.032 | −7 ± 4 |
| Glucose solution 5% w/v | 161 ± 7 * | 0.236 ± 0.04 | −31 ± 1 | 183 ± 2 * | 0.240 ± 0.025 | −25 ± 2 |
| PBS 0.01 M pH 7.4 | 180 ± 2 * | 0.301 ± 0.037 | −11 ± 1 | 465 ± 82 ** | 0.378 ± 0.035 | −5 ± 1 |
| Nanoparticles Prepared in Glucose Solution (5% w/v) | ||||||
| Medium of Analysis | Zein Nanoparticlesa | Gliadin Nanoparticlesb | ||||
| Mean Sizes (nm) | Polydispersity Index (PdI) | Z-Potential (mV) | Mean Sizes (nm) | Polydispersity Index (PdI) | Z-Potential (mV) | |
| H2O (MilliQ) | 132 ± 1 # | 0.144 ± 0.013 | −33 ± 1 | 221 ± 1 | 0.218 ± 0.006 | −25 ± 2 |
| Saline solution 0.9% w/v | 526 ± 123 ## | 0.154 ± 0.019 | −10 ± 1 | 445 ± 40 ## | 0.193 ± 0.028 | −8 ± 1 |
| Glucose solution 5% w/v | 127 ± 1 * | 0.137 ± 0.04 | −31 ± 3 | 169 ± 2 | 0.194 ± 0.01 | −22 ± 1 |
| PBS 0.01 M pH 7.4 | 257 ± 40 ## | 0.187 ± 0.038 | −12 ± 2 | 337 ± 56 ## | 0.246 ± 0.021 | −6 ± 3 |
| Cryoprotectant Concentration (% w/v) | RDI % | Cryoprotectant Concentration (% w/v) | RDI % | ||
|---|---|---|---|---|---|
| Gliadin (MilliQ water) a | - | 198 ± 10 | Zein (MilliQ water) b | - | 224 ± 11 |
| Glucose 5% | 180 ± 9 | Glucose 5% | 101 ± 5 | ||
| Glucose 10% | 152 ± 8 | Glucose 10% | 249 ± 12 | ||
| Mannose 5% | 187 ± 9 | Mannose 5% | 103 ± 5 | ||
| Mannose 10% | 119 ± 6 | Mannose 10% | 570 ± 28 | ||
| Mannitol 5% | 218 ± 11 | Mannitol 5% | 100 ± 5 | ||
| Mannitol 10% | 156 ± 8 | Mannitol 10% | 108 ± 5 | ||
| Sucrose 5% | 217 ± 11 | Sucrose 5% | 102 ± 5 | ||
| Sucrose 10% | 196 ± 10 | Sucrose 10% | 101 ± 5 | ||
| Trehalose 5% | 305 ± 15 | Trehalose 5% | 104 ± 2 | ||
| Trehalose 10% | 259 ± 16 | Trehalose 10% | 99 ± 5 | ||
| Gliadin (5% w/v glucose solution) a | - | 171 ± 9 | Zein (5% w/v glucose solution) b | - | 187 ± 9 |
| Glucose 5% | 156 ± 8 | Glucose 5% | 103 ± 5 | ||
| Glucose 10% | 115 ± 6 | Glucose 10% | 128 ± 6 | ||
| Mannose 5% | 217 ± 11 | Mannose 5% | 114 ± 6 | ||
| Mannose 10% | 106 ± 5 | Mannose 10% | 106 ± 5 | ||
| Mannitol 5% | 192 ± 10 | Mannitol 5% | 103 ± 5 | ||
| Mannitol 10% | 160 ± 8 | Mannitol 10% | 109 ± 5 | ||
| Sucrose 5% | 164 ± 8 | Sucrose 5% | 110 ± 6 | ||
| Sucrose 10% | 79 ± 4 | Sucrose 10% | 102 ± 5 | ||
| Trehalose 5% | 176 ± 9 | Trehalose 5% | 106 ± 5 | ||
| Trehalose 10% | 141 ± 7 | Trehalose 10% | 101 ± 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voci, S.; Gagliardi, A.; Salvatici, M.C.; Fresta, M.; Cosco, D. Influence of the Dispersion Medium and Cryoprotectants on the Physico-Chemical Features of Gliadin- and Zein-Based Nanoparticles. Pharmaceutics 2022, 14, 332. https://doi.org/10.3390/pharmaceutics14020332
Voci S, Gagliardi A, Salvatici MC, Fresta M, Cosco D. Influence of the Dispersion Medium and Cryoprotectants on the Physico-Chemical Features of Gliadin- and Zein-Based Nanoparticles. Pharmaceutics. 2022; 14(2):332. https://doi.org/10.3390/pharmaceutics14020332
Chicago/Turabian StyleVoci, Silvia, Agnese Gagliardi, Maria Cristina Salvatici, Massimo Fresta, and Donato Cosco. 2022. "Influence of the Dispersion Medium and Cryoprotectants on the Physico-Chemical Features of Gliadin- and Zein-Based Nanoparticles" Pharmaceutics 14, no. 2: 332. https://doi.org/10.3390/pharmaceutics14020332