Surface-Modified Multifunctional Thymol-Loaded Biodegradable Nanoparticles for Topical Acne Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
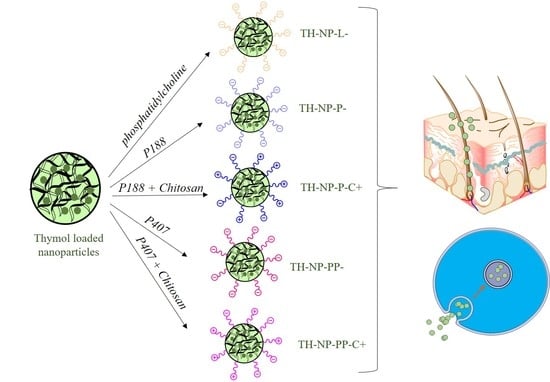
2.2.1. Preparation of Thymol Loaded Nanoparticles
2.2.2. Nanoparticles Physicochemical Characterization
2.2.3. Stability of Thymol Loaded Nanoparticles
2.2.4. Ex Vivo Skin Penetration Route of Thymol Loaded Nanoparticles
2.2.5. Ex Vivo Skin Antioxidant Activity by Methylene Blue Reduction
2.2.6. Free-Radical Scavenging by DPPH
2.2.7. Antimicrobial Efficacy of Thymol Loaded Nanoparticles
2.2.8. Cytotoxicity and Cellular Uptake of Thymol Loaded Nanoparticles
2.2.9. Anti-Inflammatory Activity in TNF-α-Induced Inflammation Model
2.2.10. Anti-Inflammatory Activity in C. acnes-Induced Inflammation Model
2.2.11. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
2.2.12. Antioxidant Activity Assessed by ROS Quantification
2.2.13. Wound Healing Activity in HaCaT Cells by the Scratch Assay
3. Results and Discussion
3.1. Thymol Loaded Nanoparticles Physicochemical Characterization
3.2. Stability of Thymol Loaded Nanoparticles
3.3. Ex Vivo Skin Penetration of Thymol Loaded Nanoparticles
3.4. Ex Vivo Methylene Blue Reduction
3.5. In Vitro Antioxidant Activity
3.6. In Vitro Antimicrobial Efficacy
3.7. Cytotoxicity and Cellular Uptake of Thymol Loaded NPs in HaCaT Cells
3.8. Anti-Inflammatory Activity of Thymol Loaded NPs in HaCaT Cells Treated with TNF-α
3.9. Anti-Inflammatory Activity of Thymol Loaded NPs in HaCaT Cells Treated with C. acnes
3.10. Antioxidant Activity via ROS Quantification in H2O2-Induce H2DCFDALabelled HaCaT
3.11. Wound Healing Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Williams, H.C.; Dellavalle, R.P.; Garner, S. Acne vulgaris. Lancet 2012, 379, 361–372. [Google Scholar] [CrossRef]
- Sachdeva, M.; Tan, J.; Lim, J.; Kim, M.; Nadeem, I.; Bismil, R. The prevalence, risk factors, and psychosocial impacts of acne vulgaris in medical students: A literature review. Int. J. Dermatol. 2021, 60, 792–798. [Google Scholar] [CrossRef]
- Well, D.; Levine, S.R. Acne vulgaris: A review of causes and treatment options. J. Dermatol. Nurses. Assoc. 2014, 6, 302–309. [Google Scholar] [CrossRef]
- Flowers, L.; Grice, E.A. The Skin Microbiota: Balancing Risk and Reward. Cell Host Microbe 2020, 28, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Chlebus, E.; Chlebus, M. Factors affecting the course and severity of adult acne. Observational cohort study. J. Dermatolog. Treat. 2017, 28, 737–744. [Google Scholar] [CrossRef]
- Bansal, P.; Sardana, K.; Vats, G.; Sharma, L.; Garga, U.C.; Khurana, A. A Prospective Study Examining Trigger Factors and Hormonal Abnormalities in Adult Female Acne. Indian Derm. Online J. 2020, 11, 544–550. [Google Scholar] [CrossRef]
- Tanghetti, E.A. The Role of Inflammation in the Pathology of Acne. J. Clin. Aesthetic Dermatol. 2013, 6, 27–35. [Google Scholar]
- Bhatia, A.; Maisonneuve, J.F.; Persing, D.H. Propionibacterium acnes and chronic diseases. In The Infectious Etiology of Chronic Diseases: Defining the Relationship, Enhancing the Research, and Mitigating the Effects: Workshop Summary; Knobler, S.L., O’Connor, S., Lemon, S.M., Eds.; National Academies Press: Washington, DC, USA, 2004. [Google Scholar]
- Platsidaki, E.; Dessinioti, C. Recent advances in understanding Propionibacterium acnes (Cutibacterium acnes) in acne. F1000Research 2018, 7, 1953. [Google Scholar] [CrossRef] [Green Version]
- Rozas, M.; de Ruijter, A.H.; Fabrega, M.J.; Zorgani, A.; Guell, M.; Paetzold, B.; Brillet, F. From dysbiosis to healthy skin: Major contributions of cutibacterium acnes to skin homeostasis. Microorganisms 2021, 9, 628. [Google Scholar] [CrossRef]
- Bharti, S.; Vadlamudi, H.C. A strategic review on the involvement of receptors, transcription factors and hormones in acne pathogenesis. J. Recept. Signal Transduct. 2021, 41, 105–116. [Google Scholar] [CrossRef]
- Kim, J. Review of the innate immune response in acne vulgaris: Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. Dermatology 2005, 211, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.B.; Byun, E.J.; Kim, H.S. Potential Role of the Microbiome in Acne: A Comprehensive Review. J. Clin. Med. 2019, 8, 987. [Google Scholar] [CrossRef] [Green Version]
- Xue, X.; Falcon, D.M. The role of immune cells and cytokines in intestinal wound healing. Int. J. Mol. Sci. 2019, 20, 6097. [Google Scholar] [CrossRef] [Green Version]
- Melnik, B.C. Acne vulgaris: The metabolic syndrome of the pilosebaceous follicle. Clin. Dermatol. 2018, 36, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Katsuta, Y.; Iida, T.; Hasegawa, K.; Inomata, S.; Denda, M. Function of oleic acid on epidermal barrier and calcium influx into keratinocytes is associated with N-methyl d-aspartate-type glutamate receptors. Br. J. Dermatol. 2009, 160, 69–74. [Google Scholar] [CrossRef]
- Dreno, B.; Gollnick, H.P.M.; Kang, S.; Thiboutot, D.; Bettoli, V.; Torres, V.; Leyden, J. Understanding innate immunity and inflammation in acne: Implications for management. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Amiri, H. Essential oils composition and antioxidant properties of three thymus species. Evid.-Based Complement Altern. Med. 2012, 2012, 728065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briganti, S.; Picardo, M. Antioxidant activity, lipid peroxidation and skin diseases. What’s new. J. Eur. Acad. Dermatol. Venereol. 2003, 17, 663–669. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Jourdan, E.; Picardo, M. Acne is an inflammatory disease and alterations of sebum composition initiate acne lesions. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 527–532. [Google Scholar] [CrossRef]
- Trombetta, D.; Castelli, F.; Sarpietro, M.G.; Venuti, V.; Cristani, M.; Daniele, C.; Saija, A.; Mazzanti, G.; Bisignano, G. Mechanisms of antibacterial action of three monoterpenes. Antimicrob. Agents Chemother. 2005, 49, 2474–2478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagoor Meeran, M.F.; Javed, H.; Al Taee, H.; Azimullah, S.; Ojha, S.K. Pharmacological properties and molecular mechanisms of thymol: Prospects for its therapeutic potential and pharmaceutical development. Front. Pharmacol. 2017, 8, 1–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Najafloo, R.; Behyari, M.; Imani, R.; Nour, S. A mini-review of Thymol incorporated materials: Applications in antibacterial wound dressing. J. Drug Deliv. Sci. Technol. 2020, 60, 101904. [Google Scholar] [CrossRef]
- Pivetta, T.P.; Simões, S.; Araújo, M.M.; Carvalho, T.; Arruda, C.; Marcato, P.D. Development of nanoparticles from natural lipids for topical delivery of thymol: Investigation of its anti-inflammatory properties. Colloids Surf. B Biointerfaces 2018, 164, 281–290. [Google Scholar] [CrossRef]
- Pires, F.Q.; Pinho, L.A.; Freire, D.O.; Silva, I.C.R.; Sa-Barreto, L.L.; Cardozo-Filho, L.; Gratieri, T.; Gelfuso, G.M.; Cunha-Filho, M. Thermal analysis used to guide the production of thymol and Lippia origanoides essential oil inclusion complexes with cyclodextrin. J. Therm. Anal. Calorim. 2019, 137, 543–553. [Google Scholar] [CrossRef]
- Tao, F.; Hill, L.E.; Peng, Y.; Gomes, C.L. Synthesis and characterization of β-cyclodextrin inclusion complexes of thymol and thyme oil for antimicrobial delivery applications. LWT-Food Sci. Technol. 2014, 59, 247–255. [Google Scholar] [CrossRef]
- Sáez-Orviz, S.; Marcet, I.; Weng, S.; Rendueles, M.; Díaz, M. PLA nanoparticles loaded with thymol to improve its incorporation into gelatine films. J. Food Eng. 2020, 269, 1–7. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Esteruelas, G.; Ortiz, A.; Espina, M.; Prat, J.; Muñoz, M.; Cano, A.; Calpena, A.C.; Ettcheto, M.; Camins, A.; et al. Dexibuprofen biodegradable nanoparticles: One step closer towards a better ocular interaction study. Nanomaterials 2020, 10, 720. [Google Scholar] [CrossRef] [Green Version]
- Mengoni, T.; Adrian, M.; Pereira, S.; Santos-Carballal, B.; Kaiser, M.; Goycoolea, F.M. A chitosan-based liposome formulation enhances the in vitro wound healing efficacy of substance P neuropeptide. Pharmaceutics 2017, 9, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chronopoulou, L.; Massimi, M.; Giardi, M.F.; Cametti, C.; Devirgiliis, L.C.; Dentini, M.; Palocci, C. Chitosan-coated PLGA nanoparticles: A sustained drug release strategy for cell cultures. Colloids Surf. B Biointerfaces 2013, 103, 310–317. [Google Scholar] [CrossRef]
- Erös, G.; Ibrahim, S.; Siebert, N.; Boros, M.; Vollmar, B. Oral phosphatidylcholine pretreatment alleviates the signs of experimental rheumatoid arthritis. Arthritis Res. Ther. 2009, 11, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Hunter, R.L.; Luo, A.Z.; Zhang, R.; Kozar, R.A.; Moore, F.A. Poloxamer 188 inhibition of ischemia/reperfusion injury: Evidence for a novel anti-adhesive mechanism. Ann. Clin. Lab. Sci. 2010, 40, 115–125. [Google Scholar] [PubMed]
- Moghimi, S.M.; Hunter, A.C. Poloxamers and poloxamines in nanoparticle engineering and experimental medicine. Trends Biotechnol. 2000, 18, 412–420. [Google Scholar] [CrossRef]
- Fessi, H.; Puisieux, F.; Devissaguet, J.P.; Ammoury, N.; Benita, S. Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int. J. Pharm. 1989, 55, R1–R4. [Google Scholar] [CrossRef]
- Ghasemi Pirbalouti, A.; Rahimmalek, M.; Malekpoor, F.; Karimi, A. Variation in antibacterial activity, thymol and carvacrol contents of wild populations of Thymus daenensis subsp. daenensis Celak. Plant Omics 2011, 4, 209–214. [Google Scholar]
- Sánchez-López, E.; Egea, M.A.; Cano, A.; Espina, M.; Calpena, A.C.; Ettcheto, M.; Camins, A.; Souto, E.B.; Silva, A.M.; García, M.L. PEGylated PLGA nanospheres optimized by design of experiments for ocular administration of dexibuprofen-in vitro, ex vivo and in vivo characterization. Colloids Surf. B Biointerfaces 2016, 145, 241–250. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Pizarro, R.; Parrotta, G.; Vera, R.; Sánchez-López, E.; Galindo, R.; Kjeldsen, F.; Badia, J.; Baldoma, L.; Espina, M.; García, M.L. Ocular penetration of fluorometholone-loaded PEG-PLGA nanoparticles functionalized with cell-penetrating peptides. Nanomedicine 2019, 14, 3089–3104. [Google Scholar] [CrossRef]
- Fernández-García, E.; Heluani-Gahete, H.; Wellinger, R.E. A new colorimetric assay for antioxidant capacity and photostability. Color. Technol. 2016, 132, 195–200. [Google Scholar] [CrossRef]
- Aman, S.; Moin, S.; Owais, M.; Siddiqui, M.U. Antioxidant activity of thymol: Protective role in AAPH-induced hemolysis in diabetic erythrocytes. Int. J. Pharm. Sci. Invent. 2013, 2, 55–60. [Google Scholar]
- Messager, S.; Goddard, P.A.; Dettmar, P.W.; Maillard, J.Y. Determination of the antibacterial efficacy of several antiseptics tested on skin by an “ex-vivo” test. J. Med. Microbiol. 2001, 50, 284–292. [Google Scholar] [CrossRef] [Green Version]
- Diaz-Garrido, N.; Fábrega, M.J.; Vera, R.; Giménez, R.; Badia, J.; Baldomà, L. Membrane vesicles from the probiotic Nissle 1917 and gut resident Escherichia coli strains distinctly modulate human dendritic cells and subsequent T cell responses. J. Funct. Foods 2019, 61, 103495. [Google Scholar] [CrossRef]
- Carvajal-Vidal, P.; Fábrega, M.J.; Espina, M.; Calpena, A.C.; García, M.L. Development of Halobetasol-loaded nanostructured lipid carrier for dermal administration: Optimization, physicochemical and biopharmaceutical behavior, and therapeutic efficacy. Nanomed. Nanotechnol. Biol. Med. 2019, 20, 102026. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Wu, W.; Yang, S.; Li, D.; Sun, D.; He, L. Polyphyllin I Inhibits Propionibacterium acnes-Induced Inflammation In Vitro. Inflammation 2019, 42, 35–44. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.H.; Lin, Y.S.; Huang, Y.W.; Fang, S.U.; Lin, S.Y.; Hou, W.C. Protective Effects of Minor Components of Curcuminoids on Hydrogen Peroxide-Treated Human HaCaT Keratinocytes. J. Agric. Food Chem. 2016, 64, 3598–3608. [Google Scholar] [CrossRef]
- Governa, P.; Carullo, G.; Biagi, M.; Rago, V.; Aiello, F. Evaluation of the in vitro wound-healing activity of calabrian honeys. Antioxidants 2019, 8, 36. [Google Scholar] [CrossRef] [Green Version]
- Jangpromma, N.; Preecharram, S.; Srilert, T.; Maijaroen, S.; Mahakunakorn, P.; Nualkaew, N.; Daduang, S.; Klaynongsruang, S. In vitro and in vivo wound healing properties of plasma and serum from Crocodylus siamensis blood. J. Microbiol. Biotechnol. 2016, 26, 1140–1147. [Google Scholar] [CrossRef]
- Vega, E.; Egea, M.A.; Garduño-Ramírez, M.L.; García, M.L.; Sánchez, E.; Espina, M.; Calpena, A.C. Flurbiprofen PLGA-PEG nanospheres: Role of hydroxy-β-cyclodextrin on ex vivo human skin permeation and in vivo topical anti-inflammatory efficacy. Colloids Surf. B Biointerfaces 2013, 110, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-López, E.; Ettcheto, M.; Egea, M.A.; Espina, M.; Cano, A.; Calpena, A.C.; Camins, A.; Carmona, N.; Silva, A.M.; Souto, E.B.; et al. Memantine loaded PLGA PEGylated nanoparticles for Alzheimer’s disease: In vitro and in vivo characterization. J. Nanobiotechnol. 2018, 16, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-López, E.; Ettcheto, M.; Egea, M.A.; Espina, M.; Calpena, A.C.; Folch, J.; Camins, A.; García, M.L. New potential strategies for Alzheimer’s disease prevention: Pegylated biodegradable dexibuprofen nanospheres administration to APPswe/PS1dE9. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1171–1182. [Google Scholar] [CrossRef]
- Kim, J.Y.; Oh, S.; Yi, B.; Kim, M.J.; Lee, J.H. Synergism of phosphatidylcholine on the antioxidant properties of α-tocopherol in corn oils under different relative humidity. Int. J. Food Sci. Technol. 2015, 50, 1421–1428. [Google Scholar] [CrossRef]
- Yukuyama, M.N.; De Araújo, G.L.B.; Bou-Chacra, N.A. Nanomaterials for hair care applications. Nanocosmetics 2020, 205–225. [Google Scholar] [CrossRef]
- Santos, G.A.; Angelo, T.; Andrade, L.M.; Silva, S.M.M.; Magalhães, P.O.; Cunha-Filho, M.; Gelfuso, G.M.; Taveira, S.F.; Gratieri, T. The role of formulation and follicular pathway in voriconazole cutaneous delivery from liposomes and nanostructured lipid carriers. Colloids Sur. B Biointerfaces 2018, 170, 341–346. [Google Scholar] [CrossRef]
- Pereira, M.N.; Schulte, H.L.; Duarte, N.; Lima, E.M.; Sá-Barreto, L.L.; Gratieri, T.; Gelfuso, G.M.; Cunha-Filho, M.S.S. Solid effervescent formulations as new approach for topical minoxidil delivery. Eur. J. Pharm. Sci. 2017, 96, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Román, R.; Naik, A.; Kalia, Y.N.; Guy, R.H.; Fessi, H. Skin penetration and distribution of polymeric nanoparticles. J. Control. Release 2004, 99, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Mollarafie, P.; Khadiv Parsi, P.; Zarghami, R.; Amini Fazl, M.; Ghafarzadegan, R. Antibacterial and wound healing properties of thymol (Thymus vulgaris Oil) and its application in a novel wound dressing. J. Med. Plants 2015, 14, 69–81. [Google Scholar]
- Pires, F.Q.; Angelo, T.; Silva, J.K.R.; Sá-Barreto, L.C.L.; Lima, E.M.; Gelfuso, G.M.; Gratieri, T.; Cunha-Filho, M.S.S. Use of mixture design in drug-excipient compatibility determinations: Thymol nanoparticles case study. J. Pharm. Biomed. Anal. 2017, 137, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Tolentino, S.; Pereira, M.N.; de Sousa, M.C.; Cunha-Filho, M.; Gelfuso, G.M.; Gratieri, T. The influence of sebaceous content on the performance of nanosystems designed for the treatment of follicular diseases. J. Drug Deliv. Sci. Technol. 2020, 59, 101895. [Google Scholar] [CrossRef]












| Zav ± SD (nm) | PI ± SD | ZP ± SD (mV) | pH ± SD | |
|---|---|---|---|---|
| NPP-P- | 180.2 ± 6.5 | 0.066 ± 0.037 | −28.9 ± 1.0 | 4.15 ± 0.10 |
| NPP-P-C+ | 316.5 ± 3.8 | 0.123 ± 0.035 | 43.4 ± 0.6 | 3.15 ± 0.10 |
| NPP-PP- | 206.9 ± 9.9 | 0.101 ± 0.041 | −17.8 ± 0.7 | 4.29 ± 0.10 |
| NPP-PP-C+ | 297.3 ± 22.9 | 0.150 ± 0.068 | 19.6 ± 0.8 | 3.16 ± 0.10 |
| NPP-L- | 235.8 ± 29.7 | 0.063 ± 0.018 | −32.1 ± 0.2 | 4.08 ± 0.10 |
| Month | Zav (nm) ± SD | PI ± SD | ZP ± SD (mV) | |
|---|---|---|---|---|
| NPP-P- | 0 | 172.9 ± 1.9 | 0.066 ± 0.037 | −24.5 ± 0.9 |
| 1 | 177.7 ± 2.2 | 0.071 ± 0.015 | −20.9 ± 0.5 | |
| 3 | 183.8 ± 3.6 | 0.082 ± 0.005 | −18.3 ± 0.7 | |
| 6 | 191.5 ± 1.5 | 0.091 ± 0.015 | −14.3 ± 0.6 | |
| NPP-P-C+ | 0 | 337.3 ± 7.4 | 0.123 ± 0.035 | 23.6 ± 0.3 |
| 1 | 365.4 ± 3.2 | 0.141 ± 0.011 | 23.2 ± 0.9 | |
| 3 | 392.9 ± 8.0 | 0.158 ± 0.037 | 21.0 ± 0.3 | |
| 6 | 419.6 ± 11.5 | 0.197 ± 0.066 | 17.2 ± 0.2 | |
| NPP-PP- | 0 | 184.0 ± 0.9 | 0.101 ± 0.041 | −22.2 ± 0.6 |
| 1 | 191.2 ± 0.7 | 0.098 ± 0.022 | −18.2 ± 0.6 | |
| 3 | 189.1 ± 10.1 | 0.099 ± 0.015 | −12.1 ± 0.4 | |
| 6 | 220.0 ± 10.8 | 0.123 ± 0.33 | −8.4 ± 0.7 | |
| NPP-PP-C+ | 0 | 221.1 ± 3.3 | 0.149 ± 0.036 | 10.5 ± 0.6 |
| 1 | 224.1 ± 5.3 | 0.150 ± 0.068 | 9.8 ± 0.6 | |
| 3 | 256.1 ± 5.3 | 0.152 ± 0.077 | 7.3 ± 0.7 | |
| 6 | 348.5 ± 17.2 | 0.189 ± 0.023 | 6.1 ± 0.5 | |
| NPP-L- | 0 | 178.5 ± 0.6 | 0.063 ± 0.018 | −41.2 ± 1.8 |
| 1 | 180.9 ± 1.4 | 0.076 ± 0.019 | −39.7 ± 0.5 | |
| 3 | 214.7 ± 2.7 | 0.114 ± 0.032 | −29.7 ± 0.5 | |
| 6 | 201.9 ± 3.4 | 0.132 ± 0.027 | −23.9 ± 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Folle, C.; Díaz-Garrido, N.; Sánchez-López, E.; Marqués, A.M.; Badia, J.; Baldomà, L.; Espina, M.; Calpena, A.C.; García, M.L. Surface-Modified Multifunctional Thymol-Loaded Biodegradable Nanoparticles for Topical Acne Treatment. Pharmaceutics 2021, 13, 1501. https://doi.org/10.3390/pharmaceutics13091501
Folle C, Díaz-Garrido N, Sánchez-López E, Marqués AM, Badia J, Baldomà L, Espina M, Calpena AC, García ML. Surface-Modified Multifunctional Thymol-Loaded Biodegradable Nanoparticles for Topical Acne Treatment. Pharmaceutics. 2021; 13(9):1501. https://doi.org/10.3390/pharmaceutics13091501
Chicago/Turabian StyleFolle, Camila, Natalia Díaz-Garrido, Elena Sánchez-López, Ana Maria Marqués, Josefa Badia, Laura Baldomà, Marta Espina, Ana Cristina Calpena, and María Luisa García. 2021. "Surface-Modified Multifunctional Thymol-Loaded Biodegradable Nanoparticles for Topical Acne Treatment" Pharmaceutics 13, no. 9: 1501. https://doi.org/10.3390/pharmaceutics13091501