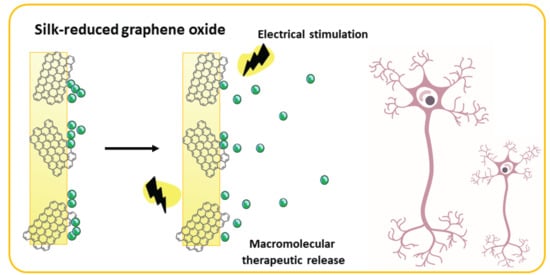
Electroresponsive Silk-Based Biohybrid Composites for Electrochemically Controlled Growth Factor Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Regenerated Silk Fibroin
2.2. Preparation of Electroconductive Biohybrid Composite Films
2.3. Characterization of the Electroconductive Biohybrid Composites
2.4. In Vitro NGF-β Loading and Release Study
2.4.1. Electrochemical Loading
2.4.2. NGF-β Release
2.4.3. NGF-β Quantification
2.5. In Silico Studies
2.6. Data Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Shah, S.A.A.; Firlak, M.; Berrow, S.R.; Halcovitch, N.R.; Baldock, S.J.; Yousafzai, B.M.; Hathout, R.M.; Hardy, J.G. Electrochemically enhanced drug delivery using polypyrrole films. Materials 2018, 11, 1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tibbitt, M.W.; Rodell, C.B.; Burdick, J.A.; Anseth, K.S. Progress in material design for biomedical applications. PNAS 2015, 112, 14444–14451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashton, M.D.; Hardy, J.G. Progress in active ingredient formulations: Towards smart stimuli-responsive formulations. Johnson Matthey Technol. Rev. 2019, 63, 211–225. [Google Scholar] [CrossRef]
- Tandon, B.; Magaz, A.; Balint, R.; Blaker, J.J.; Cartmell, S.H. Electroactive biomaterials: Vehicles for controlled delivery of therapeutic agents for drug delivery and tissue regeneration. Adv. Drug Deliv. Rev. 2018, 129, 148–168. [Google Scholar] [CrossRef] [Green Version]
- Ashton, M.D.; Appen, I.C.; Firlak, M.; Stanhope, N.E.; Schmidt, C.E.; Eisenstadt, W.R.; Hur, B.; Hardy, J.G. Wirelessly triggered bioactive molecule delivery from degradable electroactive polymer films. Polym. Int. 2018. [Google Scholar] [CrossRef]
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Conductive polymers: Towards a smart biomaterial for tissue engineering. Acta Biomater. 2014, 10, 2341–2353. [Google Scholar] [CrossRef]
- Guimard, N.K.; Gomez, N.; Schmidt, C.E. Conducting polymers in biomedical engineering. Prog. Polym. Sci. 2007, 32, 876–921. [Google Scholar] [CrossRef]
- Tseghai, G.B.; Mengistie, D.A.; Malengier, B.; Fante, K.A.; Van Langenhove, L. PEDOT:PSS-based conductive textiles and their applications. Sensors 2020, 20, 1881. [Google Scholar] [CrossRef] [Green Version]
- Magaz, A.; Faroni, A.; Gough, J.E.; Reid, A.J.; Li, X.; Blaker, J.J. Bioactive silk-based nerve guidance conduits for augmenting peripheral nerve repair. Adv. Healthc. Mater. 2018, 7, 1800308. [Google Scholar] [CrossRef] [Green Version]
- Castro Neto, A.H.; Guinea, F.; Peres, N.M.R.; Novoselov, K.S.; Geim, A.K. The electronic properties of graphene. Rev. Mod. Phys. 2009, 81, 109–162. [Google Scholar] [CrossRef] [Green Version]
- Burnstine-Townley, A.; Eshel, Y.; Amdursky, N. Conductive scaffolds for cardiac and neuronal tissue engineering: Governing factors and mechanisms. Adv. Funct. Mater. 2020, 30, 1901369. [Google Scholar] [CrossRef]
- Palza, H.; Zapata, P.A.; Angulo-Pineda, C. Electroactive smart polymers for biomedical applications. Materials 2019, 12, 277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, R.; Zhang, S.; Xiao, M.; Qian, F.; He, Z.; Li, D.; Zhang, X.; Li, H.; Yang, X.; Wang, M.; et al. Accelerating bioelectric functional development of neural stem cells by graphene coupling: Implications for neural interfacing with conductive materials. Biomaterials 2016, 106, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.J.; Kearney, C.J.; Shen, N.; Khan, U.; Kelly, A.G.; Probst, C.; Brauchle, E.; Biccai, S.; Garciarena, C.D.; Vega-Mayoral, V.; et al. Electroconductive biohybrid collagen/pristine graphene composite biomaterials with enhanced biological activity. Adv. Mater. 2018, 30, 1706442. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Lombardi, R.; Phan, T.G.; Zimmermann, C.; Lowe, D.; Jermutus, L.; Christ, D. Challenges and opportunities for non-antibody scaffold drugs. Drug Discov. Today 2015, 20, 1271–1283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carbinatto, F.M.; de Castro, A.D.; Evangelista, R.C.; Cury, B.S.F. Insights into the swelling process and drug release mechanisms from cross-linked pectin/high amylose starch matrices. Asian J. Pharm. Sci. 2014, 9, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Azad, N.; Rojanasakul, Y. Macromolecular Drug Delivery. In Biopharmaceutical Drug Design and Development; Wu-Pong, S., Rojanasakul, Y., Eds.; Humana Press: Totowa, NJ, USA, 2008; pp. 293–323. ISBN 978-1-59745-532-9. [Google Scholar]
- Mousavi, S.T.; Harper, G.R.; Municoy, S.; Ashton, M.D.; Townsend, D.; Alsharif, G.H.K.; Oikonomou, V.K.; Firlak, M.; Au-Yong, S.; Murdock, B.E.; et al. Electroactive silk fibroin films for electrochemically enhanced delivery of drugs. Macromol. Mater. Eng. 2020, 305, 2000130. [Google Scholar] [CrossRef]
- Servant, A.; Leon, V.; Jasim, D.; Methven, L.; Limousin, P.; Fernandez-Pacheco, E.V.; Prato, M.; Kostarelos, K. Graphene-based electroresponsive scaffolds as polymeric implants for on-demand drug delivery. Adv. Healthc. Mater. 2014, 3, 1334–1343. [Google Scholar] [CrossRef]
- Weaver, C.L.; LaRosa, J.M.; Luo, X.; Cui, X.T. Electrically controlled drug delivery from graphene oxide nanocomposite films. ACS Nano 2014, 8, 1834–1843. [Google Scholar] [CrossRef]
- Dang, M.; Saunders, L.; Niu, X.; Fan, Y.; Ma, P.X. Biomimetic delivery of signals for bone tissue engineering. Bone Res. 2018, 6, 1–12. [Google Scholar] [CrossRef]
- Gaudin, R.; Knipfer, C.; Henningsen, A.; Smeets, R.; Heiland, M.; Hadlock, T. Approaches to peripheral nerve repair: Generations of biomaterial conduits yielding to replacing autologous nerve grafts in craniomaxillofacial surgery. Biomed. Res. Int. 2016, 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kehoe, S.; Zhang, X.F.; Boyd, D. FDA approved guidance conduits and wraps for peripheral nerve injury: A review of materials and efficacy. Injury 2012, 43, 553–572. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Chen, H.; Qing, L.; Yang, X.; Jia, X. Biomimetic neural scaffolds: A crucial step towards optimal peripheral nerve regeneration. Biomater. Sci. 2018, 6, 1299–1311. [Google Scholar] [CrossRef] [PubMed]
- Lackington, W.A.; Ryan, A.J.; O’Brien, F.J. Advances in nerve guidance conduit-based therapeutics for peripheral nerve repair. ACS Biomater. Sci. Eng. 2017, 3, 1221–1235. [Google Scholar] [CrossRef]
- Kasper, M.; Deister, C.; Beck, F.; Schmidt, C.E. Bench-to-bedside lessons learned: Commercialization of an acellular nerve graft. Adv. Healthc. Mater. 2020, 2000174. [Google Scholar] [CrossRef]
- Levi, M.S.; Brimble, M.A. A review of neuroprotective agents. Curr. Med. Chem. 2004, 11, 2383–2397. [Google Scholar] [CrossRef]
- Mekaj, A.; Mekaj, Y. The role of pharmacological agents in nerve regeneration after peripheral nerve repair. Peripher. Nerve Regen. Surg. New Ther. Approaches Incl. Biomater. Cell-Based Ther. Dev. 2017. [Google Scholar] [CrossRef]
- Zhang, N.; Milbreta, U.; Chin, J.S.; Pinese, C.; Lin, J.; Shirahama, H.; Jiang, W.; Liu, H.; Mi, R.; Hoke, A.; et al. Biomimicking fiber scaffold as an effective in vitro and in vivo microrna screening platform for directing tissue regeneration. Adv. Sci. 2019, 6, 1800808. [Google Scholar] [CrossRef]
- Liu, C.; Wang, C.; Zhao, Q.; Li, X.; Xu, F.; Yao, X.; Wang, M. Incorporation and release of dual growth factors for nerve tissue engineering using nanofibrous bicomponent scaffolds. Biomed. Mater. 2018, 13, 044107. [Google Scholar] [CrossRef] [Green Version]
- Aloe, L.; Rocco, M.L.; Bianchi, P.; Manni, L. Nerve growth factor: From the early discoveries to the potential clinical use. J. Transl. Med. 2012, 10, 239. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.Y.; Lee, J.-W.; Schmidt, C.E. Neuroactive conducting scaffolds: Nerve growth factor conjugation on active ester-functionalized polypyrrole. J. R. Soc. Interface 2009, 6, 801–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magaz, A.; Roberts, A.D.; Faraji, S.; Nascimento, T.R.L.; Medeiros, E.S.; Zhang, W.; Greenhalgh, R.D.; Mautner, A.; Li, X.; Blaker, J.J. Porous, aligned, and biomimetic fibers of regenerated silk fibroin produced by solution blow spinning. Biomacromolecules 2018, 19, 4542–4553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitra, M.; Chatterjee, K.; Kargupta, K.; Ganguly, S.; Banerjee, D. Reduction of graphene oxide through a green and metal-free approach using formic acid. Diam. Relat. Mater. 2013, 37, 74–79. [Google Scholar] [CrossRef]
- He, L.; Tjong, S.C. Low percolation threshold of graphene/polymer composites prepared by solvothermal reduction of graphene oxide in the polymer solution. Nanoscale Res. Lett. 2013, 8, 132. [Google Scholar] [CrossRef] [Green Version]
- Ren, P.-G.; Yan, D.-X.; Ji, X.; Chen, T.; Li, Z.-M. Temperature dependence of graphene oxide reduced by hydrazine hydrate. Nanotechnology 2011, 22, 055705. [Google Scholar] [CrossRef]
- Park, S.; An, J.; Potts, J.R.; Velamakanni, A.; Murali, S.; Ruoff, R.S. Hydrazine-reduction of graphite- and graphene oxide. Carbon 2011, 49, 3019–3023. [Google Scholar] [CrossRef]
- Roshanbinfar, K.; Vogt, L.; Ruther, F.; Roether, J.A.; Boccaccini, A.R.; Engel, F.B. Nanofibrous composite with tailorable electrical and mechanical properties for cardiac tissue engineering. Adv. Funct. Mater. 2020, 30, 1908612. [Google Scholar] [CrossRef] [Green Version]
- Um, I.C.; Kweon, H.; Park, Y.H.; Hudson, S. Structural characteristics and properties of the regenerated silk fibroin prepared from formic acid. Int. J. Biol. Macromol. 2001, 29, 91–97. [Google Scholar] [CrossRef]
- Kucinska-Lipka, J.; Marzec, M.; Gubanska, I.; Janik, H. Porosity and swelling properties of novel polyurethane–ascorbic acid scaffolds prepared by different procedures for potential use in bone tissue engineering. J. Elastom. Plast. 2017, 49, 440–456. [Google Scholar] [CrossRef]
- Martins, A.M.; Eng, G.; Caridade, S.G.; Mano, J.F.; Reis, R.L.; Vunjak-Novakovic, G. Electrically conductive chitosan/carbon scaffolds for cardiac tissue engineering. Biomacromolecules 2014, 15, 635–643. [Google Scholar] [CrossRef]
- Amdursky, N.; Wang, X.; Meredith, P.; Bradley, D.D.C.; Stevens, M.M. Long-range proton conduction across free-standing serum albumin mats. Adv. Mater. 2016, 28, 2692–2698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, C.; Deng, Y.; Roudsari, A.F.; Kapetanovic, A.; Anantram, M.P.; Rolandi, M. A polysaccharide bioprotonic field-effect transistor. Nat. Commun. 2011, 2, 476. [Google Scholar] [CrossRef] [PubMed]
- Ordinario, D.D.; Phan, L.; Walkup, W.G.; Jocson, J.-M.; Karshalev, E.; Hüsken, N.; Gorodetsky, A.A. Bulk protonic conductivity in a cephalopod structural protein. Nat. Chem. 2014, 6, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Josberger, E.; Jin, J.; Roudsari, A.F.; Rousdari, A.F.; Helms, B.A.; Zhong, C.; Anantram, M.P.; Rolandi, M. H+-type and OH- -type biological protonic semiconductors and complementary devices. Sci. Rep. 2013, 3, 2481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pillay, V.; Tsai, T.-S.; Choonara, Y.E.; du Toit, L.C.; Kumar, P.; Modi, G.; Naidoo, D.; Tomar, L.K.; Tyagi, C.; Ndesendo, V.M.K. A review of integrating electroactive polymers as responsive systems for specialized drug delivery applications. J. Biomed. Mater. Res. A 2014, 102, 2039–2054. [Google Scholar] [CrossRef]
- Vadlapatla, R.; Wong, E.Y.; Gayakwad, S.G. Electronic drug delivery systems: An overview. J. Drug Deliv. Sci. Tec. 2017, 41, 359–366. [Google Scholar] [CrossRef]
- El Zein, A.; Huppé, C.; Cochrane, C. Development of a flexible strain sensor based on PEDOT:PSS for thin film structures. Sensors 2017, 17, 1337. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Lu, C.; Li, Y.; Wu, F.; Zhao, B.; Dong, X. Green fabrication of porous silk fibroin/graphene oxide hybrid scaffolds for bone tissue engineering. RSC Adv. 2015, 5, 78660–78668. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, Z.; Gu, J.; Zhou, W.; Liang, X.; Zhou, G.; Han, C.C.; Xu, S.; Liu, Y. Mechanism of a long-term controlled drug release system based on simple blended electrospun fibers. J. Control. Release 2020, 320, 337–346. [Google Scholar] [CrossRef]
- Ou, L.; Song, B.; Liang, H.; Liu, J.; Feng, X.; Deng, B.; Sun, T.; Shao, L. Toxicity of graphene-family nanoparticles: A general review of the origins and mechanisms. Part. Fibre Toxicol. 2016, 13, 57. [Google Scholar] [CrossRef] [Green Version]
- Fadeel, B.; Bussy, C.; Merino, S.; Vázquez, E.; Flahaut, E.; Mouchet, F.; Evariste, L.; Gauthier, L.; Koivisto, A.J.; Vogel, U.; et al. Safety assessment of graphene-based materials: Focus on human health and the environment. ACS Nano 2018, 12, 10582–10620. [Google Scholar] [CrossRef]
- Macmillan, D.S.; Chilton, M.L. A defined approach for predicting skin sensitisation hazard and potency based on the guided integration of in silico, in chemico and in vitro data using exclusion criteria. Regul. Toxicol. Pharmacol. 2019, 101, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Thirupathi, R.; Rao, L.P.; Atreya, H.S. Unraveling the dynamic nature of protein–graphene oxide interactions. RSC Adv. 2016, 6, 52539–52548. [Google Scholar] [CrossRef]
- Pelin, M.; Fusco, L.; León, V.; Martín, C.; Criado, A.; Sosa, S.; Vázquez, E.; Tubaro, A.; Prato, M. Differential cytotoxic effects of graphene and graphene oxide on skin keratinocytes. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelin, M.; Fusco, L.; Martín, C.; Sosa, S.; Frontiñán-Rubio, J.; González-Domínguez, J.M.; Durán-Prado, M.; Vázquez, E.; Prato, M.; Tubaro, A. Graphene and graphene oxide induce ROS production in human HaCaT skin keratinocytes: The role of xanthine oxidase and NADH dehydrogenase. Nanoscale 2018, 10, 11820–11830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fusco, L.; Garrido, M.; Martín, C.; Sosa, S.; Ponti, C.; Centeno, A.; Alonso, B.; Zurutuza, A.; Vázquez, E.; Tubaro, A.; et al. Skin irritation potential of graphene-based materials using a non-animal test. Nanoscale 2020, 12, 610–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shvedova, A.A.; Castranova, V.; Kisin, E.R.; Schwegler-Berry, D.; Murray, A.R.; Gandelsman, V.Z.; Maynard, A.; Baron, P. Exposure to carbon nanotube material: Assessment of nanotube cytotoxicity using human keratinocyte cells. J. Toxicol. Environ. Health A 2003, 66, 1909–1926. [Google Scholar] [CrossRef] [PubMed]
- Surfraz, M.B.; Fowkes, A.; Plante, J.P. A semi-automated approach to create purposeful mechanistic datasets from heterogeneous data: Data mining towards the in silico predictions for oestrogen receptor modulation and teratogenicity. Mol. Inform. 2017, 36, 1600154. [Google Scholar] [CrossRef] [PubMed]
- de Menezes, F.D.; dos Reis, S.R.R.; Pinto, S.R.; Portilho, F.L.; do Vale Chaves e Mello, F.; Helal-Neto, E.; da Silva de Barros, A.O.; Alencar, L.M.R.; de Menezes, A.S.; dos Santos, C.C.; et al. Graphene quantum dots unraveling: Green synthesis, characterization, radiolabeling with 99mTc, in vivo behavior and mutagenicity. Mater. Sci. Eng. C 2019, 102, 405–414. [Google Scholar] [CrossRef]
- Evariste, L.; Lagier, L.; Gonzalez, P.; Mottier, A.; Mouchet, F.; Cadarsi, S.; Lonchambon, P.; Daffe, G.; Chimowa, G.; Sarrieu, C.; et al. Thermal reduction of graphene oxide mitigates its in vivo genotoxicity toward xenopus laevis tadpoles. Nanomaterials 2019, 9, 584. [Google Scholar] [CrossRef] [Green Version]
- Bengtson, S.; Knudsen, K.B.; Kyjovska, Z.O.; Berthing, T.; Skaug, V.; Levin, M.; Koponen, I.K.; Shivayogimath, A.; Booth, T.J.; Alonso, B.; et al. Differences in inflammation and acute phase response but similar genotoxicity in mice following pulmonary exposure to graphene oxide and reduced graphene oxide. PLoS ONE 2017, 12, e0178355. [Google Scholar] [CrossRef] [PubMed] [Green Version]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magaz, A.; Ashton, M.D.; Hathout, R.M.; Li, X.; Hardy, J.G.; Blaker, J.J. Electroresponsive Silk-Based Biohybrid Composites for Electrochemically Controlled Growth Factor Delivery. Pharmaceutics 2020, 12, 742. https://doi.org/10.3390/pharmaceutics12080742
Magaz A, Ashton MD, Hathout RM, Li X, Hardy JG, Blaker JJ. Electroresponsive Silk-Based Biohybrid Composites for Electrochemically Controlled Growth Factor Delivery. Pharmaceutics. 2020; 12(8):742. https://doi.org/10.3390/pharmaceutics12080742
Chicago/Turabian StyleMagaz, Adrián, Mark D. Ashton, Rania M. Hathout, Xu Li, John G. Hardy, and Jonny J. Blaker. 2020. "Electroresponsive Silk-Based Biohybrid Composites for Electrochemically Controlled Growth Factor Delivery" Pharmaceutics 12, no. 8: 742. https://doi.org/10.3390/pharmaceutics12080742