Effects of Starch Incorporation on the Physicochemical Properties and Release Kinetics of Alginate-Based 3D Hydrogel Patches for Topical Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Identification of Quality Target Product Profile (QTPP) and Critical Quality Attributes (CQAs)
2.2.2. Risk Analysis of CQAs
2.2.3. Alginate-Starch Hydrogel Inks
2.2.4. Physicochemical Characterization of Alginate-Starch Hydrogel Inks
2.2.5. 3D Printing Process
2.2.6. Formula Optimization and Establishment of Design Space
2.2.7. Hydrogel 3D Patches Characterization
Gelation Time, Porosity, and Construct Integrity
Spreadability
Physicochemical Characterization
2.2.8. In vitro Release Studies
- (1)
- Zero-order kineticswhere K0 is the zero-order release constant
- (2)
- First-order kineticswhere K1 is the first-order release constant.
- (3)
- Higuchi modelwhere KH is the Higuchi release constant.
- (4)
- Korsmeyer–Peppas modelwhere KKP is the release constant incorporating structural and geometric characteristics of the drug-dosage form and n is the diffusional exponent indicating the drug-release mechanism.
- (5)
- Weibull modelwhere α defines the time scale of the process; represents the lag time before the onset of the dissolution or release process; and β is the shape parameter which characterizes the curve as either exponential (β = 1), sigmoid, S-shaped, with upward curvature followed by a turning point (β > 1), or parabolic, with a higher initial slope and after that consistent with the exponential (β < 1).
3. Results and Discussion
3.1. Risk Analysis of CQAs
3.1.1. Process Mapping
3.1.2. Risk Estimation Matrix (REM)
3.1.3. Adjustment of CPPs
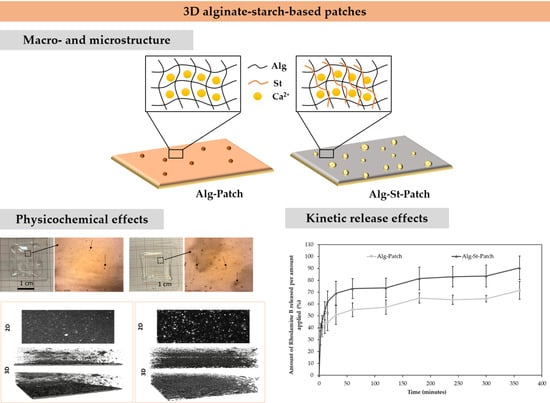
3.2. Effect of Starch on Physicochemical Properties of the Alginate-Starch-Based Inks
3.3. Formula Optimization and Establishment of Design Space
3.4. Effect of Starch on Physicochemical Properties of the 3D Alginate-Starch-Based Patches
Structural Stability of the 3D Alginate-Starch-Based Patches
3.5. In Vitro Rhodamine B Release Studies from 3D Alginate-Starch-Based Patches
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ventola, C.L. Medical Applications for 3D printing: Current and projected uses. P T 2014, 39, 704–711. [Google Scholar]
- Konta, A.A.; García-Piña, M.; Serrano, D.R. Personalised 3D printed medicines: Which techniques and polymers are more successful? Bioengineering 2017, 4, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Economidou, S.N.; Lamprou, D.A.; Douroumis, D. 3D printing applications for transdermal drug delivery. Int. J. Pharm. 2018, 544, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Amornrat, D.U.; Wang, J.; Basit, A.W.; Gaisford, S. 3D scanning and 3D printing as innovative technologies for fabricating personalized topical drug delivery systems. J. Control. Release 2016, 234, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.A.; Olawuni, D.; Kimbell, G.; Badruddoza, A.Z.M.; Hossain, M.S.; Sultana, T. Polymers for extrusion-based 3D printing of pharmaceuticals: A holistic materials–process perspective. Pharmaceutics 2020, 12, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Jiang, Q.; Zhang, Y.; Li, T.; Zhang, H.C. Sustainability of 3D printing: A critical review and recommendations. In Proceedings of the ASME 2016 11th International Manufacturing Science and Engineering Conference, Blacksburg, VA, USA, 27 June–1 July 2016; Volume 2, pp. 1–8. [Google Scholar]
- Demirtaş, T.T.; Irmak, G.; Gümüşderelioǧlu, M. A bioprintable form of chitosan hydrogel for bone tissue engineering. Biofabrication 2017, 9, 035003. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.d.S.; Tanaka, F.N.; Moura, M.d.R.; Aouada, F.A. Development of alginate/starch-based hydrogels crosslinked with different ions: Hydrophilic, kinetic and spectroscopic properties. Mater. Today Commun. 2019, 21, 100636. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Rosa, M.F.; De Sá, M.; Souza Filho, M.; Waldron, K. The use of biomass for packaging films and coatings. In Advances in Biorefineries: Biomass and Waste Supply Chain Exploitation; Waldron, K.W., Ed.; Woodhead Publishing: London, UK, 2014; pp. 819–874. ISBN 9780857095213. [Google Scholar]
- Koski, C.; Onuike, B.; Bandyopadhyay, A.; Bose, S. Starch-hydroxyapatite composite bone scaffold fabrication utilizing a slurry extrusion-based solid freeform fabricator. Addit. Manuf. 2018, 24, 47–59. [Google Scholar] [CrossRef]
- Chen, H.; Xie, F.; Chen, L.; Zheng, B. Effect of rheological properties of potato, rice and corn starches on their hot-extrusion 3D printing behaviors. J. Food Eng. 2019, 244, 150–158. [Google Scholar] [CrossRef] [Green Version]
- Maniglia, B.C.; Lima, D.C.; Matta Junior, M.D.; Le-Bail, P.; Le-Bail, A.; Augusto, P.E.D. Hydrogels based on ozonated cassava starch: Effect of ozone processing and gelatinization conditions on enhancing 3D-printing applications. Int. J. Biol. Macromol. 2019, 138, 1087–1097. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, M.; Bhandari, B.; Liu, Y. Investigation on lemon juice gel as food material for 3D printing and optimization of printing parameters. LWT Food Sci. Technol. 2018, 87, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Zheng, L.; Yu, Y.; Tong, Z.; Zou, Q.; Han, S.; Jiang, H. The characteristics of starch gels molded by 3D printing. J. Food Process. Preserv. 2019, 43, 1–11. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, D.K.; Kumar, R.; Gupta, A. Controlled release of the fungicide thiram from starch-alginate-clay based formulation. Appl. Clay Sci. 2009, 45, 76–82. [Google Scholar] [CrossRef]
- Córdoba, A.L.; Deladino, L.; Martino, M. Effect of starch filler on calcium-alginate hydrogels loaded with yerba mate antioxidants. Carbohydr. Polym. 2013, 95, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.M.; Hosseini, H.; Mohammadifar, M.A.; German, J.B.; Mortazavian, A.M.; Mohammadi, A.; Darani, K.K.; Aliabadi, S.S.; Khaksar, R. Preparation and characterization of alginate and alginate-resistant starch microparticles containing nisin. Carbohydr. Polym. 2014, 103, 573–580. [Google Scholar] [CrossRef]
- Vazquez, G.L.; Calleros, C.L.; Buendia, H.E.; Chavez, G.; Ramirez, J.A.; Carter, E.J.V. Effect of the weight ratio of alginate-modified tapioca starch on the physicochemical properties and release kinetics of chlorogenic acid containing beads. Food Hydrocoll. 2015, 48, 301–311. [Google Scholar] [CrossRef]
- Freeman, F.E.; Kelly, D.J. Tuning alginate bioink stiffness and composition for controlled growth factor delivery and to spatially direct MSC Fate within bioprinted tissues. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Sujka, M.; Jamroz, J. Characteristics of pores in native and hydrolyzed starch granules. Starch Staerke 2010, 62, 229–235. [Google Scholar] [CrossRef]
- Simpliciano, C.; Clark, L.; Asi, B.; Chu, N.; Mercado, M.; Diaz, S.; Goedert, M.; Mobed-Miremadi, M. Cross-linked alginate film pore size determination using atomic force microscopy and validation using diffusivity determinations. J. Surf. Eng. Mater. Adv. Technol. 2013, 3, 1–12. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Yang, F.; Zhao, H.; Gao, Q.; Xia, B.; Fu, J. Research on the printability of hydrogels in 3D bioprinting. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef]
- Marto, J.; Pinto, P.; Fitas, M.; Gonçalves, L.M.; Almeida, A.J.; Ribeiro, H.M. Safety assessment of starch-based personal care products: Nanocapsules and pickering emulsions. Toxicol. Appl. Pharm. 2018, 342, 14–21. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marto, J.; Gouveia, L.F.; Gonçalves, L.M.; Gaspar, D.P.; Pinto, P.; Carvalho, F.A.; Oliveira, E.; Ribeiro, H.M.; Almeida, A.J. A quality by design (QbD) approach on starch-based nanocapsules: A promising platform for topical drug delivery. Colloids Surf. B Biointerfaces 2016, 143, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.L.; Tsavaris, A.M.; Lo, W.M.; Bonassar, L.J.; Lipson, H. Improved quality of 3D-printed tissue constructs through enhanced mixing of alginate hydrogels. Res. Gate 2008, 1, 676–685. [Google Scholar]
- Piskounova, S.; Rojas, R.; Bergman, K.; Hilborn, J. The effect of mixing on the mechanical properties of hyaluronan-based injectable hydrogels. Macromol. Mater. Eng. 2011, 296, 944–951. [Google Scholar] [CrossRef]
- Guo, T.R.; Holzber, T.G.; Lim, C.; Gao, F.; Gargava, A.E.; Trachtenberg, J.G.; Mikos, A.P.; Fisher, J. 3D printing PLGA: A quantitative examination of the effects of polymer composition and printing parameters on print resolution. Biofabrication 2017, 9, 3–21. [Google Scholar] [CrossRef] [Green Version]
- Farzadi, A.; Waran, V.; Solati-Hashjin, M.; Rahman, Z.A.A.; Asadi, M.; Osman, N.A.A. Effect of layer printing delay on mechanical properties and dimensional accuracy of 3D printed porous prototypes in bone tissue engineering. Ceram. Int. 2015, 41, 8320–8330. [Google Scholar] [CrossRef] [Green Version]
- Farrés, I.F.; Norton, I.T. Formation kinetics and rheology of alginate fluid gels produced by in-situ calcium release. Food Hydrocoll. 2014, 40, 76–84. [Google Scholar] [CrossRef] [Green Version]
- Kuo, C.K.; Ma, P.X. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: Part 1. Structure, gelation rate and mechanical properties. Biomaterials 2001, 22, 511–521. [Google Scholar] [CrossRef]
- Viyoch, J.; Sudedmark, T.; Srema, W.; Suwongkrua, W. Development of hydrogel patch for controlled release of alpha-hydroxy acid contained in tamarind fruit pulp extract. Int. J. Cosmet. Sci. 2005, 27, 89–99. [Google Scholar] [CrossRef]
- Ceylan Tuncaboylu, D.; Abdurrahmanoglu, S.; Gazioglu, I. Rheological characterization of starch gels: A biomass based sorbent for removal of polycyclic aromatic hydrocarbons (PAHs). J. Hazard. Mater. 2019, 371, 406–414. [Google Scholar] [CrossRef]
- Zhu, B.; Ma, D.; Wang, J.; Zhang, S. Structure and properties of semi-interpenetrating network hydrogel based on starch. Carbohydr. Polym. 2015, 133, 448–455. [Google Scholar] [CrossRef]
- Voo, W.P.; Lee, B.B.; Idris, A.; Islam, A.; Tey, B.T.; Chan, E.S. Production of ultra-high concentration calcium alginate beads with prolonged dissolution profile. RSC Adv. 2015, 5, 36687–36695. [Google Scholar] [CrossRef]
- Costa, M.J.; Marques, A.M.; Pastrana, L.M.; Teixeira, J.A.; Sillankorva, S.M.; Cerqueira, M.A. Physicochemical properties of alginate-based films: Effect of ionic crosslinking and mannuronic and guluronic acid ratio. Food Hydrocoll. 2018, 81, 442–448. [Google Scholar] [CrossRef] [Green Version]
- Onyido, I.; Sha’Ato, R.; Nnamonu, L.A. Environmentally friendly formulations of trifluralin based on alginate modified starch. J. Environ. Prot. 2012, 03, 1085–1093. [Google Scholar] [CrossRef] [Green Version]
- Szekalska, M.; Sosnowska, K.; Kósnik, A.C.; Winnicka, K. Calcium chloride modified alginate microparticles formulated by the spray drying process: A strategy to prolong the release of freely soluble drugs. Materials 2018, 11, 1522. [Google Scholar] [CrossRef] [Green Version]
- Bruschi, M.L. Mathematical models of drug release. In Strategies to Modify Drug Release from Pharmaceutical Systems; Bruschi, M.L., Ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 63–86. ISBN 9780081000922. [Google Scholar]
- Kosmidis, K.; Argyrakis, P.; Macheras, P. Fractal kinetics in drug release from finite fractal matrices. J. Chem. Phys. 2003, 119, 6373–6377. [Google Scholar] [CrossRef] [Green Version]
- Papadopoulou, V.; Kosmidis, K.; Vlachou, M.; Macheras, P. On the use of the Weibull function for the discernment of drug release mechanisms. Int. J. Pharm. 2006, 309, 44–50. [Google Scholar] [CrossRef]
- Bunde, A.; Havlin, S.; Nossal, R.; Stanley, H.E.; Weiss, G.H. On controlled diffusion-limited drug release from a leaky matrix. J. Chem. Phys. 1985, 83, 5909–5913. [Google Scholar] [CrossRef]
- Cojocaru, V.; Ranetti, A.E.; Hinescu, L.G.; Ionescu, M.; Cosmescu, C.; Poștoarcă, A.G.; Cinteză, L.O. Formulation and evaluation of in vitro release kinetics of na3cadtpa decorporation agent embedded in microemulsion-based gel formulation for topical delivery. Farmacia 2015, 63, 656–664. [Google Scholar]
- Gupta, B.; Chaurasia, U.; Chakraborty, P. Design and development of oral transmucosal film for delivery of salbutamol sulphate. J. Pharm. Chem. Biol. Sci. 2014, 2, 118–129. [Google Scholar]







| QTPP Element | Target |
|---|---|
| Dosage form | 3D hydrogel patch |
| Route of administration | Topical |
| Gelation time | 10–30 min [19] |
| Porosity | 1–20 µm [20,21] |
| Printing accuracy | High [22] |
| Elasticity | Medium |
| Adhesiveness | High |
| Biological effects | Non-sensitizing and nonirritant [23] |
| Failure Modes | 3D Hydrogel Patch CQAs | ||||||
|---|---|---|---|---|---|---|---|
| Gelation Time | Porosity | Printing Accuracy | Elasticity | Adhesiveness | |||
| Raw Materials Attributes | Solvent (Water) | Amount | Medium | High | Medium | Medium | Low |
| Gelation Agent (CaCl2·2H2O) | Amount | Medium | High | High | Medium | Low | |
| Polymer (Alginate/Starch) | Amount | Medium | High | High | Medium | Low | |
| Molecular weight | Medium | High | High | Medium | Low | ||
| Viscosity | Medium | High | High | Medium | Low | ||
| Drug | Solubility | Medium | Low | Low | Low | Low | |
| Amount | Medium | Low | Low | Low | Low | ||
| Process Parameters | Pre-manufacturing (Formulation) | Mixing time and rate | Medium | Medium | Medium | Low | Low |
| Design and slicing | Printing setups | Medium | High | High | Low | Low | |
| Dimensions (x,y,z) | Medium | Medium | Medium | Low | Low | ||
| Number of layers and contour strategy | Medium | Medium | Medium | Low | Low | ||
| Feed rate | Low | Low | Low | Low | Low | ||
| 3D Printing | Needle material and diameter | Low | Low | Low | Low | Low | |
| Distance between the needle and the platform | Low | Low | Low | Low | Low | ||
| Ink viscosity | Medium | High | Medium | High | High | ||
| Surface adhesion materials | Low | Low | Medium | Low | Low | ||
| Extrusion chamber filling | Low | Low | Low | Low | Low | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bom, S.; Santos, C.; Barros, R.; Martins, A.M.; Paradiso, P.; Cláudio, R.; Pinto, P.C.; Ribeiro, H.M.; Marto, J. Effects of Starch Incorporation on the Physicochemical Properties and Release Kinetics of Alginate-Based 3D Hydrogel Patches for Topical Delivery. Pharmaceutics 2020, 12, 719. https://doi.org/10.3390/pharmaceutics12080719
Bom S, Santos C, Barros R, Martins AM, Paradiso P, Cláudio R, Pinto PC, Ribeiro HM, Marto J. Effects of Starch Incorporation on the Physicochemical Properties and Release Kinetics of Alginate-Based 3D Hydrogel Patches for Topical Delivery. Pharmaceutics. 2020; 12(8):719. https://doi.org/10.3390/pharmaceutics12080719
Chicago/Turabian StyleBom, Sara, Catarina Santos, Rita Barros, Ana M. Martins, Patrizia Paradiso, Ricardo Cláudio, Pedro Contreiras Pinto, Helena M. Ribeiro, and Joana Marto. 2020. "Effects of Starch Incorporation on the Physicochemical Properties and Release Kinetics of Alginate-Based 3D Hydrogel Patches for Topical Delivery" Pharmaceutics 12, no. 8: 719. https://doi.org/10.3390/pharmaceutics12080719