Fibroblast Growth Factor 2—A Review of Stabilisation Approaches for Clinical Applications
Abstract
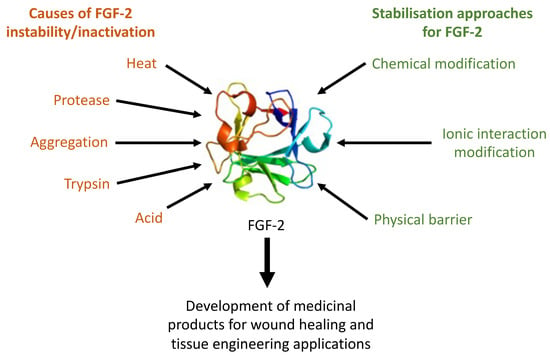
:1. Introduction
2. FGF-2 Structure and Stability
3. Strategies for Preserving FGF-2 Functionality in Aqueous Solutions
3.1. Modulating Ionic Interactions in Solutions
3.1.1. Conjugation to Heparin and Heparin-Like Molecules
3.1.2. Coacervation
3.2. Chemical Modifications
3.2.1. Genetic Engineering
3.2.2. Chemical Conjugation to Inorganic Materials
3.3. Physical Barrier Strategies
3.3.1. Entrapment in Hydrogel Scaffolds
3.3.2. Microencapsulation
3.3.3. Adsorption
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Kawai, K.; Suzuki, S.; Tabata, Y.; Ikada, Y.; Nishimura, Y. Accelerated tissue regeneration through incorporation of basic fibroblast growth factor-impregnated gelatin microspheres into artificial dermis. Biomaterials 2000, 21, 489–499. [Google Scholar] [CrossRef]
- Gao, Y.; Zhu, S.; Luo, E.; Li, J.; Feng, G.; Hu, J. Basic fibroblast growth factor suspended in Matrigel improves titanium implant fixation in ovariectomized rats. J. Control. Release 2009, 139, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Takafuji, H.; Suzuki, T.; Okubo, Y.; Fujimura, K.; Bessho, K. Regeneration of articular cartilage defects in the temporomandibular joint of rabbits by fibroblast growth factor-2: A pilot study. Int. J. Oral Maxillofac. Surg. 2007, 36, 934–937. [Google Scholar] [CrossRef] [PubMed]
- Hankemeier, S.; Keus, M.; Zeichen, J.; Jagodzinski, M.; Barkhausen, T.; Bosch, U.; Krettek, C.; Van Griensven, M. Modulation of proliferation and differentiation of human bone marrow stromal cells by fibroblast growth factor 2: Potential implications for tissue engineering of tendons and ligaments. Tissue Eng. 2005, 11, 41–49. [Google Scholar] [CrossRef]
- Kasai, M.; Jikoh, T.; Fukumitsu, H.; Furukawa, S. FGF-2-responsive and spinal cord-resident cells improve locomotor function after spinal cord injury. J. Neurotrauma 2014, 31, 1584–1598. [Google Scholar] [CrossRef] [Green Version]
- Gospodarowicz, D. Purification of a fibroblast growth factor from bovine pituitary. J. Biol. Chem. 1975, 250, 2515–2520. [Google Scholar]
- Fox, G.M. Production, biological activity, and structure of recombinant basic fibroblast growth factor and an analog with cysteine replaced by serine. J. Biol. Chem. 1988, 263, 18452–18458. [Google Scholar]
- Plouët, J.; Schilling, J.; Gospodarowicz, D. Isolation and characterization of a newly identified endothelial cell mitogen produced by AtT-20 cells. EMBO J. 1989, 8, 3801. [Google Scholar] [CrossRef]
- Abraham, J.A.; Whang, J.L.; Tumolo, A.; Mergia, A.; Friedman, J.; Gospodarowicz, D.; Fiddes, J.C. Human basic fibroblast growth factor: Nucleotide sequence and genomic organization. EMBO J. 1986, 5, 2523. [Google Scholar] [CrossRef]
- Simpson, R.J.; Moritz, R.L.; Lloyd, C.J.; Fabri, L.J.; Nice, E.C.; Rubira, M.R.; Burgess, A.W. Primary structure of ovine pituitary basic fibroblast growth factor. FEBS Lett. 1987, 224, 128–132. [Google Scholar] [CrossRef] [Green Version]
- Shimasaki, S.; Emoto, N.; Koba, A.; Mercado, M.; Shibata, F.; Cooksey, K.; Baird, A.; Ling, N. Complementary DNA cloning and sequencing of rat ovarian basic fibroblast growth factor and tissue distribution study of its mRNA. Biochem. Biophys. Res. Commun. 1988, 157, 256–263. [Google Scholar] [CrossRef]
- Beenken, A.; Mohammadi, M. The FGF family: Biology, pathophysiology and therapy. Nat. Rev. Drug Discov. 2009, 8, 235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, Y.-R.; Won, J.E.; Jeon, E.; Lee, S.; Kang, W.; Jo, H.; Jang, J.-H.; Shin, U.S.; Kim, H.-W. Fibroblast growth factors: Biology, function, and application for tissue regeneration. J. Tissue Eng. 2010, 2010, 218142. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Hwang, S.R.; Yoon, I.S. Advanced growth factor delivery systems in wound management and skin regeneration. Molecules 2017, 22, 1259. [Google Scholar] [CrossRef] [Green Version]
- Xuan, X.; Zhou, Y.; Chen, A.; Zheng, S.; An, Y.; He, H.; Huang, W.; Chen, Y.; Yang, Y.; Li, S.; et al. Silver crosslinked injectable bFGF-eluting supramolecular hydrogels speed up infected wound healing. J. Mater. Chem. B 2020, 8, 1359–1370. [Google Scholar] [CrossRef]
- Washio, A.; Teshima, H.; Yokota, K.; Kitamura, C.; Tabata, Y. Preparation of gelatin hydrogel sponges incorporating bioactive glasses capable for the controlled release of fibroblast growth factor-2. J. Biomater. Sci. Polym. Ed. 2019, 30, 49–63. [Google Scholar] [CrossRef]
- Kanemaru, S.-I.; Umeda, H.; Kitani, Y.; Nakamura, T.; Hirano, S.; Ito, J. Regenerative treatment for tympanic membrane perforation. Otol. Neurotol. 2011, 32, 1218–1223. [Google Scholar] [CrossRef]
- Lou, Z.; Huang, P.; Yang, J.; Xiao, J.; Chang, J. Direct application of bFGF without edge trimming on human subacute tympanic membrane perforation. Am. J. Otolaryngol. 2016, 37, 156–161. [Google Scholar] [CrossRef]
- Lou, Z.; Tang, Y.; Wu, X. Analysis of the effectiveness of basic fibroblast growth factor treatment on traumatic perforation of the tympanic membrane at different time points. Am. J. Otolaryngol. 2012, 33, 244–249. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, Y. Evaluation of the optimum time for direct application of fibroblast growth factor to human traumatic tympanic membrane perforations. Growth Factors 2015, 33, 65–70. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, Y.; Yu, G. Effects of basic fibroblast growth factor dose on traumatic tympanic membrane perforation. Growth Factors 2014, 32, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.-C.; Lou, Z.-H. The short- and long-term adverse effects of FGF-2 on tympanic membrane perforations. Acta Otorhinolaryngol. Ital. 2018, 38, 264. [Google Scholar] [CrossRef]
- Lou, Z.C.; He, J.G. A randomised controlled trial comparing spontaneous healing, gelfoam patching and edge-approximation plus gelfoam patching in traumatic tympanic membrane perforation with inverted or everted edges. Clin. Otolaryngol. 2011, 36, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.C.; Wang, Y.B.Z. Healing outcomes of large (>50%) traumatic membrane perforations with inverted edges following no intervention, edge approximation and fibroblast growth factor application; a sequential allocation, three-armed trial. Clin. Otolaryngol. 2013, 38, 289–296. [Google Scholar] [CrossRef]
- Acharya, A.N.; Coates, H.; Tavora-Vièira, D.; Rajan, G.P. A pilot study investigating basic fibroblast growth factor for the repair of chronic tympanic membrane perforations in pediatric patients. Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 332–335. [Google Scholar] [CrossRef]
- Chen, J.-H. Comparison of FGF-2, FLOX, and gelfoam patching for traumatic tympanic membrane perforation. Otol. Neurotol. 2016, 37, 1679–1680. [Google Scholar] [CrossRef]
- Hakuba, N.; Hato, N.; Okada, M.; Mise, K. Preoperative factors affecting tympanic membrane regeneration therapy using an atelocollagen and basic fibroblast growth factor. JAMA Otolaryngol. Head Neck Surg. 2015, 141, 60–66. [Google Scholar] [CrossRef] [Green Version]
- Hakuba, N.; Iwanaga, M.; Tanaka, S.; Hiratsuka, Y.; Kumabe, Y.; Konishi, M.; Okanoue, Y.; Hiwatashi, N.; Wada, T. Basic fibroblast growth factor combined with atelocollagen for closing chronic tympanic membrane perforations in 87 patients. Otol. Neurotol. 2010, 31, 118–121. [Google Scholar] [CrossRef]
- Omae, K.; Kanemaru, S.-I.; Nakatani, E.; Kaneda, H.; Nishimura, T.; Tona, R.; Naito, Y.; Kawamoto, A.; Fukushima, M. Regenerative treatment for tympanic membrane perforation using gelatin sponge with basic fibroblast growth factor. Auris Nasus Larynx 2017, 44, 664–671. [Google Scholar] [CrossRef]
- Matsumine, H.; Fujimaki, H.; Takagi, M.; Mori, S.; Iwata, T.; Shimizu, M.; Takeuchi, M. Full-thickness skin reconstruction with basic fibroblast growth factor-impregnated collagen-gelatin sponge. Regen. Ther. 2019, 11, 81–87. [Google Scholar] [CrossRef]
- Lou, Z.C.; Lou, Z.H.; Zhang, Q.P. Traumatic tympanic membrane perforations: A study of etiology and factors affecting outcome. Am. J. Otolaryngol. 2012, 33, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.C. Spontaneous healing of traumatic eardrum perforation: Outward epithelial cell migration and clinical outcome. Otolaryngol. Head Neck Surg. 2012, 147, 1114–1119. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Yang, J.; Tang, Y.; Xiao, J. Risk factors affecting human traumatic tympanic membrane perforation regeneration therapy using fibroblast growth factor-2. Growth Factors 2015, 33, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.C.; Lou, Z.H.; Liu, Y.C.; Chang, J. Healing human moderate and large traumatic tympanic membrane perforations using basic fibroblast growth factor, 0.3% ofloxacin eardrops, and gelfoam patching. Otol. Neurotol. 2016, 37, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Lou, Z.; Tang, Y.; Xiao, J. The effect of ofloxacin otic drops on the regeneration of human traumatic tympanic membrane perforations. Clin. Otolaryngol. 2016, 41, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, N.; Yoshimura, K.; Niimi, M.; Ito, T.; Aya, R.; Fujitaka, J.; Tada, H.; Teramukai, S.; Murayama, T.; Toyooka, C.; et al. Novel collagen/gelatin scaffold with sustained release of basic fibroblast growth factor: Clinical Trial for chronic skin ulcers. Tissue Eng. Part A 2013, 19, 1931–1940. [Google Scholar] [CrossRef] [Green Version]
- Burgess, W.H.; Maciag, T. The Heparin-binding (fibroblast) growth factor family of proteins. Annu. Rev. Biochem. 1989, 58, 575–602. [Google Scholar] [CrossRef]
- Andreopoulos, F.M.; Persaud, I. Delivery of basic fibroblast growth factor (bFGF) from photoresponsive hydrogel scaffolds. Biomaterials 2006, 27, 2468–2476. [Google Scholar] [CrossRef]
- Cai, S.; Liu, Y.; Zheng Shu, X.; Prestwich, G.D. Injectable glycosaminoglycan hydrogels for controlled release of human basic fibroblast growth factor. Biomaterials 2005, 26, 6054–6067. [Google Scholar] [CrossRef]
- Freudenberg, U.; Hermann, A.; Welzel, P.B.; Stirl, K.; Schwarz, S.C.; Grimmer, M.; Zieris, A.; Panyanuwat, W.; Zschoche, S.; Meinhold, D.; et al. A star-PEG–heparin hydrogel platform to aid cell replacement therapies for neurodegenerative diseases. Biomaterials 2009, 30, 5049–5060. [Google Scholar] [CrossRef]
- Zhang, J.D.; Cousens, L.S.; Barr, P.J.; Sprang, S.R. Three-dimensional structure of human basic fibroblast growth factor, a structural homolog of interleukin 1 beta. Proc. Natl. Acad. Sci. USA 1991, 88, 3446–3450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Shahrokh, Z.; Vemuri, S.; Eberlein, G.; Beylin, I.; Busch, M. Characterization, stability, and formulations of basic fibroblast growth factor. In Formulation, Characterization, and Stability of Protein Drugs: Case Histories; Pearlman, R., Wang, J.Y., Eds.; Springer: New York, NY, USA, 2002; pp. 141–180. [Google Scholar] [CrossRef]
- Vemuri, S.; Beylin, I.; Sluzky, V.; Stratton, P.A.; Eberlein, G.A.; Wang, Y.J. The stability of bFGF against thermal denaturation. J. Pharm. Pharmacol. 1994, 46, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Furue, M.K.; Na, J.; Jackson, J.P.; Okamoto, T.; Jones, M.; Baker, D.; Hata, R.-I.; Moore, H.D.; Sato, J.D.; Andrews, P.W. Heparin promotes the growth of human embryonic stem cells in a defined serum-free medium. Proc. Natl. Acad. Sci. USA 2008, 105, 13409–13414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akl, M.R.; Nagpal, P.; Ayoub, N.M.; Tai, B.; Prabhu, S.A.; Capac, C.M.; Gliksman, M.; Goy, A.; Suh, K.S. Molecular and clinical significance of fibroblast growth factor 2 (FGF2 /bFGF) in malignancies of solid and hematological cancers for personalized therapies. Oncotarget 2016, 7, 44735–44762. [Google Scholar] [CrossRef] [Green Version]
- Ohtake, S.; Kita, Y.; Arakawa, T. Interactions of formulation excipients with proteins in solution and in the dried state. Adv. Drug Deliv. Rev. 2011, 63, 1053–1073. [Google Scholar] [CrossRef]
- Fang, H.Z.; Zheng, Z.S.; Zhu, A.T.; Lan, X.; Huang, X.K. Recombinant Bovine Basic Fibroblast Growth Factor Freeze-Dried Formulation for External Application. CN Patent CN102389402B, 19 December 2012. [Google Scholar]
- Fang, H.Z.; Zheng, Z.S.; Zhu, A.T.; Lan, X.; Huang, X.K. External Recombinant Bovine Basic Fibroblast Growth Factor Lyophilized Preparation. CN Patent CN104586778A, 23 March 2016. [Google Scholar]
- Shah, D. Effect of Various Additives on the Stability of Basic Fibroblast Growth Factor and Development of an Intradermal Injectable Formulation. Ph.D. Thesis, University of Missouri, Kansas City, MO, USA, 1998. [Google Scholar]
- Chen, G.; Gulbranson, D.R.; Yu, P.; Hou, Z.; Thomson, J.A. Thermal stability of fibroblast growth factor protein is a determinant factor in regulating self-renewal, differentiation, and reprogramming in human pluripotent stem cells. Stem Cells 2012, 30, 623–630. [Google Scholar] [CrossRef] [Green Version]
- Chu, H.; Gao, J.; Chen, C.-W.; Huard, J.; Wang, Y. Injectable fibroblast growth factor-2 coacervate for persistent angiogenesis. Proc. Natl. Acad. Sci. USA 2011, 108, 13444. [Google Scholar] [CrossRef] [Green Version]
- Paluck, S.J.; Nguyen, T.H.; Lee, J.P.; Maynard, H.D. A heparin-mimicking block copolymer both stabilizes and increases the activity of fibroblast growth factor 2 (FGF2). Biomacromolecules 2016, 17, 3386–3395. [Google Scholar] [CrossRef]
- Wu, J.; Mao, Z.; Hong, Y.; Han, L.; Gao, C. Conjugation of basic fibroblast growth factor on a heparin gradient for regulating the migration of different types of cells. Bioconj. Chem. 2013, 24, 1302–1313. [Google Scholar] [CrossRef]
- Macdonald, M.L.; Rodriguez, N.M.; Shah, N.J.; Hammond, P.T. Characterization of tunable FGF-2 releasing polyelectrolyte multilayers. Biomacromolecules 2010, 11, 2053–2059. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Zhang, Z.; Linhardt, R.J. Lessons learned from the contamination of heparin. Nat. Prod. Rep. 2009, 26, 313–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warkentin, T.E.; Sheppard, J.-A.I.; Moore, J.C.; Cook, R.J.; Kelton, J.G. Studies of the immune response in heparin-induced thrombocytopenia. Blood 2009, 113, 4963. [Google Scholar] [CrossRef] [PubMed]
- Hovingh, P.; Linker, A. The enzymatic degradation of heparin and heparitin sulfate: III. Purification of a heparitinase and a heparinase from flavobacteria. J. Biol. Chem. 1970, 245, 6170–6175. [Google Scholar] [PubMed]
- Merli, G.J.; Groce, J.B. Pharmacological and clinical differences between low-molecular-weight heparins: Implications for prescribing practice and therapeutic interchange. Pharm. Ther. 2010, 35, 95–105. [Google Scholar]
- Papy-Garcia, D.; Barbier-Chassefière, V.; Rouet, V.; Kerros, M.-E.; Klochendler, C.; Tournaire, M.-C.; Barritault, D.; Caruelle, J.-P.; Petit, E. Nondegradative sulfation of polysaccharides. Synthesis and structure characterization of biologically active heparan sulfate mimetics. Macromolecules 2005, 38, 4647–4654. [Google Scholar] [CrossRef]
- Mauzac, M.; Jozefonvicz, J. Anticoagulant activity of dextran derivatives. Part I: Synthesis and characterization. Biomaterials 1984, 5, 301–304. [Google Scholar] [CrossRef]
- Mammadov, R.; Mammadov, B.; Guler, M.O.; Tekinay, A.B. Growth factor binding on heparin mimetic peptide nanofibers. Biomacromolecules 2012, 13, 3311–3319. [Google Scholar] [CrossRef]
- Coombe, D.R.; Kett, W.C. Heparin mimetics. Handb. Exp. Pharmacol. 2012, 361–383. [Google Scholar] [CrossRef]
- Hassan, H.H. Chemistry and biology of heparin mimetics that bind to fibroblast growth factors. Mini Rev. Med. Chem. 2007, 7, 1206–1235. [Google Scholar] [CrossRef]
- Kim, S.H.; Kiick, K.L. Heparin-mimetic sulfated peptides with modulated affinities for heparin-binding peptides and growth factors. Peptides 2007, 28, 2125–2136. [Google Scholar] [CrossRef] [Green Version]
- Logeart-Avramoglou, D.; Jozefonvicz, J. Carboxymethyl benzylamide sulfonate dextrans (CMDBS), a family of biospecific polymers endowed with numerous biological properties: A review. J. Biomed. Mater. Res. 1999, 48, 578–590. [Google Scholar] [CrossRef]
- Maynard, H.D.; Hubbell, J.A. Discovery of a sulfated tetrapeptide that binds to vascular endothelial growth factor. Acta Biomater. 2005, 1, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Tardieu, M.; Gamby, C.; Avramoglou, T.; Jozefonvicz, J.; Barritault, D. Derivatized dextrans mimic heparin as stabilizers, potentiators, and protectors of acidic or basic FGF. J. Cell. Physiol. 1992, 150, 194–203. [Google Scholar] [CrossRef]
- Liekens, S.; Leali, D.; Neyts, J.; Esnouf, R.; Rusnati, M.; Dell’Era, P.; Maudgal, P.C.; De Clercq, E.; Presta, M. Modulation of fibroblast growth factor-2 receptor binding, signaling, and mitogenic activity by heparin-mimicking polysulfonated compounds. Mol. Pharmacol. 1999, 56, 204–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, T.H.; Paluck, S.J.; McGahran, A.J.; Maynard, H.D. Poly(vinyl sulfonate) Facilitates bFGF-Induced Cell Proliferation. Biomacromolecules 2015, 16, 2684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, T.H.; Kim, S.-H.; Decker, C.G.; Wong, D.Y.; Loo, J.A.; Maynard, H.D. A heparin-mimicking polymer conjugate stabilizes basic fibroblast growth factor. Nat. Chem. 2013, 5, 221–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dvorak, P.; Bednar, D.; Vanacek, P.; Balek, L.; Eiselleova, L.; Stepankova, V.; Sebestova, E.; Kunova Bosakova, M.; Konecna, Z.; Mazurenko, S.; et al. Computer-assisted engineering of hyperstable fibroblast growth factor 2. Biotechnol. Bioeng. 2017, 115, 850–862. [Google Scholar] [CrossRef]
- Moon, K.-S.; Choi, E.-J.; Oh, S.; Kim, S. The Effect of Covalently Immobilized FGF-2 on Biphasic Calcium Phosphate Bone Substitute on Enhanced Biological Compatibility and Activity. BioMed Res. Int. 2015, 2015, 10. [Google Scholar] [CrossRef] [Green Version]
- Nolle, V. Fibroblast Growth Factor Muteins with Increased Activity. U.S. Patent 20150284443A1, 8 October 2015. [Google Scholar]
- Jeong, S.S. Thermostable Variants of Fibroblast Growth Factors. U.S. Patent No. 9,169,309, 27 October 2015. [Google Scholar]
- Koledova, Z.; Sumbal, J.; Rabata, A.; de La Bourdonnaye, G.; Chaloupkova, R.; Hrdlickova, B.; Damborsky, J.; Stepankova, V. Fibroblast Growth Factor 2 Protein Stability Provides Decreased Dependence on Heparin for Induction of FGFR Signaling and Alters ERK Signaling Dynamics. Front. Cell Dev. Biol. 2019, 7. [Google Scholar] [CrossRef] [Green Version]
- Valastyan, J.S.; Lindquist, S. Mechanisms of protein-folding diseases at a glance. Dis. Models Mech. 2014, 7, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Myerowitz, R.; Costigan, F.C. The major defect in Ashkenazi Jews with Tay-Sachs disease is an insertion in the gene for the alpha-chain of beta-hexosaminidase. J. Biol. Chem. 1988, 263, 18587–18589. [Google Scholar] [PubMed]
- Lisignoli, G.; Zini, N.; Remiddi, G.; Piacentini, A.; Puggioli, A.; Trimarchi, C.; Fini, M.; Maraldi, N.M.; Facchini, A. Basic fibroblast growth factor enhances in vitro mineralization of rat bone marrow stromal cells grown on non-woven hyaluronic acid based polymer scaffold. Biomaterials 2001, 22, 2095–2105. [Google Scholar] [CrossRef]
- Tanihara, M.; Suzuki, Y.; Yamamoto, E.; Noguchi, A.; Mizushima, Y. Sustained release of basic fibroblast growth factor and angiogenesis in a novel covalently crosslinked gel of heparin and alginate. J. Biomed. Mater. Res. 2001, 56, 216–221. [Google Scholar] [CrossRef]
- Zellin, G.; Linde, A. Effects of recombinant human fibroblast growth factor-2 on osteogenic cell populations during orthopic osteogenesis in vivo. Bone 2000, 26, 161–168. [Google Scholar] [CrossRef]
- Jeong, I.; Yu, H.S.; Kim, M.K.; Jang, J.H.; Kim, H.W. FGF2-adsorbed macroporous hydroxyapatite bone granules stimulate in vitro osteoblastic gene expression and differentiation. J. Mater. Sci. Mater. Med. 2010, 21, 1335–1342. [Google Scholar] [CrossRef]
- Sogo, Y.; Ito, A.; Onoguchi, M.; Oyane, A.; Tsurushima, H.; Ichinose, N. Formation of a FGF-2 and calcium phosphate composite layer on a hydroxyapatite ceramic for promoting bone formation. Biomed. Mater. (Bristol Engl.) 2007, 2, S175–S180. [Google Scholar] [CrossRef]
- Ali, Z.; Islam, A.; Sherrell, P.; Le-Moine, M.; Lolas, G.; Syrigos, K.; Rafat, M.; Jensen, L.D. Adjustable delivery of pro-angiogenic FGF-2 by alginate:collagen microspheres. Biol. Open 2018, 7. [Google Scholar] [CrossRef] [Green Version]
- Galderisi, U.; Peluso, G.; Di Bernardo, G.; Calarco, A.; D’Apolito, M.; Petillo, O.; Cipollaro, M.; Fusco, F.R.; Melone, M.A.B. Efficient cultivation of neural stem cells with controlled delivery of FGF-2. Stem Cell Res. 2013, 10, 85–94. [Google Scholar] [CrossRef] [Green Version]
- Layman, H.; Spiga, M.-G.; Brooks, T.; Pham, S.; Webster, K.A.; Andreopoulos, F.M. The effect of the controlled release of basic fibroblast growth factor from ionic gelatin-based hydrogels on angiogenesis in a murine critical limb ischemic model. Biomaterials 2007, 28, 2646–2654. [Google Scholar] [CrossRef] [Green Version]
- Lotz, S.; Goderie, S.; Tokas, N.; Hirsch, S.E.; Ahmad, F.; Corneo, B.; Le, S.; Banerjee, A.; Kane, R.S.; Stern, J.H.; et al. Sustained levels of FGF2 maintain undifferentiated stem cell cultures with biweekly feeding. PLoS ONE 2013, 8, e56289. [Google Scholar] [CrossRef] [Green Version]
- Yoon, J.-Y.; Kim, J.-J.; El-Fiqi, A.; Jang, J.-H.; Kim, H.-W. Ultrahigh protein adsorption capacity and sustained release of nanocomposite scaffolds: Implication for growth factor delivery systems. RSC Adv. 2017, 7, 16453–16459. [Google Scholar] [CrossRef] [Green Version]
- Fukunaga, K.; Hijikata, S.; Ishimura, K.; Ohtani, Y.; Kimura, K.; Fuji, M.; Hata, Y. Composition of Stabilized Fibroblast Growth Factor. U.S. Patent 5482929, 9 January 1996. [Google Scholar]
- Mayfield, A.E.; Tilokee, E.L.; Latham, N.; McNeill, B.; Lam, B.-K.; Ruel, M.; Suuronen, E.J.; Courtman, D.W.; Stewart, D.J.; Davis, D.R. The effect of encapsulation of cardiac stem cells within matrix-enriched hydrogel capsules on cell survival, post-ischemic cell retention and cardiac function. Biomaterials 2014, 35, 133–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gelmi, A.; Cieslar-Pobuda, A.; de Muinck, E.; Los, M.; Rafat, M.; Jager, E.W.H. Direct mechanical stimulation of stem cells: A beating electromechanically active scaffold for cardiac tissue engineering. Adv. Healthc. Mater. 2016, 5, 1471–1480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwakura, A.; Tabata, Y.; Koyama, T.; Doi, K.; Nishimura, K.; Kataoka, K.; Fujita, M.; Komeda, M. Gelatin sheet incorporating basic fibroblast growth factor enhances sternal healing after harvesting bilateral internal thoracic arteries. J. Thorac. Cardiovasc. Surg. 2003, 126, 1113–1120. [Google Scholar] [CrossRef] [Green Version]
- Pieper, J.S.; Hafmans, T.; van Wachem, P.B.; van Luyn, M.J.A.; Brouwer, L.A.; Veerkamp, J.H.; van Kuppevelt, T.H. Loading of collagen-heparan sulfate matrices with bFGF promotes angiogenesis and tissue generation in rats. J. Biomed. Mater. Res. 2002, 62, 185. [Google Scholar] [CrossRef]
- Lee, H.; Lim, S.; Birajdar, M.S.; Lee, S.H.; Park, H. Fabrication of FGF-2 immobilized electrospun gelatin nanofibers for tissue engineering. Int. J. Biol. Macromol. 2016, 93, 1559–1566. [Google Scholar] [CrossRef]
- Tokunaga, T.; Arimura, H.; Yonemitsu, R.; Karasugi, T.; Ide, J.; Mizuta, H. Effect of FGF-2-impregnated gelatin hydrogel sheet incorporation into the bony trough on rotator cuff healing: A rabbit model. J. Shoulder Elb. Surg. 2017, 26, e110. [Google Scholar] [CrossRef]
- Gounis, M.J.; Spiga, M.G.; Graham, R.M.; Wilson, A.; Haliko, S.; Lieber, B.B.; Wakhloo, A.K.; Webster, K.A. Angiogenesis is confined to the transient period of VEGF expression that follows adenoviral gene delivery to ischemic muscle. Gene Ther. 2005, 12, 762. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, H.S.; Rasmussen, C.S.; Macko, J. VEGF gene therapy for coronary artery disease and peripheral vascular disease. Cardiovasc. Radiat. Med. 2002, 3, 114–117. [Google Scholar] [CrossRef]
- Sherrell, P.C.; Elmén, K.; Cieślar-Pobuda, A.; Wiecheć, E.; Lemoine, M.; Arzhangi, Z.; Silverå Ejneby, M.; Brask, J.; Daka, J.N.; Rafat, M. Cardiac and stem cell-cocooned hybrid microspheres: A multi factorial design approach. Sens. Actuators B Chem. 2016, 236, 480–489. [Google Scholar] [CrossRef]
- Ayala, P.; Caves, J.; Dai, E.; Siraj, L.; Liu, L.; Chaudhuri, O.; Haller, C.A.; Mooney, D.J.; Chaikof, E.L. Engineered composite fascia for stem cell therapy in tissue repair applications. Acta Biomater. 2015, 26, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khorshidi, S.; Solouk, A.; Mirzadeh, H.; Mazinani, S.; Lagaron, J.M.; Sharifi, S.; Ramakrishna, S. A review of key challenges of electrospun scaffolds for tissue-engineering applications. J. Tissue Eng. Regen. Med. 2015, 10, 715–738. [Google Scholar] [CrossRef] [PubMed]
- Tabata, Y.; Nagano, A.; Muniruzzaman, M.; Ikada, Y. In vitro sorption and desorption of basic fibroblast growth factor from biodegradable hydrogels. Biomaterials 1998, 19, 1781–1789. [Google Scholar] [CrossRef]
- Bower, V.E.; Paabo, M.; Bates, R. A Standard for the Measurement of the pH of Blood and Other Physiological Media. J. Res. Natl. Bur. Stand. 1961, 65, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Schneider, L.A.; Korber, A.; Grabbe, S.; Dissemond, J. Influence of pH on wound-healing: A new perspective for wound-therapy? Arch. Dermatol. Res. 2007, 298, 413–420. [Google Scholar] [CrossRef] [PubMed]
| Strategy | Technique | Comparative Stability | Potential Application | Model for Functional Assessment | Ref |
|---|---|---|---|---|---|
| Conjugation with a heparin-mimicking polymer | Conjugation with p(SS-co-PEGMA)-b-VS1, which contains a segment that enhances FGF-2 stability and another that binds FGF-2 receptor. | Biological activity extended to 7 days at 4 °C and 23 °C. Facilitated binding to receptor and increased migration of endothelial cells by 80% compared to free FGF-2. | Tissue engineering and wound healing | Human dermal fibroblasts Human umbilical vein endothelial cells | [52] |
| Complexation with heparin | Complexation with heparin immobilised on glass/silicone slides | Maintained bioactivity for up to 12 h at 37 °C. Rate of cell migration increased by 14 µm/h for vascular smooth muscle cells compared to free FGF-2 | Tissue engineering and wound healing | Human vascular smooth muscle cells, endothelial cells and Mesenchyme stem cells | [53] |
| Complex coacervation | Coacervation in the presence of a polycation (PEAD)2 and heparin | Maintained in vivo bioactivity for over 4 weeks from a single injection. Increased proliferation of endothelial and mural cells by 47% at 37 °C, when compared with free FGF-2. | Tissue engineering | BALB/cJ mice, murine endothelial and mural cells | [51] |
| Strategy | Technique | Comparative Stability | Potential Application | Model for Functional Assessment | Ref |
|---|---|---|---|---|---|
| Genetic engineering | Nine-point mutant of a low molecular weight isoform of FGF-2 | In vitro functional half-life at 37 °C improved from 10 h to more than 20 days. | Cell culture research | Human embryonic stem cells | [71] |
| Covalent grafting | Conjugation onto biphasic calcium phosphate scaffolds 1 | Increase alkaline phosphatase activity at 37 °C by up to 200% when compared with free FGF-2. | Tissue engineering | Human mesenchyme stem cells | [72] |
| Strategy | Technique | Comparative Stability | Potential Application | Model for Functional Assessment | Ref |
|---|---|---|---|---|---|
| Entrapment within hydrogel scaffolds | Gelatin-polylysine and Gelatin-polyglutamic acid hydrogels loaded with FGF-2 | FGF-2 release was sustained over 28 days at 37 °C. Limb reperfusion and angiogenesis was 69.3% higher in the hydrogel treated mice than in mice treated with free FGF-2. | Tissue engineering | Human neo-natal fibroblasts, BALB/c mice | [85] |
| Entrapment within polymer microspheres | FGF-2, magnesium hydroxide, heparin, PLGA and PVA1 were mixed to create a water/oil/water emulsion, from which microspheres were fabricated. | FGF-2 levels sustained for 3-4 days at 37 °C, compared with no activity remaining at 24 h for free FGF-2. | Cell culture research | Human embryonic stem cells, human neural stem cells, induced pluripotent stem cells | [86] |
| Alginate:collagen microspheres loaded with FGF-2 | Sustained release over 7 days at 37 °C | Tissue engineering | C57/Bl6 mice, porcine aortic endothelial cells | [83] | |
| Entrapment within polyelectrolyte polymer | HEMA2 hydrogel was loaded with either FGF-2 or FGF-2 and heparin. | Hydrogel containing only FGF-2 released FGF-2 over 6 days at 37 °C. Addition of heparin to the matrix prolonged the FGF-2 release to 12 days | Tissue engineering | Neural stem cells | [84] |
| Ionic entrapment within biodegradable multilayer thin film | Thin film consisting of 10–50 tetralayers made up of beta aminoester/ heparin/FGF-2/ heparin. | Up to 5 days sustained release of FGF-2 from the film, with in vitro biological activity sustained for 12 days at 37 °C. | Tissue engineering or wound healing | Murine MC3T3 pre-osteoblast cells | [54] |
| Adsorption onto polymer carriers | Bioactive glass nanoparticles were fabricated with the biodegradable polylactic acid into highly porous scaffolds. FGF-2 was then adsorbed onto the scaffold surface. | FGF-2 release was sustained over 4 weeks at 37 °C with an almost linear release pattern after the initial 3 days. Biological effect was observed over 3 weeks. | Tissue engineering | Mesenchymal stem cells | [87] |
| FGF-2 adsorbed onto the aluminium salt of cyclodextrin sulphate | FGF-2 retained activity following exposure to pepsin for 6 h or chromotrypsin for 24 h. Showed 62% improved efficacy against gastric ulcers in a rat model compared to 15% improvement with free FGF-2. | Wound healing | BHK-21 cells, Male Jal:Wistar rats | [88] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benington, L.; Rajan, G.; Locher, C.; Lim, L.Y. Fibroblast Growth Factor 2—A Review of Stabilisation Approaches for Clinical Applications. Pharmaceutics 2020, 12, 508. https://doi.org/10.3390/pharmaceutics12060508
Benington L, Rajan G, Locher C, Lim LY. Fibroblast Growth Factor 2—A Review of Stabilisation Approaches for Clinical Applications. Pharmaceutics. 2020; 12(6):508. https://doi.org/10.3390/pharmaceutics12060508
Chicago/Turabian StyleBenington, Leah, Gunesh Rajan, Cornelia Locher, and Lee Yong Lim. 2020. "Fibroblast Growth Factor 2—A Review of Stabilisation Approaches for Clinical Applications" Pharmaceutics 12, no. 6: 508. https://doi.org/10.3390/pharmaceutics12060508