New Trends in Bio-Based Aerogels
Abstract
:1. Introduction
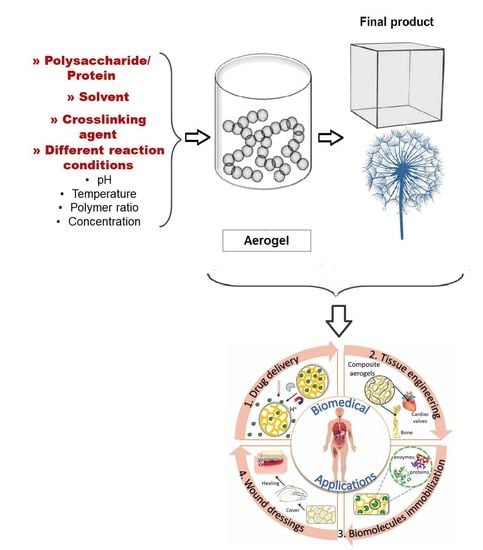
2. Methods of Preparation:
3. Drying Procedures for Obtaining Aerogels
3.1. Supercritical Conditions
3.2. Ambient Pressure Drying
3.3. Freeze-Drying (Lyophilization)
3.4. Other Methods
4. Bio-Based Aerogels
4.1. Cellulose-Based Aerogels
Type of Cellulose
4.2. Lignin-Based Aerogels
4.3. Pectin-Based Aerogels
4.4. Alginate-Based Aerogels
4.5. Starch-Based Aerogels
4.6. Chitosan-Based Aerogels
4.7. Protein-Based Aerogels
4.7.1. Albumin-Based Aerogels
4.7.2. Casein-Based Aerogels
4.7.3. Gelatin-Based Aerogels
5. Application in the Biomedical Field
5.1. Aerogel in Drug Delivery
5.2. Aerogel for Tissue Engineering
5.3. Aerogel for Biomolecules Immobilization
5.4. Aerogel for Wound Care
6. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Wan, W.; Zhang, R.; Ma, M.; Zhou, Y. Monolithic aerogel photocatalysts: A review. J. Mat. Chem. A 2018, 6, 754–775. [Google Scholar] [CrossRef]
- Shimizu, T.; Kanamori, K.; Nakanishi, K. Silicone-based organic-inorganic hybrid aerogels and xerogels. Chem. A Eur. J. 2017, 23, 5176–5187. [Google Scholar] [CrossRef]
- Zhao, S.; Malfait, W.J.; Guerrero-Alburquerque, N.; Koebel, M.M.; Nyström, G. Biopolymer aerogels and foams: Chemistry, properties, and applications. Angew. Chem. Int. Ed. 2018, 57, 7580–7608. [Google Scholar] [CrossRef] [PubMed]
- Subrahmanyam, R.; Gurikov, P.; Meissner, I.; Smirnova, I. Preparation of biopolymer aerogels using green solvents. J. Vis. Exp. 2016, 113, 22718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maleki, H.; Montes, S.; Hayati-Roodbari, N.; Putz, F.; Huesing, N. Compressible, thermally insulating, and fire retardant aerogels through self-assembling silk fibroin biopolymers inside a silica structure—An approach towards 3D printing of aerogels. ACS Appl. Mater. Interfaces 2018, 10, 22718–22730. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, I.; Gurikov, P. Aerogels in chemical engineering: Strategies toward tailor-made aerogels. Annu. Rev. Chem. Biomol. Eng. 2017, 8, 307–334. [Google Scholar] [CrossRef] [PubMed]
- Maleki, H.; Durães, L.; García-González, C.A.; del Gaudio, P.; Portugal, A.; Mahmoudi, M. Synthesis and biomedical applications of aerogels: Possibilities and challenges. Adv. Colloid Interface Sci. 2016, 236, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Rege, A.; Preibisch, I.; Schestakow, M.; Ganesan, K.; Gurikov, P.; Milow, B.; Itskov, M. Correlating synthesis parameters to morphological entities: Predictive modeling of biopolymer aerogels. Materials 2018, 11, 1670. [Google Scholar] [CrossRef] [Green Version]
- Randall, J.P.; Meador, M.A.B.; Jana, S.C. Tailoring mechanical properties of aerogels for aerospace applications. ACS Appl. Mater. Interfaces 2011, 3, 613–626. [Google Scholar] [CrossRef]
- Mitropoulos, A.; Burpo, F.; Nguyen, C.; Nagelli, E.; Ryu, M.; Wang, J.; Wickiser, J. Noble metal composite porous silk fibroin aerogel fibers. Materials 2019, 12, 894. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Su, Y.; Wang, W.; Fang, Y.; Riffat, S.B.; Jiang, F. The advances of polysaccharide-based aerogels: Preparation and potential application. Carbohydr. Polym. 2019, 226, 115242. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Zhang, Y.; Zhang, L.; Miao, Y.-E.; Fan, W.; Liu, T. Polymer/carbon-based hybrid aerogels: Preparation, properties and applications. Materials 2015, 8, 6806–6848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aerogel Market Size, Share & Trends Analysis Report By Form (Blanket, Particle, Panel, Monolith), By Product (Silica, Carbon, Polymers), By End Use, By Technology, By Region, And Segment Forecasts. Available online: https://www.researchandmarkets.com/reports/4613368/aerogel-market-size-share-and-trends-analysis#rela4-4894101 (accessed on 21 February 2020).
- Aerogel Market will reach CAGR of 18.63% in 2023, Current Position of key Vendors by Their Size & Share in Chemicals & Advanced Materials sector. Available online: https://menafn.com/1099566680/Aerogel-Market-will-reach-CAGR-of-1863-in-2023-Current-Position-of-key-Vendors-by-Their-Size-Share-in-Chemicals-Advanced-Materials-sector (accessed on 21 February 2020).
- Baumann, T.F.; Worsley, M.A.; Han, T.Y.J.; Satcher, J.H. High surface area carbon aerogel monoliths with hierarchical porosity. J. Non-Cryst. Solids 2008, 354, 3513–3515. [Google Scholar] [CrossRef]
- Karaaslan, M.A.; Kadla, J.F.; Ko, F.K. Lignin in Polymer Composites. In Lignin in Polymer Composites Lignin-Based Aerogels; Faruk, O., Sain, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 67–93. [Google Scholar]
- Kistler, S.S. Coherent Expanded Aerogels and Jellies. Nature 1931, 127, 741. [Google Scholar] [CrossRef]
- Fan, Y.; Ma, W.; Han, D.; Gan, S.; Dong, X.; Niu, L. Convenient recycling of 3D AGX/graphene aerogels (X = Br, Cl) for efficient photocatalytic degradation of water pollutants. Adv. Mater. 2015, 27, 3767–3773. [Google Scholar] [CrossRef] [PubMed]
- Bi, H.; Yin, Z.; Cao, X.; Xie, X.; Tan, C.; Huang, X.; Chen, B.; Chen, F.; Yang, Q.; Bu, X.; et al. Carbon fiber aerogel made from raw cotton: A novel, efficient and recyclable sorbent for oils and organic solvents. Adv. Mater. 2013, 25, 5916–5921. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, J.; Hu, Y.; Cheng, H.; Hu, C.; Jiang, C.; Jiang, L.; Cao, A.; Qu, L. Highly compression-tolerant supercapacitor based on polypyrrole-mediated graphene foam electrodes. Adv. Mater. 2013, 25, 591–595. [Google Scholar] [CrossRef]
- Moon, I.K.; Yoon, S.; Chun, K.Y.; Oh, J. Highly elastic and conductive n-doped monolithic graphene aerogels for multifunctional applications. Adv. Funct. Mater. 2015, 25, 6976–6984. [Google Scholar] [CrossRef]
- Zu, G.; Shen, J.; Wang, W.; Zou, L.; Lian, Y.; Zhang, Z.; Liu, B.; Zhang, F. Robust, Highly thermally stable, core-shell nanostructured metal oxide aerogelsas high-temperature thermal superinsulators, adsorbents, and catalysts. Chem. Mater. 2014, 26, 5761–5772. [Google Scholar] [CrossRef]
- Shang, K.; Yang, J.-C.; Cao, Z.-J.; Liao, W.; Wang, Y.-Z.; Schiraldi, D.A. Novel polymer aerogel toward high dimensional stability, mechanical property, and fire safety. ACS Appl. Mater. Interfaces 2017, 9, 22985–22993. [Google Scholar] [CrossRef]
- Thapliyal, P.C.; Singh, K. Aerogels as promising thermal insulating materials: An overview. J. Mater. 2014, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Tan, C.; Fung, B.M.; Newman, J.K.; Vu, C. Organic aerogels with very high impact strength. Adv. Mater. 2001, 13, 644–646. [Google Scholar] [CrossRef]
- Chen, H.-B.; Chiou, B.-S.; Wang, Y.-Z.; Schiraldi, D.A. Biodegradable pectin/clay aerogels. ACS Appl. Mater. Interfaces 2013, 5, 1715–1721. [Google Scholar] [CrossRef] [PubMed]
- Budtova, T. Cellulose II aerogels: A review. Cellulose 2019, 26, 81–121. [Google Scholar] [CrossRef]
- Silva, S.S.; Duarte, A.R.C.; Carvalho, A.P.; Mano, J.F.; Reis, R.L. Green processing of porous chitin structures for biomedical applications combining ionic liquids and supercritical fluid technology. Acta Biomater. 2011, 7, 1166–1172. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.; Bao, W.; Xu, S.; Wang, X.; Sun, R. Porous Cellulose Aerogels with High Mechanical Performance and their Absorption Behaviors. BioResources 2015, 11, 8–20. [Google Scholar] [CrossRef] [Green Version]
- Oshima, T.; Sakamoto, T.; Ohe, K.; Baba, Y. Cellulose aerogel regenerated from ionic liquid solution for immobilized metal affinity adsorption. Carbohydr. Polym. 2014, 103, 62–69. [Google Scholar] [CrossRef]
- Mi, Q.-Y.; Ma, S.-R.; Yu, J.; He, J.-S.; Zhang, J. Flexible and Transparent Cellulose Aerogels with Uniform Nanoporous Structure by a Controlled Regeneration Process. ACS Sustain. Chem. Eng. 2016, 4, 656–660. [Google Scholar] [CrossRef]
- Luo, Q.; Huang, X.; Gao, F.; Li, D.; Wu, M. Preparation and Characterization of High Amylose Corn Starch–Microcrystalline Cellulose Aerogel with High Absorption. Materials 2019, 12, 1420. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Xiong, Y.; Fan, B.; Yao, Q.; Wang, H.; Jin, C.; Sun, Q. Cellulose as an adhesion agent for the synthesis of lignin aerogel with strong mechanical performance, Sound-absorption and thermal Insulation. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Lopes, J.M.; Mustapa, A.N.; Pantić, M.; Bermejo, M.D.; Martín, Á.; Novak, Z.; Knez, Ž.; Cocero, M.J. Preparation of cellulose aerogels from ionic liquid solutions for supercritical impregnation of phytol. J. Supercrit. Fluids 2017, 130, 17–22. [Google Scholar] [CrossRef]
- Chiriac, A.P.; Neamtu, I.; Nita, L.E.; Nistor, M.T. Sol-Gel Based Materials for Biomedical Applications, the Sol-Gel Process: Uniformity, Polymers and Applications; Morris, R.E., Ed.; NovaPublisher: Hauppauge, NY, USA, 2010; pp. 1–68. ISBN 978-1-61761-621-1. [Google Scholar]
- Nistor, M.T.; Vasile, C.; Chiriac, A.P.; Rusu, A.; Zgardan, C.; Nita, L.E.; Neamtu, I. Hybrid Sensitive Hydrogels for Medical Applications. In Polymer Materials with Smart Properties; Chapter, 3, Bercea, M., Eds.; Nova Science Publ.: Hauppauge, NY, USA, 2013; pp. 67–89. ISBN 978-1-62808-876-2. [Google Scholar]
- Nita, L.E.; Chiriac, A.P.; Neamtu., I. Sol-Gel Technique Implemented for Biomedical Applications. In Polymer Materials with smart Properties; Chapter 8; Bercea, M., Ed.; Nova Science Publ.: Hauppauge, NY, USA, 2013; pp. 189–204. ISBN 978-1-62808-876-2. [Google Scholar]
- Chiriac, A.P.; Nita, L.E.; Neamtu, I.; Nistor, M.T. Sol gel method performed for biomedical products implementation. Mini-Rev. Med. 2010, 10, 990–1013. [Google Scholar] [CrossRef] [PubMed]
- Long, L.-Y.; Weng, Y.-X.; Wang, Y.-Z. Cellulose aerogels: Synthesis, applications, and prospects. Polymers 2018, 10, 623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gawryla, M.; Arndt, E.; Sánchez-Soto, M.; Schiraldi, D. Poly(amide-imide) aerogel materials produced via an ice templating process. Materials 2018, 11, 233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierre, A.C.; Pajonk, G.M. Chemistry of Aerogels and Their Applications. Chem. Rev. 2002, 102, 4243–4266. [Google Scholar] [CrossRef] [PubMed]
- Sehaqui, H.; Zhou, Q.; Berglund, L.A. High-porosity aerogels of high specific surface area prepared from nanofibrillated cellulose (NFC). Compos. Sci. Technol. 2011, 71, 1593–1599. [Google Scholar] [CrossRef]
- Schiraldi, D.A. Polymer Aerogels. In Encyclopedia of Polymer Science and Technology; Wiley: Hoboken, NJ, USA, 2015; pp. 1–18. [Google Scholar]
- Subrahmanyam, R.; Gurikov, P.; Dieringer, P.; Sun, M.; Smirnova, I. On the Road to Biopolymer Aerogels—Dealing with the Solvent. Gels 2015, 1, 291–313. [Google Scholar] [CrossRef] [Green Version]
- Baldino, L.; Concilio, S.; Cardea, S.; Reverchon, E. Interpenetration of natural polymer aerogels by supercritical drying. Polymers 2016, 8, 106. [Google Scholar] [CrossRef]
- Carroll, M.K.; Anderson, A.M.; Gorka, C.A. Preparing silica aerogel monoliths via a rapid supercritical extraction method. J. Vis. Exp. 2014, 84, e51421. [Google Scholar] [CrossRef] [Green Version]
- Quiño, J.; Ruehl, M.; Klima, T.; Ruiz, F.; Will, S.; Braeuer, A. Supercritical drying of aerogel: In Situ analysis of concentration profiles inside the gel and derivation of the effective binary diffusion coefficient using Raman spectroscopy. J. Supercrit Fluid. 2016, 108, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Błaszczyński, T.; Ślosarczyk, A.; Morawski, M. Synthesis of silica aerogel by supercritical drying method. Procedia Eng. 2013, 57, 200–206. [Google Scholar] [CrossRef] [Green Version]
- Lazrag, M.; Lemaitre, C.; Castel, C.; Hannachi, A.; Barth, D. Aerogel production by supercritical drying of organogels: Experimental study and modelling investigation of drying kinetics. J. Supercrit Fluid. 2018, 140, 394–405. [Google Scholar] [CrossRef]
- Şahin, İ.; Özbakır, Y.; İnönü, Z.; Ulker, Z.; Erkey, C. Kinetics of supercritical drying of gels. Gels 2017, 4, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preibisch, I.; Niemeyer, P.; Yusufoglu, Y.; Gurikov, P.; Milow, B.; Smirnova, I. Polysaccharide-based aerogel bead production via jet cutting method. Materials 2018, 11, 1287. [Google Scholar] [CrossRef] [Green Version]
- Druel, L.; Bardl, R.; Vorwerg, W.; Budtova, T. Starch aerogels: A member of the family of thermal superinsulating materials. Biomacromolecules 2017, 18, 4232–4239. [Google Scholar] [CrossRef]
- Yagoub, H.; Zhu, L.; Shibraen, M.H.M.A.; Altam, A.A.; Babiker, D.M.D.; Liang, S.; Yang, S. Complex aerogels generated from nano-polysaccharides and its derivatives for oil–water separation. Polymers 2019, 11, 1593. [Google Scholar] [CrossRef] [Green Version]
- Cai, J.; Kimura, S.; Wada, M.; Kuga, S.; Zhang, L. Cellulose aerogels from aqueous alkali hydroxide–urea solution. ChemSusChem 2008, 1, 149–154. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, X.; Lu, C.; Lan, L.; Yuan, G. Polyaniline-decorated cellulose aerogel nanocomposite with strong interfacial adhesion and enhanced photocatalytic activity. RSC Adv. 2014, 4, 8966. [Google Scholar] [CrossRef]
- Jiang, F.; Hsieh, Y.-L. Amphiphilic superabsorbent cellulose nanofibril aerogels. J. Mater. Chem. A 2014, 2, 6337–6342. [Google Scholar] [CrossRef] [Green Version]
- Qian, Z.; Wang, Z.; Zhao, N.; Xu, J. Aerogels derived from polymer nanofibers and their applications. Macromol. Rapid Commun. 2018, 39, 1700724. [Google Scholar] [CrossRef]
- Nguyen, B.N.; Cudjoe, E.; Douglas, A.; Scheiman, D.; McCorkle, L.; Meador, M.A.B.; Rowan, S.J. Polyimide cellulose nanocrystal composite aerogels. Macromolecules 2016, 49, 1692–1703. [Google Scholar] [CrossRef] [Green Version]
- Mulyadi, A.; Zhang, Z.; Deng, Y. Fluorine-Free oil absorbents made from cellulose nanofibril aerogels. ACS Appl. Mater. Interfaces 2016, 8, 2732–2740. [Google Scholar] [CrossRef] [PubMed]
- Demilecamps, A.; Beauger, C.; Hildenbrand, C.; Rigacci, A.; Budtova, T. Cellulose–silica aerogels. Carbohydr. Polym. 2015, 122, 293–300. [Google Scholar] [CrossRef]
- Seantier, B.; Bendahou, D.; Bendahou, A.; Grohens, Y.; Kaddami, H. Multi-scale cellulose based new bio-aerogel composites with thermal super-insulating and tunable mechanical properties. Carbohydr. Polym. 2015, 138, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Shulga, G.; Vitolina, S.; Shakels, V.; Belkova, L.; Cazacu, G.; Vasile, C.; Nita, L.E. Lignin separated from the hydrolyzate of the hydrothermal treatment of birch wood and its surface properties. Cell Chem Technol. 2012, 46, 287–318. [Google Scholar]
- Grishechko, L.I.; Amaral-Labat, G.; Szczurek, A.; Fierro, V.; Kuznetsov, B.N.; Pizzi, A.; Celzard, A. New tannin–lignin aerogels. Ind Crop. Prod. 2013, 41, 347–355. [Google Scholar] [CrossRef]
- Amaral-Labat, G.; Szczurek, A.; Fierro, V.; Pizzi, A.; Celzard, A. Systematic studies of tannin–formaldehyde aerogels: Preparation and properties. Sci. Technol. Adv. Mat. 2013, 14, 015001. [Google Scholar] [CrossRef] [Green Version]
- Szczurek, A.; Amaral-Labat, G.; Fierro, V.; Pizzi, A.; Masson, E.; Celzard, A. The use of tannin to prepare carbon gels. Part I: Carbon aerogels. Carbon 2011, 49, 2773–2784. [Google Scholar]
- Chen, F.; Xu, M.; Wang, L.; Li, J. Preparation and characterization of organic aerogels by the lignin-resorcinol-formaldehyde copolymer. BioResources 2011, 6, 1262–1272. [Google Scholar]
- Saito, T.; Brown, R.H.; Hunt, M.A.; Pickel, D.L.; Pickel, J.M.; Messman, J.M.; Baker, F.S.; Keller, M.; Naskar, A.K. Turning renewable resources into value-added polymer: Development of lignin-based thermoplastic. Green Chem. 2012, 14, 3295–3303. [Google Scholar] [CrossRef]
- Sriamornsak, P. Pectin: The role in helath. J. Silpakorn Univ. 2001, 21–22, 60–77. [Google Scholar]
- García-González, C.A.; Carenza, E.; Zeng, M.; Smirnova, I.; Roig, A. Design of biocompatible magnetic pectin aerogel monoliths and microspheres. RSC Adv. 2012, 2, 9816. [Google Scholar] [CrossRef]
- Veronovski, A.; Tkalec, G.; Knez, Ž.; Novak, Z. Characterisation of biodegradable pectin aerogels and their potential use as drug carriers. Carbohydr. Polym. 2014, 113, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Groult, S.; Budtova, T. Thermal conductivity/structure correlations in thermal super-insulating pectin aerogels. Carbohydr. Polym. 2018, 196, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Stanford, E.C.C. On algin: A new substance obtained from some of the commoner species of marine algae. Chem. News 1883, 47, 254. [Google Scholar] [CrossRef]
- Vasile, C.; Nita, L.E. Novel multi-stimuli responsive sodium alginate-grafted-poly(N-isopropylacrylamide) copolymers: II. Dilute solution properties. Carbohydr. Polym. 2011, 86, 77–84. [Google Scholar] [CrossRef]
- Mehling, T.; Smirnova, I.; Guenther, U.; Neubert, R.H.H. Polysaccharide-based aerogels as drug carriers. J. Non-Cryst. Solids 2009, 355, 2472–2479. [Google Scholar] [CrossRef]
- Veronovski, A.; Knez, Ž.; Novak, Z. Preparation of multi-membrane alginate aerogels used for drug delivery. J. Supercrit Fluids 2013, 79, 209–215. [Google Scholar] [CrossRef]
- Paques, J.P.; van der Linden, E.; van Rijn, C.J.M.; Sagis, L.M.C. Preparation methods of alginate nanoparticles. Adv. Colloid Interface Sci. 2014, 209, 163–171. [Google Scholar] [CrossRef]
- Paques, J.P.; Sagis, L.M.C.; van Rijn, C.J.M.; van der Linden, E. Nanospheres of alginate prepared through w/o emulsification and internal gelation with nanoparticles of CaCO3. Food Hydrocoll. 2014, 40, 182–188. [Google Scholar] [CrossRef]
- Rodríguez-Dorado, R.; López-Iglesias, C.; García-González, C.; Auriemma, G.; Aquino, R.; Del Gaudio, P. Design of aerogels, cryogels and xerogels of alginate: Effect of molecular weight, gelation conditions and drying method on particles’ micromeritics. Molecules 2019, 24, 1049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mustapa, A.N.; Martín, Á.; Cocero, M.J. Alginate aerogels dried by supercritical CO2 as herbal delivery carrier. Malays. J. Anal. Sci. 2018, 22, 522–531. [Google Scholar]
- Baudron, V.; Gurikov, P.; Smirnova, I. A continuous approach to the emulsion gelation method for the production of aerogel micro-particle. Colloids Surf. Phys. Eng. Asp. 2018, 566, 58–69. [Google Scholar] [CrossRef]
- Ahmad, F.; Ulker, Z.; Erkey, C. A novel composite of alginate aerogel with PET nonwoven with enhanced thermal resistance. J. Non-Cryst. Solids 2018, 491, 7–13. [Google Scholar] [CrossRef]
- Martins, M.; Barros, A.A.; Quraishi, S.; Gurikov, P.; Raman, S.P.; Smirnova, I.; Reis, R.L. Preparation of macroporous alginate-based aerogels for biomedical applications. J. Supercrit Fluids 2015, 106, 152–159. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Zhang, H. Alginate/pectin aerogel microspheres for controlled release of proanthocyanidins. Int. J. Biol. Macromol. 2019, 136, 936–943. [Google Scholar] [CrossRef]
- Dosta, M.; Jarolin, K.; Gurikov, P. Modelling of mechanical behavior of biopolymer alginate aerogels using the bonded-particle model. Molecules 2019, 24, 2543. [Google Scholar] [CrossRef] [Green Version]
- García-González, C.A.; Alnaief, M.; Smirnova, I. Polysaccharide-based aerogels—promising biodegradable carriers for drug delivery systems. Carbohydr. Polym. 2011, 86, 14–25. [Google Scholar] [CrossRef]
- Tudorachi, N.; Chiriac, A.P.; Nita, L.E.; Mustata, F.; Diaconu, A.; Balan, V.; Rusu, A.G.; Lisa, G. Studies on the nanocomposites based on carboxymethyl starch-g-lactic acid-co-glycolic acid copolymer and magnetite. J. Anal. Calorim. 2018, 131, 1867–1880. [Google Scholar] [CrossRef]
- Ubeyitogullari, A.; Ciftci, O.N. Formation of nanoporous aerogels from wheat starch. Carbohydr. Polym. 2016, 147, 125–132. [Google Scholar] [CrossRef]
- Wang, L.; Sánchez-Soto, M.; Abt, T.; Maspoch, M.L.; Santana, O.O. Microwave-crosslinked bio-based starch/clay aerogels. Polym. Int. 2016, 65, 899–904. [Google Scholar] [CrossRef] [Green Version]
- Kenar, J.A.; Eller, F.J.; Felker, F.C.; Jackson, M.A.; Fanta, G.F. Starch aerogel beads obtained from inclusion complexes prepared from high amylose starch and sodium palmitate. Green Chem. 2014, 16, 1921–1930. [Google Scholar] [CrossRef]
- Ganesan, K.; Budtova, T.; Ratke, L.; Gurikov, P.; Baudron, V.; Preibisch, I.; Milow, B. Review on the production of polysaccharide aerogel particles. Materials 2018, 11, 2144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos-Rosales, V.; Ardao, I.; Alvarez-Lorenzo, C.; Ribeiro, N.; Oliveira, A.L.; García-González, C.A. Sterile and dual-porous aerogels scaffolds obtained through a multistep supercritical CO2-based approach. Molecules 2019, 24, 871. [Google Scholar] [CrossRef] [Green Version]
- El Kadib, A.; Bousmina, M. Chitosan bio-based organic-inorganic hybrid aerogel microspheres. Chem. Eur. J. 2011, 18, 8264–8277. [Google Scholar] [CrossRef]
- Ghimici, L.; Brunchi, C.E.; Diaconu, A. Removal of some commercial pesticides containing α-cypermethrin, deltamethrin and mancozeb as active ingredients by chitosan solution. Cellulose 2018, 23, 3837–3846. [Google Scholar] [CrossRef]
- Dumitriu, R.P.; Profire, L.; Nita, L.E.; Dragostin, O.M.; Ghetu, N.; Pieptu, D.; Vasile, C. Sulfadiazine-chitosan conjugates and their polyelectrolyte complexes with hyaluronate destined to the management of burn wounds. Material 2015, 8, 317–338. [Google Scholar] [CrossRef] [Green Version]
- Rusu, A.G.; Chiriac, A.P.; Nita, L.E.; Bercea, M.; Tudorachi, N.; Ghilan, A.; Pamfil, D.; Rusu, D.; Cojocaru, F.D. Interpenetrated polymer network with modified chitosan in composition and self-healing properties. Int. J. Biol. Macromol. 2019, 132, 374–384. [Google Scholar] [CrossRef]
- Chiriac, A.P.; Ghilan, A.; Neamtu, I.; Nita, L.E.; Rusu, A.G. Advancement in the biomedical applications of the (nano)gel structures based on particular polysaccharides. Macromol. Biosci. 2019, 19, 1900187. [Google Scholar] [CrossRef]
- Rinki, K.; Dutta, P.K.; Hunt, A.J.; Macquarrie, D.J.; Clark, J.H. Chitosan aerogels exhibiting high surface area for biomedical application: Preparation, characterization, and antibacterial study. Int J. Polym Mater. 2011, 60, 988–999. [Google Scholar] [CrossRef]
- Zhang, S.; Feng, J.; Feng, J.; Jiang, Y.; Li, L. Ultra-low shrinkage chitosan aerogels trussed with polyvinyl alcohol. Mater. Des. 2018, 156, 398–406. [Google Scholar] [CrossRef]
- Takeshita, S.; Yoda, S. Upscaled preparation of trimethylsilylated chitosan aerogel. Ind. Eng. Chem. Res. 2018, 57, 10421–10430. [Google Scholar] [CrossRef]
- Santos-López, G.; Argüelles-Monal, W.; Carvajal-Millan, E.; López-Franco, Y.; Recillas-Mota, M.; Lizardi-Mendoza, J. Aerogels from chitosan solutions in ionic liquids. Polymers 2017, 9, 722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keshipour, S.; Mirmasoudi, S.S. Cross-linked chitosan aerogel modified with Au: Synthesis, characterization and catalytic application. Carbohydr. Polym. 2018, 196, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Radwan-Pragłowska, J.; Piątkowski, M.; Janus, Ł.; Bogdał, D.; Matysek, D. Biodegradable, pH-responsive chitosan aerogels for biomedical applications. RSC Adv. 2017, 7, 32960–32965. [Google Scholar] [CrossRef] [Green Version]
- Gong, Y.; Yu, Y.; Kang, H.; Chen, X.; Liu, H.; Zhang, Y.; Song, H. Synthesis and characterization of graphene oxide/chitosan composite aerogels with high mechanical performance. Polymers 2019, 11, 777. [Google Scholar] [CrossRef] [Green Version]
- Betz, M.; García-González, C.A.; Subrahmanyam, R.P.; Smirnova, I.; Kulozik, U. Preparation of novel whey protein-based aerogels as drug carriers for life science applications. J. Supercrit Fluids 2012, 72, 111–119. [Google Scholar] [CrossRef]
- Mallepally, R.R.; Marin, M.A.; Surampudi, V.; Subia, B.; Rao, R.R.; Kundu, S.C.; McHugh, M.A. Silk fibroin aerogels: Potential scaffolds for tissue engineering applications. Biomed. Mater. 2015, 10, 035002. [Google Scholar] [CrossRef]
- Arboleda, J.C.; Hughes, M.; Lucia, L.A.; Laine, J.; Ekman, K.; Rojas, O.J. Soy protein–nanocellulose composite aerogels. Cellulose 2013, 20, 2417–2426. [Google Scholar] [CrossRef]
- Oh, J.K.; Perez, K.; Kohli, N.; Kara, V.; Li, J.; Min, Y.; Akbulut, M. Hydrophobically-modified silica aerogels: Novel food-contact surfaces with bacterial anti-adhesion properties. Food Control. 2015, 52, 132–141. [Google Scholar] [CrossRef]
- Kleemann, C.; Selmer, I.; Smirnova, I.; Kulozik, U. Tailor made protein based aerogel particles from egg white protein, whey protein isolate and sodium caseinate: Influence of the preceding hydrogel characteristics. Food Hydrocoll. 2018, 83, 365–374. [Google Scholar] [CrossRef]
- Ghilan, A.; Chiriac, A.P.; Nita, L.E. Magnetic composites based on bovine serum albumin and poly(aspartic acid). Polym. Eng. Sci. 2019, 59, 1409–1415. [Google Scholar] [CrossRef]
- Rusu, A.G.; Chiriac, A.P.; Nita, L.E.; Mititelu-Tartau, L.; Tudorachi, N.; Ghilan, A.; Rusu, D. Multifunctional BSA scaffolds prepared with a novel combination of UV-crosslinking systems. Macromol. Chem. Phys. 2019, 1900378. [Google Scholar] [CrossRef]
- Diaconu, A.; Nita, L.E.; Chiriac, A.P.; Butnaru, M. Investigation of the magnetic field effect upon interpolymeric complexes formation based on bovine serum albumin and poly(aspartic acid). Int. J. Biol. Macromol. 2018, 119, 974–981. [Google Scholar] [CrossRef]
- Nita, L.E.; Chiriac, A.P.; Bercea, M.; Asandulesa, M.; Wolf, B.A. Self-assembling of poly(aspartic acid) with bovine serum albumin in aqueous solutions. Int. J. Biol. Macromol. 2017, 95, 412–420. [Google Scholar] [CrossRef]
- Li, X.; Pizzi, A.; Cangemi, M.; Navarrete, P.; Segovia, C.; Fierro, V.; Celzard, A. Insulation rigid and elastic foams based on albumin. Ind. Crop. Prod. 2012, 37, 149–154. [Google Scholar] [CrossRef]
- Lacoste, C.; Basso, M.C.; Pizzi, A.; Celzard, A.; Laborie, M.-P. Natural albumin/tannin 978-1-78262-765-4; PDF eISBN cellular foams. Ind Crop. Prod. 2015, 73, 41–48. [Google Scholar] [CrossRef]
- Muthuraj, R.; Jimenez-Saelices, C.; Grohens, Y.; Seantier, B. Applications of polysaccharide and protein based aerogels in thermal insulation. In in Biobased Aerogels: Polysaccharide and Protein-Based Materials; Chapter 15; Royal Society of Chemistry: London, UK, 2018. [Google Scholar]
- Patni, N.; Tripathi, N.; Bosmia, S. Biodegradable polymer: A casein aerogel composite; casein Extraction from various milk samples and its role as a viable substitute for conventional plastics. Int. J. Appl. Eng. Res. 2013, 8, 10–13. [Google Scholar]
- Wang, J.; Zhao, D.; Shang, K.; Wang, Y.-T.; Ye, D.-D.; Kang, A.-H.; Wang, Y.-Z. Ultrasoft gelatin aerogels for oil contaminant removal. J. Mater. Chem. A 2016, 4, 9381–9389. [Google Scholar] [CrossRef]
- Kuttor, A.; Szalóki, M.; Rente, T.; Kerényi, F.; Bakó, J.; Fábián, I.; Hegedüs, C. Preparation and application of highly porous aerogel-based bioactive materials in dentistry. Front. Mater. Sci. 2014, 8, 46–52. [Google Scholar] [CrossRef]
- Glenn, G.M.; Irving, D.W. Starch-Based Microcellular Foams. Cereal Chem. 1995, 72, 155–161. [Google Scholar]
- Te Wierik, G.H.P.; Bergsma, J.; Arends, A.W.; Boersma, T.; Eissens, A.C.; Lerk, C.F. A new generation of starch products as excipient in pharmaceutical tablets. I. Preparation and binding properties of high surface area potato starch products. Int. J. Pharm. 1996, 134, 27–36. [Google Scholar] [CrossRef]
- García-González, C.A.; Smirnova, I. Use of supercritical fluid technology for the production of tailor-made aerogel particles for delivery systems. J. Supercrit Fluids 2013, 79, 152–158. [Google Scholar] [CrossRef]
- Starbird, R.; García-González, C.A.; Smirnova, I.; Krautschneider, W.H.; Bauhofer, W. Synthesis of an organic conductive porous material using starch aerogels as template for chronic invasive electrodes. Mater. Sci. Eng. C 2014, 37, 177–183. [Google Scholar] [CrossRef]
- Cai, H.; Sharma, S.; Liu, W.; Mu, W.; Liu, W.; Zhang, X.; Deng, Y. Aerogel microspheres from natural cellulose nanofibrils and their application as cell culture scaffold. Biomacromolecules 2014, 15, 2540–2547. [Google Scholar] [CrossRef]
- In, E.; Naguib, H. Fabrication and characterization of silica aerogel as synthetic tissues for medical imaging phantoms. AIP Conf. Proc. 2015, 1664, 130002. [Google Scholar] [CrossRef]
- Nita, L.E.; Chiriac, A.P.; Bercea, M.; Ghilan, A.; Rusu, A.G.; Tudorachi, N. Multifunctional hybrid 3D network based on hyaluronic acid and a copolymer containing pendant spiroacetal moieties. Int. J. Biol. Macromol. 2019, 125, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Nita, L.E.; Chiriac, A.P.; Rusu, A.G.; Bercea, M.; Ghilan, A.; Dumitriu, R.P.; Mititelu-Tartau, L. New self-healing hydrogels based on reversible physical interactions and their potential applications. Eur. Polym. J. 2019, 118, 176–185. [Google Scholar] [CrossRef]
- Diaconu, A.; Nita, L.E.; Bercea, M.; Chiriac, A.P.; Rusu, A.G.; Rusu, D. Hyaluronic acid gels with tunable properties by conjugating with a synthetic copolymer. Biochem. Eng. J. 2017, 125, 135–143. [Google Scholar] [CrossRef]
- Desfrançois, C.; Auzély, R.; Texier, I. Lipid nanoparticles and their hydrogel composites for drug delivery: A Review. Pharmaceuticals 2018, 11, 118. [Google Scholar] [CrossRef] [Green Version]
- Naguib, H.E.; Al Jahwari, F. An accurate higher order plate theory for tailoring the properties of functionally graded porous media. AIP Conf. Proc. 2015, 1664, 040006. [Google Scholar] [CrossRef] [Green Version]
- Wan, C.; Jiao, Y.; Sun, Q.; Li, J. Preparation, characterization, and antibacterial properties of silver nanoparticles embedded into cellulose aerogels. Polym. Compos. 2014, 37, 1137–1142. [Google Scholar] [CrossRef]
- El-Naggar, M.E.; Othman, S.I.; Allam, A.A.; Morsy, O.M. Synthesis, drying process and medical application of polysaccharide-based aerogels. Int. J. Biol. Macromol. 2020, 145, 1115–1128. [Google Scholar] [CrossRef] [PubMed]
- Malik, E.; Dennison, S.R.; Harris, F.; Phoenix, D.A. pH dependent antimicrobial peptides and proteins, their mechanisms of action and potential as therapeutic agents. Pharmaceuticals 2016, 9, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ulker, Z.; Erkey, C. A novel hybrid material: An inorganic silica aerogel core encapsulated with a tunable organic alginate aerogel layer. RSC Adv. 2014, 4, 62362–62366. [Google Scholar] [CrossRef] [Green Version]
- Lovskaya, D.D.; Lebedev, A.E.; Menshutina, N.V. Aerogels as drug delivery systems: In vitro and in vivo evaluations. J. Supercrit. Fluids 2015, 106, 115–121. [Google Scholar] [CrossRef]
- Yan, N.; Zhou, Y.; Zheng, Y.; Qiao, S.; Yu, Q.; Li, Z.; Lu, H. Antibacterial properties and cytocompatibility of bio-based nanostructured carbon aerogels derived from silver nanoparticles deposited onto bacterial cellulose. RSC Adv. 2015, 5, 97467–97476. [Google Scholar] [CrossRef]
- Rudaz, C.; Courson, R.M.; Bonnet, L.; Calas-Etienne, S.; Sallée, H.; Budtova, T. Aeropectin: Fully biomass-based mechanically strong and thermal superinsulating aerogel. Biomacromolecules 2014, 15, 2188–2195. [Google Scholar] [CrossRef]
- Vilela, C.; Pinto, R.J.B.; Pinto, S.; Marques, P.; Silvestre, A.; Barros, C.S.R.F. Polysaccharides-Based Hybrids with Carbon Nanotubes, Polysaccharide Based Hybrid Materials; Springer: Berlin, Germany, 2018; pp. 95–114. [Google Scholar]
- De Cicco, F.; Russo, P.; Reverchon, E.; García-González, C.A.; Aquino, R.P.; Del Gaudio, P. Prilling and supercritical drying: A successful duo to produce core-shell polysaccharide aerogel beads for wound healing. Carbohydr. Polym. 2016, 147, 482–489. [Google Scholar] [CrossRef]
- López-Iglesias, C.; Barros, J.; Ardao, I.; Monteiro, F.J.; Alvarez-Lorenzo, C.; GómezAmoza, J.L.; García-González, C.A. Vancomycin-loaded chitosan aerogel particles for chronic wound applications. Carbohydr. Polym. 2019, 204, 223–231. [Google Scholar] [CrossRef]
- Concha, M.; Vidal, A.; Giacaman, A.; Ojeda, J.; Pavicic, F.; Oyarzun-Ampuero, F.A.; Torres, C.; Cabrera, M.; Moreno-Villoslada, I.; Orellana, S.L. Aerogels made of chitosan and 37 chondroitin sulfate at high degree of neutralization: Biological properties toward wound healing. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 2464–2471. [Google Scholar] [CrossRef]
- Ulker, Z.; Erkey, C. An emerging platform for drug delivery: Aerogel based systems. J. Control. Release 2014, 177, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Du, A.; Zhou, B.; Zhang, Z.; Shen, J. A special material or a new state of matter: A review and reconsideration of the aerogel. Materials 2013, 6, 941–968. [Google Scholar] [CrossRef] [Green Version]
- Selmer, I.; Kleemann, C.; Kulozik, U.; Heinrich, S.; Smirnova, I. Development of egg white protein aerogels as new matrix material for microencapsulation in food. J. Supercrit. Fluids 2015, 106, 42–49. [Google Scholar] [CrossRef]
- Tkalec, G.; Knez, Ž.; Novak, Z. Formation of polysaccharide aerogels in ethanol. RSC Adv. 2015, 5, 77362–77371. [Google Scholar] [CrossRef]
- Veronovski, A.; Knez, Ž.; Novak, Z. Comparison of ionic and non-ionic drug release from multi-membrane spherical aerogels. Int. J. Pharm 2013, 454, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Veres, P.; López-Periago, A.M.; Lázár, I.; Saurina, J.; Domingo, C. Hybrid aerogel preparations as drug delivery matrices for low water-solubility drugs. Int. J. Pharm 2015, 496, 360–370. [Google Scholar] [CrossRef] [Green Version]
- García-González, C.A.; Jin, M.; Gerth, J.; Alvarez-Lorenzo, C.; Smirnova, I. Polysaccharide-based aerogel microspheres for oral drug delivery. Carbohydr. Polym. 2015, 117, 797–806. [Google Scholar] [CrossRef] [Green Version]
- Obaidat, R.M.; Tashtoush, B.M.; Bayan, M.F. Drying using supercritical fluid technology as a potential method for preparation of chitosan aerogel microparticles. AAPS Pharm Sci Tech. 2015, 16, 1235–1244. [Google Scholar] [CrossRef] [Green Version]
- Raman, S.P.; Gurikov, P.; Smirnova, I. Hybrid alginate based aerogels by carbon dioxide induced gelation: Novel technique for multiple applications. J. Supercrit. Fluids 2015, 106, 23–33. [Google Scholar] [CrossRef]
- Ratke, L.; Pierre, A.C. Monoliths and fibrous cellulose aerogels, 173–191. In Aerogels Handbook; Chapter 9; Series: Advances in Sol-Gel Derived Materials and Technologies; Aegerter, M.A., Leventis, N., Koebel, M.M., Eds.; Springer: New York, NY, USA, 2011; ISBN: 1441974776,9781441974778. [Google Scholar]
- Zhao, J.; Lu, C.; He, X.; Zhang, X.; Zhang, W.; Zhang, X. Polyethylenimine-grafted cellulose nanofibril aerogels as versatile vehicles for drug delivery. ACS Appl. Mater. Interfaces 2015, 7, 2607–2615. [Google Scholar] [CrossRef] [PubMed]
- Nagy, G.; Király, G.; Veres, P.; Lázár, I.; Fábián, I.; Bánfalvi, G.; Kalmár, J. Controlled release of methotrexate from functionalized silica-gelatin aerogel microparticles applied against tumor cell growth. Int. J. Pharm 2019, 558, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Tkalec, G.; Knez, Ž.; Novak, Z. Fast production of high-methoxyl pectin aerogels for enhancing the bioavailability of low-soluble drugs. J. Supercrit Fluids 2015, 106, 16–22. [Google Scholar] [CrossRef]
- Horvat, G.; Knez, Ž.; Novak, Z. Encapsulation of pharmaceuticals into pectin aerogels for controlled drug release. Adv. Tech. 2015, 4, 49–52. [Google Scholar]
- Marin, M.A.; Mallepally, R.R.; McHugh, M.A. Silk fibroin aerogels for drug delivery applications. J. Supercrit. Fluids 2014, 91, 84–89. [Google Scholar] [CrossRef]
- Stergar, J.; Maver, U. Review of aerogel-based materials in biomedical applications. J. Sol.-Gel. Sci. Techn. 2016, 77, 738–752. [Google Scholar] [CrossRef]
- Owens, G.J.; Singh, R.K.; Foroutan, F.; Alqaysi, M.; Han, C.-M.; Mahapatra, C.; Knowles, J.C. Sol–gel based materials for biomedical applications. Prog. Mater. Sci. 2016, 77, 1–79. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Ruíz, A.; Escobar-García, D.M.; Quintana, M.; Pozos-Guillén, A.; Flores, H. Synthesis and characterization of a new collagen-alginate aerogel for tissue engineering. J. Nanomat. 2019. [Google Scholar] [CrossRef] [Green Version]
- Silva, S.S.; Duarte, A.R.C.; Oliveira, J.M.; Mano, J.F.; Reis, R.L. Alternative methodology for chitin–hydroxyapatite composites using ionic liquids and supercritical fluid technology. J. Bioact. Compat. Polym. 2013, 28, 481–491. [Google Scholar] [CrossRef] [Green Version]
- Quraishi, S.; Martins, M.; Barros, A.A.; Gurikov, P.; Raman, S.P.; Smirnova, I.; Reis, R.L. Novel non-cytotoxic alginate–lignin hybrid aerogels as scaffolds for tissue engineering. J. Supercrit Fluids 2015, 105, 1–8. [Google Scholar] [CrossRef]
- Lu, T.; Li, Q.; Chen, W.; Yu, H. Composite aerogels based on dialdehyde nanocellulose and collagen for potential applications as wound dressing and tissue engineering scaffold. Compos. Sci Technol. 2014, 94, 132–138. [Google Scholar] [CrossRef]
- Reverchon, E.; Pisanti, P.; Cardea, S. Nanostructured PLLA−hydroxyapatite scaffolds produced by a supercritical assisted technique. Ind. Eng. Chem. Res. 2009, 48, 5310–5316. [Google Scholar] [CrossRef]
- Ge, J.; Li, M.; Zhang, Q.; Yang, C.Z.; Wooley, P.H.; Chen, X.; Yang, S.-Y. Silica aerogel improves the biocompatibility in a poly-ε-caprolactone composite used as a tissue engineering scaffold. Int. J. Polym. Sci. 2013, 2013, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.K.; Yang, D.-K.; Chen, Y.-C.; Su, H.-J.; Wu, J.-C.; Chen-Yang, Y.W. A novel three-dimensional aerogel biochip for molecular recognition of nucleotide acids. Acta Biomater. 2010, 6, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Xie, Y.; Wang, Y.; Qi, W.; Huang, R.; Su, R.; He, Z. Self-assembled microporous peptide-polysaccharide aerogels for oil-water separation. Langmuir 2018, 34, 10732–10738. [Google Scholar] [CrossRef] [PubMed]
- Leventis, N.; Sadekar, A.; Chandrasekaran, N.; Sotiriou-Leventis, C. Click synthesis of monolithic silicon carbide aerogels from polyacrylonitrile-coated 3D silica networks. Chem Mater. 2010, 22, 2790–2803. [Google Scholar] [CrossRef]
- Leventis, N.; Chandrasekaran, N.; Sadekar, A.G.; Mulik, S.; Sotiriou-Leventis, C. The effect of compactness on the carbothermal conversion of interpenetrating metal oxide/resorcinol-formaldehyde nanoparticle networks to porous metals and carbides. J. Mater. Chem. 2010, 20, 7456. [Google Scholar] [CrossRef]
- Duan, Y.; Jana, S.C.; Lama, B.; Espe, M.P. Reinforcement of silica aerogels using silane-end-capped polyurethanes. Langmuir 2013, 29, 6156–6165. [Google Scholar] [CrossRef] [PubMed]
- White, L.S.; Bertino, M.F.; Kitchen, G.; Young, J.; Newton, C.; Al-Soubaihi, R.; Saoud, K. Shortened aerogel fabrication times using an ethanol–water azeotrope as a gelation and drying solvent. J. Mater. Chem. A 2015, 3, 762–772. [Google Scholar] [CrossRef]
- Leventis, N.; Sotiriou-Leventis, C.; Chandrasekaran, N.; Mulik, S.; Larimore, Z.J.; Lu, H.; Mang, J.T. Multifunctional polyurea aerogels from isocyanates and water. A structure−property case study. Chem. Mater. 2010, 22, 6692–6710. [Google Scholar] [CrossRef]
- Zhou, L.; Zhai, Y.-M.; Yang, M.-B.; Yang, W. Flexible and Tough Cellulose Nanocrystal/Polycaprolactone Hybrid Aerogel Based on the Strategy of Macromolecule Cross-Linking via Click Chemistry. ACS Sustain. Chem. Eng. 2019, 7, 15617–15627. [Google Scholar] [CrossRef]
- Hosseini, H.; Zirakjou, A.; Goodarzi, V.; Mousavi, S.M.; Khonakdar, H.A.; Zamanlui, S. Lightweight aerogels based on bacterial cellulose/silver nanoparticles/polyaniline with tuning morphology of polyaniline and application in soft tissue engineering. Int. J. Biol. Macromol. 2020, 152, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.; Fontenot, K.; Liebner, F.; Condon, B. Peptide-Cellulose Conjugates on Cotton-Based Materials Have Protease Sensor/Sequestrant Activity. Sensors 2018, 18, 2334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bacakova, L.; Pajorova, J.; Bacakova, M.; Skogberg, A.; Kallio, P.; Kolarova, K.; Svorcik, V. Versatile application of nanocellulose: From industry to skin tissue engineering and wound healing. Nanomaterials 2019, 9, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubo, S.; Kadla, J.F. The formation of strong intermolecular interactions in immiscible blends of poly (vinyl alcohol)(PVA) and lignin. Biomacromolecules 2003, 4, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Bacakova, L.; Pajorova, J.; Tomkova, M.; Matejka, R.; Broz, A.; Stepanovska, J.; Prazak, S.; Skogberg, A.; Siljander, S.; Kallio, P. Applications of Nanocellulose/Nanocarbon Composites: Focus on Biotechnology and Medicine. Nanomaterials 2020, 10, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Cheng, F.; Liu, J.; Smått, J.-H.; Gepperth, D.; Lastusaari, M.; Xu, C.; Hupa, L. Biocomposites of copper-containing mesoporous bioactive glass and nanofibrillated cellulose: Biocompatibility and angiogenic promotion in chronic wound healing application. Acta Biomater. 2016, 46, 286–298. [Google Scholar] [CrossRef]
- Si, Y.; Yu, J.; Tang, X.; Ge, J.; Ding, B. Ultralight nanofibre-assembled cellular aerogels with superelasticity and multifunctionality. Nat. Commun. 2014, 5, 5802. [Google Scholar] [CrossRef] [Green Version]
- Cook, H.; Stephens, P.; Davies, K.J.; Thomas, D.W.; Harding, K.G. Defective Extracellular Matrix Reorganization by Chronic Wound Fibroblasts is Associated with Alterations in TIMP-1, TIMP-2, and MMP-2 Activity. J. Investig. Dermatol. 2000, 115, 225–233. [Google Scholar] [CrossRef] [Green Version]
- Franco, P.; Pessolano, E.; Belvedere, R.; Petrella, A.; Marco, I.D. Supercritical impregnation of mesoglycan into calcium alginate aerogel for wound healing. J. Supercrit. Fluids 2020, 157, 104711. [Google Scholar] [CrossRef]
- Dharunya, G.; Duraipandy, N.; Lakra, R.; Korapatti, P.S.; Jayavel, R.; Kiran, M.S. Curcumin cross-linked collagen aerogels with controlled anti-proteolytic and pro-angiogenic efficacy. Biomed. Mater. 2016, 11, 045011. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, D.; Duraipandy, N.; Srivatsan, K.V.; Lakra, R.; Korapatti, P.S.; Jayavel, R.; Kiran, M.S. Fabrication of Hybrid Collagen Aerogels Reinforced with Wheat Grass Bioactives as Instructive Scaffolds for Collagen Turnover and Angiogenesis for Wound Healing Applications. ACS Appl. Mater. Interfaces 2017, 9, 16939–16950. [Google Scholar] [CrossRef] [PubMed]
- García-González, C.A.; Concheiro, A.; Alvarez-Lorenzo, C. Processing of materials for regenerative medicine using supercritical fluid technology. Bioconjugate Chem. 2015, 26, 1159–1171. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Venkitachalam, S.M.; Jarrett, E.; Staggs, S.; Leventis, N.; Lu, H.; Rubenstein, D.A. Biocompatibility of surfactant-templated polyurea-nanoencapsulated macroporous silica aerogels with plasma platelets and endothelial cells. J. Biomed. Mater. Res. A 2009, 92, 1431–1439. [Google Scholar] [CrossRef]
- Barrios, E.; Fox, D.; Li Sip, Y.Y.; Catarata, R.; Calderon, J.E.; Azim, N.; Afrin, S.; Zhang, Z.; Zhai, L. Nanomaterials in advanced, high-performance aerogel composites: A review. Polymers 2019, 11, 726. [Google Scholar] [CrossRef] [Green Version]
- García-González, C.A.; Budtova, T.; Durães, L.; Erkey, C.; Del Gaudio, P.; Gurikov, P.; Smirnova, I. An opinion paper on aerogels for biomedical and environmental applications. Molecules 2019, 24, 1815. [Google Scholar] [CrossRef] [Green Version]






| Biobased Aerogels | References |
|---|---|
| Cellulose-based aerogels | [12,23,25,27,39,53,54,55,56,57,58,59,60,61] |
| Lignin-based aerogels | [62,63,64,65,66,67,68] |
| Pectin-based aerogels | [4,26,68,69,70,71] |
| Alginate-based aerogels | [3,13,72,73,74,75,76,77,78,79,80,81,82,83,84] |
| Starch-based aerogels | [85,86,87,88,89,90,91] |
| Chitosan-based aerogels | [12,92,93,94,95,96,97,98,99,100,101,102,103] |
| Protein-based aerogels | [7,26,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118] |
| Fields of Applications of Aerogels | References |
|---|---|
| Aerogel in drug delivery | [7,74,144,145,146,147] |
| chitosan | [98,100,102,148] |
| alginate | [75,133,149] |
| celulose | [150,151] |
| gelatin | [152] |
| pectine | [68,153,154] |
| protein | [104,143,155] |
| Aerogel for tissue engineering | [121,156,157] |
| collagen/alginate | [158] |
| chitin-hydroxyapatite composites | [159] |
| alginate-lignin | [160] |
| nanocellulose | [161] |
| silica | [162,163] |
| chitosan | [148] |
| Aerogel for biomolecules immobilization | [158,164,165] |
| Aerogel for wound care | [166,167,168,169,170] |
| cellulose | [171,172,173] |
| nanocellulose | [174,175,176,177] |
| chitosan | [178,179] |
| alginate | [180] |
| collagen | [181,182] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nita, L.E.; Ghilan, A.; Rusu, A.G.; Neamtu, I.; Chiriac, A.P. New Trends in Bio-Based Aerogels. Pharmaceutics 2020, 12, 449. https://doi.org/10.3390/pharmaceutics12050449
Nita LE, Ghilan A, Rusu AG, Neamtu I, Chiriac AP. New Trends in Bio-Based Aerogels. Pharmaceutics. 2020; 12(5):449. https://doi.org/10.3390/pharmaceutics12050449
Chicago/Turabian StyleNita, Loredana Elena, Alina Ghilan, Alina Gabriela Rusu, Iordana Neamtu, and Aurica P. Chiriac. 2020. "New Trends in Bio-Based Aerogels" Pharmaceutics 12, no. 5: 449. https://doi.org/10.3390/pharmaceutics12050449