Exposure-Response and Clinical Outcome Modeling of Inhaled Budesonide/Formoterol Combination in Asthma Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Subjects
2.2. Sampling Time and Analysis
2.3. Model Development
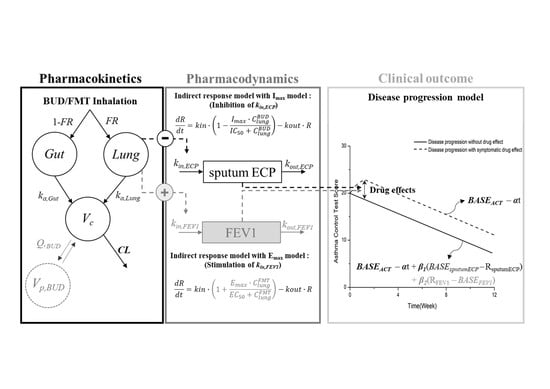
2.3.1. Structural Model Development
2.3.2. Covariate Model Development
2.3.3. Statistical Model Development
2.3.4. Modeling Methodology and Software
2.4. Model Evaluation
2.5. Simulation for Dose Optimization
3. Results
3.1. Clinical Trial Results
3.2. Population PK/PD/CO Analysis
3.3. Simulation Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kroegel, C. Global Initiative for Asthma (GINA) guidelines: 15 years of application. Expert Rev. Clin. Immunol. 2009, 5, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.; Pereira, A.M.; Morais-Almeida, M. Asthma costs and social impact. Asthma Res. Pract. 2017, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddel, H.K.; Bateman, E.D.; Becker, A.; Boulet, L.P.; Cruz, A.A.; Drazen, J.M.; Haahtela, T.; Hurd, S.S.; Inoue, H.; de Jongste, J.C.; et al. A summary of the new GINA strategy: A roadmap to asthma control. Eur. Respir. J. 2015, 46, 622–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCracken, J.L.; Veeranki, S.P.; Ameredes, B.T.; Calhoun, W.J. Diagnosis and management of Asthma in adults: A review. JAMA 2017, 318, 279–290. [Google Scholar] [CrossRef]
- Beasley, R.; Semprini, A.; Mitchell, E.A. Risk factors for asthma: Is prevention possible? Lancet 2015, 386, 1075–1085. [Google Scholar] [CrossRef]
- Lambrecht, B.N.; Hammad, H. The immunology of asthma. Nat. Immunol. 2015, 16, 45–56. [Google Scholar] [CrossRef]
- Bystrom, J.; Amin, K.; Bishop-Bailey, D. Analysing the eosinophil cationic protein—A clue to the function of the eosinophil granulocyte. Respir. Res. 2011, 12, 10. [Google Scholar] [CrossRef] [Green Version]
- Khakzad, M.R.; Mirsadraee, M.; Sankian, M.; Varasteh, A.; Meshkat, M. Is serum or sputum eosinophil cationic protein level adequate for diagnosis of mild asthma? Iran. J. Allergy Asthma Immunol. 2009, 8, 155–160. [Google Scholar]
- McGavin, J.K.; Goa, K.L.; Jarvis, B. Inhaled budesonide/formoterol combination. Drugs 2001, 61, 71–78. [Google Scholar] [CrossRef]
- Nathan, R.A.; Sorkness, C.A.; Kosinski, M.; Schatz, M.; Li, J.T.; Marcus, P.; Murray, J.J.; Pendergraft, T.B. Development of the asthma control test: A survey for assessing asthma control. J. Allergy Clin. Immunol. 2004, 113, 59–65. [Google Scholar] [CrossRef]
- Melosini, L.; Dente, F.L.; Bacci, E.; Bartoli, M.L.; Cianchetti, S.; Costa, F.; Di Franco, A.; Malagrino, L.; Novelli, F.; Vagaggini, B.; et al. Asthma control test (ACT): Comparison with clinical, functional, and biological markers of asthma control. J. Asthma 2012, 49, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, J.; Clark, T.J.; Hurd, S.; Khaltaev, N.; Lenfant, C.; O’Byrne, P.; Sheffer, A. GINA guidelines on asthma and beyond. Allergy 2007, 62, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Schatz, M.; Sorkness, C.A.; Li, J.T.; Marcus, P.; Murray, J.J.; Nathan, R.A.; Kosinski, M.; Pendergraft, T.B.; Jhingran, P. Asthma control test: Reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J. Allergy Clin. Immunol. 2006, 117, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Lotvall, J. Combination therapy in asthma—Fixed or variable dosing in different patients? Curr. Med. Res. Opin. 2004, 20, 1711–1727. [Google Scholar] [CrossRef]
- Stallberg, B.; Naya, I.; Ekelund, J.; Eckerwall, G. Real-life use of budesonide/formoterol in clinical practice: A 12-month follow-up assessment in a multi-national study of asthma patients established on single-inhaler maintenance and reliever therapy. Int. J. Clin. Pharm. 2015, 53, 447–455. [Google Scholar] [CrossRef] [Green Version]
- Kuna, P.; Creemers, J.P.; Vondra, V.; Black, P.N.; Lindqvist, A.; Nihlen, U.; Vogelmeier, C. Once-daily dosing with budesonide/formoterol compared with twice-daily budesonide/formoterol and once-daily budesonide in adults with mild to moderate asthma. Respir. Med. 2006, 100, 2151–2159. [Google Scholar] [CrossRef] [Green Version]
- Ind, P.W.; Haughney, J.; Price, D.; Rosen, J.P.; Kennelly, J. Adjustable and fixed dosing with budesonide/ formoterol via a single inhaler in asthma patients: The ASSURE study. Respir. Med. 2004, 98, 464–475. [Google Scholar] [CrossRef] [Green Version]
- National Institutes for Health; Lung and Blood Institute. Global Strategy for Asthma Management and Prevention; Publication Number 02-3659; Global Initiative for Asthma (GINA): Fontana, WI, USA, 2002. [Google Scholar]
- British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma. Thorax 2003, 58 (Suppl. 1), 1–94. [Google Scholar] [CrossRef]
- Chen, Q.; Hu, C.; Yu, H.; Shen, K.; Assam, P.N.; Gillen, M.; Liu, Y.; Dorinsky, P. Pharmacokinetics and tolerability of budesonide/glycopyrronium/formoterol fumarate dihydrate and glycopyrronium/formoterol fumarate dihydrate metered dose inhalers in healthy chinese adults: A randomized, double-blind, parallel-group study. Clin. Ther. 2019, 41, 897–909. [Google Scholar] [CrossRef] [Green Version]
- Soulele, K.; Macheras, P.; Karalis, V. On the pharmacokinetics of two inhaled budesonide/formoterol combinations in asthma patients using modeling approaches. Pulm. Pharmacol. 2018, 48, 168–178. [Google Scholar] [CrossRef]
- Gaz, C.; Cremona, G.; Panunzi, S.; Patterson, B.; De Gaetano, A. A geometrical approach to the PKPD modelling of inhaled bronchodilators. J. Pharm. Pharmacol. 2012, 39, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Looby, M.; Pillai, G.; Pinault, G.; Drollman, A.F.; Pascoe, S. Population pharmacodynamic model of the longitudinal FEV1 response to an inhaled long-acting anti-muscarinic in COPD patients. J. Pharm. Pharmacol. 2011, 38, 105–119. [Google Scholar] [CrossRef] [PubMed]
- FDA. Symbicort Label; AstraZeneca: White Oak, MD, USA, 2006. [Google Scholar]
- Mascher, D.G.; Zech, K.; Nave, R.; Kubesch, K.M.; Mascher, H.J. Ultra-sensitive determination of Formoterol in human serum by high performance liquid chromatography and electrospray tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2006, 830, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Szeitz, A.; Manji, J.; Riggs, K.W.; Thamboo, A.; Javer, A.R. Validated assay for the simultaneous determination of cortisol and budesonide in human plasma using ultra high performance liquid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2014, 90, 198–206. [Google Scholar] [CrossRef]
- Jusko, W.J.; Ko, H.C. Physiologic indirect response models characterize diverse types of pharmacodynamic effects. Clin. Pharmacol. 1994, 56, 406–419. [Google Scholar] [CrossRef]
- Mirsadraee, M.; Khashkhashi Moghaddam, S.; Saeedi, P.; Ghaffari, S. Effect of borago officinalis extract on moderate persistent Asthma: A phase two randomized, double blind, placebo-controlled clinical trial. Tanaffos 2016, 15, 168–174. [Google Scholar]
- Lindbom, L.; Pihlgren, P.; Jonsson, E.N. PsN-Toolkit—A collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput. Methods Progr. Biomed. 2005, 79, 241–257. [Google Scholar] [CrossRef]
- Jonsson, E.N.; Karlsson, M.O. Xpose—An S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput. Methods. Progr. Biomed. 1999, 58, 51–64. [Google Scholar] [CrossRef]
- Post, T.M.; Freijer, J.I.; Ploeger, B.A.; Danhof, M. Extensions to the visual predictive check to facilitate model performance evaluation. J. Pharm. Pharmacol. 2008, 35, 185–202. [Google Scholar] [CrossRef] [Green Version]
- Mould, D.R.; Upton, R.N. Basic concepts in population modeling, simulation, and model-based drug development-part 2: Introduction to pharmacokinetic modeling methods. CPT Pharm. Syst. Pharmacol. 2013, 2, e38. [Google Scholar] [CrossRef]
- Thomas, M.; Kay, S.; Pike, J.; Williams, A.; Rosenzweig, J.R.; Hillyer, E.V.; Price, D. The Asthma Control Test (ACT) as a predictor of GINA guideline-defined asthma control: Analysis of a multinational cross-sectional survey. Prim. Care Respir. J. 2009, 18, 41–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AstraZeneca Canada. Symbicort Turbuhaler Product Monograph; AstraZeneca Canada: Mississauga, ON, Canada, 2016. [Google Scholar]
- De Buck, S.S.; Sinha, V.K.; Fenu, L.A.; Nijsen, M.J.; Mackie, C.E.; Gilissen, R.A. Prediction of human pharmacokinetics using physiologically based modeling: A retrospective analysis of 26 clinically tested drugs. Drug Metab. Dispos. 2007, 35, 1766–1780. [Google Scholar] [CrossRef] [PubMed]
- Kelly, H.W. Comparison of inhaled corticosteroids. Ann. Pharmacother. 1998, 32, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Nials, A.T.; Coleman, R.A.; Johnson, M.; Magnussen, H.; Rabe, K.F.; Vardey, C.J. Effects of beta-adrenoceptor agonists in human bronchial smooth muscle. Br. J. Pharmacol. 1993, 110, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Beta-adrenergic receptors and their regulation. Am. J. Respir. Crit. Care Med. 1995, 152, 838–860. [Google Scholar] [CrossRef] [PubMed]
- Pelaia, G.; Vatrella, A.; Busceti, M.T.; Fabiano, F.; Terracciano, R.; Matera, M.G.; Maselli, R. Molecular and cellular mechanisms underlying the therapeutic effects of budesonide in asthma. Pulm. Pharmacol. 2016, 40, 15–21. [Google Scholar] [CrossRef]
- Derendorf, H. Pharmacokinetic and pharmacodynamic properties of inhaled ciclesonide. J. Clin. Pharmacol. 2007, 47, 782–789. [Google Scholar] [CrossRef]
- Derendorf, H.; Nave, R.; Drollmann, A.; Cerasoli, F.; Wurst, W. Relevance of pharmacokinetics and pharmacodynamics of inhaled corticosteroids to asthma. Eur. Respir. J. 2006, 28, 1042–1050. [Google Scholar] [CrossRef]
- Borghardt, J.M.; Weber, B.; Staab, A.; Kloft, C. Pharmacometric models for characterizing the pharmacokinetics of orally inhaled drugs. AAPS J. 2015, 17, 853–870. [Google Scholar] [CrossRef] [Green Version]
- Koh, G.C.; Shek, L.P.; Goh, D.Y.; Van Bever, H.; Koh, D.S. Eosinophil cationic protein: Is it useful in asthma? A systematic review. Respir. Med. 2007, 101, 696–705. [Google Scholar] [CrossRef] [Green Version]
- Virchow, J.C., Jr.; Holscher, U.; Virchow, C., Sr. Sputum ECP levels correlate with parameters of airflow obstruction. Am. Rev. Respir. Dis. 1992, 146, 604–606. [Google Scholar] [CrossRef] [PubMed]
- Pavord, I.D.; Pizzichini, M.M.; Pizzichini, E.; Hargreave, F.E. The use of induced sputum to investigate airway inflammation. Thorax 1997, 52, 498–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deykin, A.; Lazarus, S.C.; Fahy, J.V.; Wechsler, M.E.; Boushey, H.A.; Chinchilli, V.M.; Craig, T.J.; Dimango, E.; Kraft, M.; Leone, F.; et al. Sputum eosinophil counts predict asthma control after discontinuation of inhaled corticosteroids. J. Allergy Clin. Immunol. 2005, 115, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Derendorf, H.; Meibohm, B. Modeling of pharmacokinetic/pharmacodynamic (PK/PD) relationships: Concepts and perspectives. Pharm. Res. 1999, 16, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Meibohm, B.; Hochhaus, G.; Mollmann, H.; Barth, J.; Wagner, M.; Krieg, M.; Stockmann, R.; Derendorf, H. A pharmacokinetic/pharmacodynamic approach to predict the cumulative cortisol suppression of inhaled corticosteroids. J. Pharm. Biopharm. 1999, 27, 127–147. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Goyal, N.; Stark, J.G.; Hochhaus, G. Evaluation of the administration time effect on the cumulative cortisol suppression and cumulative lymphocytes suppression for once-daily inhaled corticosteroids: A population modeling/simulation approach. J. Clin. Pharmacol. 2008, 48, 1069–1080. [Google Scholar] [CrossRef]
- Auclair, B.; Wainer, I.W.; Fried, K.; Koch, P.; Jerussi, T.P.; Ducharme, M.P. A population analysis of nebulized (R)-albuterol in dogs using a novel mixed gut-lung absorption PK-PD model. Pharm. Res. 2000, 17, 1228–1235. [Google Scholar] [CrossRef]
- Strandberg, K.; Blidberg, K.; Sahlander, K.; Palmberg, L.; Larsson, K. Effect of formoterol and budesonide on chemokine release, chemokine receptor expression and chemotaxis in human neutrophils. Pulm. Pharmacol. 2010, 23, 316–323. [Google Scholar] [CrossRef] [Green Version]
- Rossios, C.; To, Y.; Osoata, G.; Ito, M.; Barnes, P.J.; Ito, K. Corticosteroid insensitivity is reversed by formoterol via phosphoinositide-3-kinase inhibition. Br. J. Pharmacol. 2012, 167, 775–786. [Google Scholar] [CrossRef] [Green Version]
- Vestbo, J.; Sorensen, T.; Lange, P.; Brix, A.; Torre, P.; Viskum, K. Long-term effect of inhaled budesonide in mild and moderate chronic obstructive pulmonary disease: A randomised controlled trial. Lancet 1999, 353, 1819–1823. [Google Scholar] [CrossRef]
- Frey, U.; Suki, B. Complexity of chronic asthma and chronic obstructive pulmonary disease: Implications for risk assessment, and disease progression and control. Lancet 2008, 372, 1088–1099. [Google Scholar] [CrossRef] [Green Version]
- Frey, U.; Brodbeck, T.; Majumdar, A.; Taylor, D.R.; Town, G.I.; Silverman, M.; Suki, B. Risk of severe asthma episodes predicted from fluctuation analysis of airway function. Nature 2005, 438, 667–670. [Google Scholar] [CrossRef] [PubMed]
- Eid, N.; Yandell, B.; Howell, L.; Eddy, M.; Sheikh, S. Can peak expiratory flow predict airflow obstruction in children with asthma? Pediatrics 2000, 105, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.L.; Kittelson, J.; Cowan, J.O.; Flannery, E.M.; Hancox, R.J.; McLachlan, C.R.; Taylor, D.R. The predictive value of exhaled nitric oxide measurements in assessing changes in asthma control. Am. J. Respir. Crit. Care Med. 2001, 164, 738–743. [Google Scholar] [CrossRef]
- Thamrin, C.; Zindel, J.; Nydegger, R.; Reddel, H.K.; Chanez, P.; Wenzel, S.E.; FitzPatrick, S.; Watt, R.A.; Suki, B.; Frey, U. Predicting future risk of asthma exacerbations using individual conditional probabilities. J. Allergy Clin. Immunol. 2011, 127, 1494–1502. [Google Scholar] [CrossRef]
- Bateman, E.D.; Reddel, H.K.; Eriksson, G.; Peterson, S.; Ostlund, O.; Sears, M.R.; Jenkins, C.; Humbert, M.; Buhl, R.; Harrison, T.W.; et al. Overall asthma control: The relationship between current control and future risk. J. Allergy Clin. Immunol. 2010, 125, 600–608. [Google Scholar] [CrossRef]
- Bleecker, E.R.; Meyers, D.A.; Bailey, W.C.; Sims, A.M.; Bujac, S.R.; Goldman, M.; Martin, U.J. ADRB2 polymorphisms and budesonide/formoterol responses in COPD. Chest 2012, 142, 320–328. [Google Scholar] [CrossRef] [Green Version]
- Rebordosa, C.; Kogevinas, M.; Guerra, S.; Castro-Giner, F.; Jarvis, D.; Cazzoletti, L.; Pin, I.; Siroux, V.; Wjst, M.; Anto, J.M.; et al. ADRB2 Gly16Arg polymorphism, asthma control and lung function decline. Eur. Respir. J. 2011, 38, 1029–1035. [Google Scholar] [CrossRef] [Green Version]
- de Paiva, A.C.; Marson, F.A.; Ribeiro, J.D.; Bertuzzo, C.S. Asthma: Gln27Glu and Arg16Gly polymorphisms of the beta2-adrenergic receptor gene as risk factors. Allergy Asthma Clin. Immunol. 2014, 10, 8. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Li, Q.; Liu, R.; He, J.; Wu, D.; Wang, Y.; Zhang, J. ADRB2 Arg16Gly polymorphism and pulmonary function response of inhaled corticosteroids plus long-acting beta agonists for Asthma treatment: A systematic review and meta-analysis. Can. Respir. J. 2018, 2018, 5712805. [Google Scholar] [CrossRef] [Green Version]
- Pauwels, R.A.; Lofdahl, C.G.; Postma, D.S.; Tattersfield, A.E.; O’Byrne, P.; Barnes, P.J.; Ullman, A. Effect of inhaled formoterol and budesonide on exacerbations of asthma. Formoterol and Corticosteroids Establishing Therapy (FACET) International Study Group. N. Engl. J. Med. 1997, 337, 1405–1411. [Google Scholar] [CrossRef] [PubMed]
- Wallin, A.; Sandstrom, T.; Soderberg, M.; Howarth, P.; Lundback, B.; Della-Cioppa, G.; Wilson, S.; Judd, M.; Djukanovic, R.; Holgate, S.; et al. The effects of regular inhaled formoterol, budesonide, and placebo on mucosal inflammation and clinical indices in mild asthma. Am. J. Respir. Crit. Care Med. 1999, 159, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Zhou, L.; Ni, Y.; Yu, Y.; Wu, F.; Shi, G. Inhaled corticosteroids improve lung function, airway hyper-responsiveness and airway inflammation but not symptom control in patients with mild intermittent asthma: A meta-analysis. Exp. Ther. Med. 2017, 14, 1594–1608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badar, A.; Saeed, W.; Hussain, M.M.; Aslam, M. Correlation of eosinophil cationic protein with severity of asthma. J. Ayub Med. Coll. Abbottabad 2004, 16, 66–71. [Google Scholar] [PubMed]




| Variables | All Patients (n = 53) |
|---|---|
| Sex (male/female), n/n (%/%) | 33/20 (62%/38%) |
| Age (years), mean ± SD | 41 ± 13 |
| Weight (kg), mean ± SD | 70 ± 14 |
| Height (cm), mean ± SD | 168 ± 9 |
| ADRB2 genotype (n), AA/AG/GG | 16/25/12 |
| Asthma duration (years), mean ± SD | 7.0 ± 6.9 |
| Baseline FEV1 (%), mean ± SD | 85.1 ± 13.8 |
| Baseline sputum eosinophil cationic protein (μg·L−1), mean ± SD | 51.3 ± 83.2 |
| Baseline Asthma control test score, mean ± SD | 19.7 ± 4.1 |
| Budesonide | Formoterol | ||||||||
| PK Parameters | Value (%RSE) | IIV (%RSE) | Bootstrapped IIV (5th–95th percentile) | Value (%RSE) | IIV (%RSE) | Bootstrapped IIV (5th–95th percentile) | |||
| Vc (L) | 216 # (20.5%) | 93.3% (11.6%) | 77.4% (69.6–85.0%) | 1250 # (12.0%) | 85.8% (20%) | 71.6% (64.7–78.3%) | |||
| CL (L·hr−1) | 18.4 # (29.5%) | - | - | 292 # (16.0%) | 91.1% (19.3%) | 125.0% (95.3–156.1%) | |||
| ka,Lung (hr−1) | 19.7 * | - | - | 14.8 # (26.5%) | - | - | |||
| ka,Gut (hr−1) | 0.00076# (67.5%) | - | - | 0.0524 # (52.0%) | - | - | |||
| FR | 0.38 * | 6% * | 6% * | 0.385 * | - | - | |||
| Q (L·hr−1) | 88.3 # (48%) | - | - | - | - | ||||
| Vp,BUD (L) | 106 # (37.2%) | - | - | - | - | ||||
| RV | 64.2% (9.0%) | - | 80.8% (10.0%) | - | |||||
| PD Parameters | Sputum ECP | Bootstrapped IIV (5th–95th percentile) | %FEV1 | Bootstrapped IIV (5th–95th percentile) | |||||
| BASE | 19.7 ng/mL (38.9%) | 113.9% (8.2%) | 147.4% (128.9–166.9%) | 85.8% (2.0%) | 12.9% (10%) | 12.7% (11.7–13.7%) | |||
| IC50 (ng·mL−1) | 0.025 * | - | - | - | - | ||||
| EC50 (pg·mL−1) | - | - | 0.081 * | - | - | ||||
| kout (hr−1) | 0.00598 (77.9%) | 32.4% (5450%) | 26.3% (23.2–30.0) | 0.000951 (183%) | 110.9% (12.4%) | 90.7% (68.1–112.7) | |||
| RV | 72.1% (5.0%) | - | 6.35% (8.0%) | - | |||||
| CO Parameters | Asthma Control Test | ||||||||
| Value (% RSE) | IIV (% RSE) | Bootstrapped IIV (5th–95th percentile) | |||||||
| BASEACT (score) | 19.70 * | 14.9% (9%) | 14.8% (13.7–15.8%) | ||||||
| A | 0.00083 * | - | |||||||
| β1 | 0.00008 (46.9%) | - | |||||||
| β2 | 0.644 (80.9%) | - | |||||||
| RV | 2.32 (10.7%) | - | - | ||||||
| Week | Scenario | Number of Patients | Percentage (%) | ||||
|---|---|---|---|---|---|---|---|
| ACT Score | ACT Score | ||||||
| ≥20 | 15–20 | <15 | ≥20 | 15–20 | <15 | ||
| 0 | Observation | 29 | 13 | 6 | 60.4 | 27.1 | 12.5 |
| 1puff BID | 472 | 423 | 105 | 47.2 | 42.3 | 10.5 | |
| 2puff BID | 472 | 423 | 105 | 47.2 | 42.3 | 10.5 | |
| 2puff QD | 472 | 423 | 105 | 47.2 | 42.3 | 10.5 | |
| 4 | Observation | 29 | 17 | 3 | 59.2 | 34.7 | 6.1 |
| 1puff BID | 552 | 388 | 60 | 55.2 | 38.8 | 6.0 | |
| 2puff BID | 561 | 382 | 57 | 56.1 | 38.2 | 5.7 | |
| 2puff QD | 495 | 422 | 83 | 49.5 | 42.2 | 8.3 | |
| 8 | Observation | 36 | 11 | 3 | 72.0 | 22.0 | 6.0 |
| 1puff BID | 569 | 359 | 72 | 56.9 | 35.9 | 7.2 | |
| 2puff BID | 585 | 349 | 66 | 58.5 | 34.9 | 6.6 | |
| 2puff QD | 465 | 431 | 104 | 46.5 | 43.1 | 10.4 | |
| 12 | Observation | 35 | 12 | 3 | 70.0 | 24.0 | 6.0 |
| 1puff BID | 551 | 370 | 79 | 55.1 | 37.0 | 7.9 | |
| 2puff BID | 571 | 354 | 75 | 57.1 | 35.4 | 7.5 | |
| 2puff QD | 417 | 456 | 127 | 41.7 | 45.6 | 12.7 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Back, H.-m.; Lee, J.B.; Kim, A.; Park, S.-J.; Kim, J.; Chae, J.-w.; Sheen, S.S.; Kagan, L.; Park, H.-S.; Ye, Y.-M.; et al. Exposure-Response and Clinical Outcome Modeling of Inhaled Budesonide/Formoterol Combination in Asthma Patients. Pharmaceutics 2020, 12, 336. https://doi.org/10.3390/pharmaceutics12040336
Back H-m, Lee JB, Kim A, Park S-J, Kim J, Chae J-w, Sheen SS, Kagan L, Park H-S, Ye Y-M, et al. Exposure-Response and Clinical Outcome Modeling of Inhaled Budesonide/Formoterol Combination in Asthma Patients. Pharmaceutics. 2020; 12(4):336. https://doi.org/10.3390/pharmaceutics12040336
Chicago/Turabian StyleBack, Hyun-moon, Jong Bong Lee, Anhye Kim, Seon-Jong Park, Junyeong Kim, Jung-woo Chae, Seung Soo Sheen, Leonid Kagan, Hae-Sim Park, Young-Min Ye, and et al. 2020. "Exposure-Response and Clinical Outcome Modeling of Inhaled Budesonide/Formoterol Combination in Asthma Patients" Pharmaceutics 12, no. 4: 336. https://doi.org/10.3390/pharmaceutics12040336