Nanohydrogels Based on Self-Assembly of Cationic Pullulan and Anionic Dextran Derivatives for Efficient Delivery of Piroxicam
Abstract
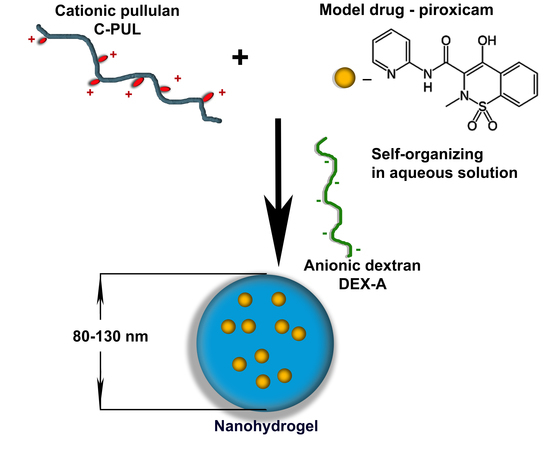
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Cationic Pullulan (C-PUL)
2.3. Preparation of C-PUL/DEX-A Nanohydrogel Particles (NGs)
2.4. Preparation of the Piroxicam Loaded Nanogel NG-PIXs
2.5. Characterization of Pullulan Derivative
2.6. Determination of the Nanohydrogel Particles Size and Morphology (DLS, SEM, TEM, AFM)
2.7. UV/Vis Absorption Spectra Measurements
2.8. Determination of the Entrapment Efficiency of the Nanogel
2.9. Piroxicam Release from NG-PIX
2.10. Biological Studies
2.11. Langmuir Trough Experiments
3. Results and Discussion
3.1. Synthesis and Characterization of Cationic Pullulan Derivative
3.2. Preparation of Nanohydrogel (NGs)
3.3. C-PUL-Binding Constant of Piroxicam
3.4. Piroxicam-Loaded Nanohydrogel Particles (PIROX-NGs)
3.5. Studies on the Intercalation of NGs Systems into the Lipid Monolayer
3.6. Piroxicam Release
3.7. Biological Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dalwadi, C.; Patel, G. Application of nanohydrogels in drug delivery systems: Recent patents review. Recent Pat. Nanotechnol. 2015, 9, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Akram, M.; Hussain, R. Nanohydrogels: History, Development, and Applications in Drug Delivery; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 297–330. ISBN 9783527803835. [Google Scholar]
- Akhter, K.F.; Mumin, M.A.; Lui, E.M.K.; Charpentier, P.A. Immunoengineering with ginseng polysaccharide nanobiomaterials through oral administration in mice. ACS Biomater. Sci. Eng. 2019, 5, 2916–2925. [Google Scholar] [CrossRef]
- Milewska, A.; Ciejka, J.; Kaminski, K.; Karewicz, A.; Bielska, D.; Zeglen, S.; Karolak, W.; Nowakowska, M.; Potempa, J.; Bosch, B.J.; et al. Novel polymeric inhibitors of HCoV-NL63. Antivir. Res. 2013, 97, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Tahara, Y.; Akiyoshi, K. Current advances in self-assembled nanogel delivery systems for immunotherapy. Adv. Drug Deliv. Rev. 2015, 95, 65–76. [Google Scholar] [CrossRef]
- Soni, G.; Yadav, K.S. Nanogels as potential nanomedicine carrier for treatment of cancer: A mini review of the state of the art. Saudi Pharm. J. 2016, 24, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Kłodzińska, S.N.; Pletzer, D.; Rahanjam, N.; Rades, T.; Hancock, R.E.W.; Nielsen, H.M. Hyaluronic acid-based nanogels improve in vivo compatibility of the anti-biofilm peptide DJK-5. Nanomed. Nanotechnol. Biol. Med. 2019, 20. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, L.; Lin, L.; Chen, J.; Tian, H.; Chen, X.; Maruyama, A. In situ dual-crosslinked nanoparticles for tumor targeting gene delivery. Acta Biomater. 2018, 65, 349–362. [Google Scholar] [CrossRef]
- Zhang, H.; Zhai, Y.; Wang, J.; Zhai, G. New progress and prospects: The application of nanogel in drug delivery. Mater. Sci. Eng. C 2016, 60, 560–568. [Google Scholar] [CrossRef]
- García, M.C.; Cuggino, J.C. Stimulus-responsive nanogels for drug delivery. In Stimuli Responsive Polymeric Nanocarriers for Drug Delivery Applications; Woodhead Publishing: Cambridge, UK, 2018; Volume 1, pp. 321–341. [Google Scholar] [CrossRef]
- Morimoto, N.; Hirano, S.; Takahashi, H.; Loethen, S.; Thompson, D.H.; Akiyoshi, K. Self-assembled pH-sensitive cholesteryl pullulan nanogel as a protein delivery vehicle. Biomacromolecules 2013, 14, 56–63. [Google Scholar] [CrossRef]
- Kabb, C.P.; Bryan, C.S.O.; Deng, C.C.; Thomas, E.; Sumerlin, B.S. Photoreversible covalent hydrogels for soft-matter additive manufacturing. ACS Appl. Mater. Interfaces 2018, 10, 16793–16801. [Google Scholar] [CrossRef]
- Conde, J. Handbook of Nanomaterials for Cancer Theranostics; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Lin, X.; Ma, Q.; Su, J.; Wang, C.; Kankala, R.K.; Zeng, M.; Lin, H.; Zhou, S.-F. Dual-responsive alginate hydrogels for controlled release of therapeutics. Molecules 2019, 24, 2089. [Google Scholar] [CrossRef] [PubMed]
- Sahu, P.; Kashaw, S.K.; Sau, S.; Kushwah, V.; Jain, S.; Agrawal, R.K.; Iyer, A.K. pH triggered and charge attracted nanogel for simultaneous evaluation of penetration and toxicity against skin cancer: In-vitro and ex-vivo study. Int. J. Biol. Macromol. 2019, 128, 740–751. [Google Scholar] [CrossRef] [PubMed]
- Amrita; Arora, A.; Sharma, P.; Katti, D.S. Pullulan-based composite scaffolds for bone tissue engineering: Improved osteoconductivity by pore wall mineralization. Carbohydr. Polym. 2015, 123, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Lazaridou, A.; Biliaderis, C.G.; Kontogiorgos, V. Molecular weight effects on solution rheology of pullulan and mechanical properties of its films. Carbohydr. Polym. 2003, 52, 151–166. [Google Scholar] [CrossRef]
- Su, T.; Wu, L.; Pan, X.; Zhang, C.; Shi, M.; Gao, R.; Qi, X.; Dong, W. Pullulan-derived nanocomposite hydrogels for wastewater remediation: Synthesis and characterization. J. Colloid Interface Sci. 2019, 542, 253–262. [Google Scholar] [CrossRef]
- Hennink, W.E.; van Nostrum, C.F. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 2012, 64, 223–236. [Google Scholar] [CrossRef]
- Buwalda, S.J.; Boere, K.W.M.; Dijkstra, P.J.; Feijen, J.; Vermonden, T.; Hennink, W.E. Hydrogels in a historical perspective: From simple networks to smart materials. J. Control. Release 2014, 190, 254–273. [Google Scholar] [CrossRef]
- Ravi, P.R.; Vats, R.; Balija, J.; Adapa, S.P.N.; Aditya, N. Modified pullulan nanoparticles for oral delivery of lopinavir: Formulation and pharmacokinetic evaluation. Carbohydr. Polym. 2014, 110, 320–328. [Google Scholar] [CrossRef]
- Cheng, Y.; Yu, S.; Zhen, X.; Wang, X.; Wu, W.; Jiang, X. Alginic acid nanoparticles prepared through counterion complexation method as a drug delivery system. ACS Appl. Mater. Interfaces 2012, 4, 5325–5332. [Google Scholar] [CrossRef]
- Hong, J.S.; Vreeland, W.N.; Depaoli Lacerda, S.H.; Locascio, L.E.; Gaitan, M.; Raghavan, S.R. Liposome-templated supramolecular assembly of responsive alginate nanogels. Langmuir 2007, 24, 4092–4096. [Google Scholar] [CrossRef]
- Nesamony, J.; Singh, P.R.; Nada, S.E.; Shah, Z.A.; Kolling, W.M. Calcium alginate nanoparticles synthesized through a novel interfacial cross-linking method as a potential protein drug delivery system. J. Pharm. Sci. 2012, 101, 2177–2184. [Google Scholar] [CrossRef] [PubMed]
- Karewicz, A.; Zasada, K.; Bielska, D.; Douglas, T.E.L.; Jansen, J.A.; Leeuwenburgh, S.C.G.; Nowakowska, M. Alginate-hydroxypropylcellulose hydrogel microbeads for alkaline phosphatase encapsulation. J. Microencapsul. 2014, 31, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Sorasitthiyanukarn, F.N.; Muangnoi, C.; Ratnatilaka Na Bhuket, P.; Rojsitthisak, P.; Rojsitthisak, P. Chitosan/alginate nanoparticles as a promising approach for oral delivery of curcumin diglutaric acid for cancer treatment. Mater. Sci. Eng. C 2018, 93, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Bielska, D.; Karewicz, A.; Kamiński, K.; Kiełkowicz, I.; Lachowicz, T.; Szczubiałka, K.; Nowakowska, M. Self-organized thermo-responsive hydroxypropyl cellulose nanoparticles for curcumin delivery. Eur. Polym. J. 2013, 49, 2485–2494. [Google Scholar] [CrossRef]
- Lapitsky, Y. Ionically crosslinked polyelectrolyte nanocarriers: Recent advances and open problems. Curr. Opin. Colloid Interface Sci. 2014, 19, 122–130. [Google Scholar] [CrossRef]
- Szafraniec, J.; Błazejczyk, A.; Kus, E.; Janik, M.; Zajac, G.; Wietrzyk, J.; Chlopicki, S.; Zapotoczny, S. Robust oil-core nanocapsules with hyaluronate-based shells as promising nanovehicles for lipophilic compounds. Nanoscale 2017, 9, 18867–18880. [Google Scholar] [CrossRef]
- Sawtarie, N.; Cai, Y.; Lapitsky, Y. Preparation of chitosan/tripolyphosphate nanoparticles with highly tunable size and low polydispersity. Colloids Surf. B Biointerfaces 2017, 157, 110–117. [Google Scholar] [CrossRef]
- Cai, Y.; Lapitsky, Y. Formation and dissolution of chitosan/pyrophosphate nanoparticles: Is the ionic crosslinking of chitosan reversible? Colloids Surf. B Biointerfaces 2014, 115, 100–108. [Google Scholar] [CrossRef]
- Cai, Y.; Lapitsky, Y. Analysis of chitosan/tripolyphosphate micro- and nanogel yields is key to understanding their protein uptake performance. J. Colloid Interface Sci. 2017, 494, 242–254. [Google Scholar] [CrossRef]
- Gadalla, H.H.; El-Gibaly, I.; Soliman, G.M.; Mohamed, F.A.; El-Sayed, A.M. Amidated pectin/sodium carboxymethylcellulose microspheres as a new carrier for colonic drug targeting: Development and optimization by factorial design. Carbohydr. Polym. 2016, 153, 526–534. [Google Scholar] [CrossRef]
- Swierczewska, M.; Han, H.S.; Kim, K.; Park, J.H.; Lee, S. Polysaccharide-based Nanoparticles for Theranostic Nanomedicine HHS Public Access. Adv. Drug. Deliv. Rev. 2016, 99, 70–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giacalone, G.; Hillaireau, H.; Capiau, P.; Chacun, H.; Reynaud, F.; Fattal, E. Stabilization and cellular delivery of chitosan-polyphosphate nanoparticles by incorporation of iron. J. Control. Release 2014, 194, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Maleh, H.; Sheikhshoaie, I.; Samadzadeh, A. Simultaneous electrochemical determination of levodopa and piroxicam using a glassy carbon electrode modified with a ZnO–Pd/CNT nanocomposite. RSC Adv. 2018, 8, 26707–26712. [Google Scholar] [CrossRef] [Green Version]
- Brogden, R.N.; Heel, R.C.; Speight, T.M.; Avery, G.S. Piroxicam: A review of its pharmacological properties and therapeutic efficacy. Drugs 1981, 22, 165–187. [Google Scholar] [CrossRef]
- Brogden, R.N.; Heel, R.C.; Speight, T.M.; Avery, G.S. Piroxicam. Drugs 1984, 28, 292–323. [Google Scholar] [CrossRef]
- Sotomayor, R.G.; Holguín, A.R.; Cristancho, D.M.; Delgado, D.R.; Martínez, F. Extended hildebrand solubility approach applied to piroxicam in ethanol + water mixtures. J. Mol. Liq. 2013, 180, 34–38. [Google Scholar] [CrossRef]
- Mihalić, M.; Hofman, H.; Kuftinec, J.; Krile, B.; Čaplar, V.; Kajfež, F.; Blažević, N. Piroxicam. In Analytical Profiles of Drug Substances; Academic Press: Cambridge, MA, USA, 1986; pp. 509–531. [Google Scholar]
- Karewicz, A.; Bielska, D.; Gzyl-Malcher, B.; Kepczynski, M.; Lach, R.; Nowakowska, M. Interaction of curcumin with lipid monolayers and liposomal bilayers. Colloids Surf. B Biointerfaces 2011, 88, 231–239. [Google Scholar] [CrossRef]
- Patnaik, S.; Avinash Chunduri, L.A.; Sai Akilesh, M.; Srimadh Bhagavatham, S.; Kamisetti, V. Enhanced dissolution characteristics of piroxicam-Soluplus® nanosuspensions. Saikrishna Srimadh Bhagavatham Venkataramaniah Kamisetti 2016, 11, 916–929. [Google Scholar] [CrossRef] [Green Version]
- Ustün Alkan, F.; Ustüner, O.; Bakırel, T.; Erten, G.; Deniz, G. The effects of piroxicam and deracoxib on canine mammary tumour cell line. Sci. World J. 2012, 2012. [Google Scholar] [CrossRef] [Green Version]
- Kępczyński, M.; Pandian, R.P.; Smith, K.M.; Ehrenberg, B. Do liposome-binding constants of porphyrins correlate with their measured and predicted partitioning between octanol and water? Photochem. Photobiol. 2007, 76, 127–134. [Google Scholar] [CrossRef]
- Aricov, L.; Angelescu, D.G.; Băran, A.; Leontieş, A.R.; Popa, V.T.; Precupaş, A.; Sandu, R.; Stîngă, G.; Anghel, D.-F. Interaction of piroxicam with bovine serum albumin investigated by spectroscopic, calorimetric and computational molecular methods. J. Biomol. Struct. Dyn. 2019, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Karewicz, A.; Bielska, D.; Loboda, A.; Gzyl-Malcher, B.; Bednar, J.; Jozkowicz, A.; Dulak, J.; Nowakowska, M. Curcumin-containing liposomes stabilized by thin layers of chitosan derivatives. Colloids Surf. B Biointerfaces 2013, 109, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Monnery, B.D.; Wright, M.; Cavill, R.; Hoogenboom, R.; Shaunak, S.; Steinke, J.H.G.; Thanou, M. Cytotoxicity of polycations: relationship of molecular weight and the hydrolytic theory of the mechanism of toxicity. Int. J. Pharm. 2017, 521, 249–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]










| C-PUL:DEX-A (w/w) | dz (nm) | PDI | ζ (mV) |
|---|---|---|---|
| 1:1 | 108 ± 2 | 0.359 | −28.6 ± 3.3 |
| 1:2 | 106 ± 1 | 0.351 | −22.4 ± 2.3 |
| 1:4 | 337 ± 2 | 0.298 | −21.0 ± 2.0 |
| 1:6 | 165 ± 6 | 0.432 | −38.4 ± 3.9 |
| 1:10 | 130 ± 8 | 0.733 | −42.3 ± 4.3 |
| 2:1 | 156 ± 1 | 0.297 | −16.2 ± 3.4 |
| 3:1 | 117 ± 2 | 0.195 | +11.1 ± 1.0 |
| 4:1 | 127 ± 1 | 0.228 | +19.7 ± 0.7 |
| 6:1 | 152 ± 10 | 0.445 | +35.2 ± 1.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lachowicz, D.; Mielczarek, P.; Wirecka, R.; Berent, K.; Karewicz, A.; Szuwarzyński, M.; Zapotoczny, S. Nanohydrogels Based on Self-Assembly of Cationic Pullulan and Anionic Dextran Derivatives for Efficient Delivery of Piroxicam. Pharmaceutics 2019, 11, 622. https://doi.org/10.3390/pharmaceutics11120622
Lachowicz D, Mielczarek P, Wirecka R, Berent K, Karewicz A, Szuwarzyński M, Zapotoczny S. Nanohydrogels Based on Self-Assembly of Cationic Pullulan and Anionic Dextran Derivatives for Efficient Delivery of Piroxicam. Pharmaceutics. 2019; 11(12):622. https://doi.org/10.3390/pharmaceutics11120622
Chicago/Turabian StyleLachowicz, Dorota, Przemyslaw Mielczarek, Roma Wirecka, Katarzyna Berent, Anna Karewicz, Michał Szuwarzyński, and Szczepan Zapotoczny. 2019. "Nanohydrogels Based on Self-Assembly of Cationic Pullulan and Anionic Dextran Derivatives for Efficient Delivery of Piroxicam" Pharmaceutics 11, no. 12: 622. https://doi.org/10.3390/pharmaceutics11120622