Development and Evaluation of Alginate Membranes with Curcumin-Loaded Nanoparticles for Potential Wound-Healing Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PCL Nanoparticles Loaded with Curcumin (CNp)
2.3. Physicochemical Characterization of CNp
2.3.1. Particle Size and Zeta Potential Assessment
2.3.2. Atomic Force Microscopy (AFM)
2.3.3. Drug Loading and Entrapment Efficiency of CNp
2.4. Preparation of Polymer Gels and Membranes
2.4.1. Preparation of Nanoparticle-Coated Alginate Membranes (CNp‒M4)
2.5. Physicochemical Characterization of Membranes
2.5.1. Swelling Test
2.5.2. Mechanical Test
2.5.3. Thermogravimetric Analysis (TGA)
2.5.4. Differential Scanning Calorimetry (DSC)
2.5.5. pH Values
2.5.6. Structure and Morphology of M4 and CNp‒M4 Membranes
2.5.7. In Vitro Release Study of Drug Dispersion, CNp and CNp‒M4 Membrane
2.6. Permeation Assays
2.6.1. Ex Vivo Permeation Assay
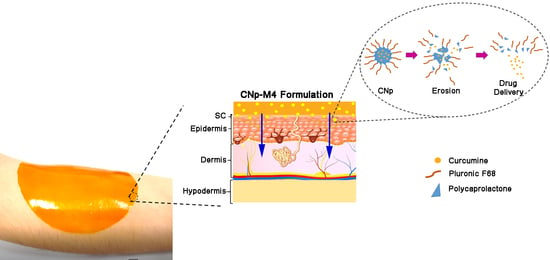
2.6.2. In Vivo Permeation Assay
3. Results and Discussion
3.1. Physicochemical Characterization of CNp
3.1.1. Particle Size and Zeta Potential Assessment
3.1.2. Atomic Force Microscopy (AFM)
3.1.3. Drug Loading and Entrapment Efficiency of Nanoparticles
3.2. Physicochemical and Mechanical Characterization of Nanoparticle-Coated Alginate Membranes as Wound Dressings
3.2.1. Swelling Test
3.2.2. Mechanical Test
3.2.3. Thermogravimetric Analysis (TGA)
3.2.4. Differential Scanning Calorimetry (DSC)
3.2.5. pH Determination
3.2.6. Structure and Morphology of M4 and CNp‒M4 Membranes
3.2.7. In Vitro Release Study of Drug Dispersion, CNp and CNp‒M4
3.3. Curcumin Permeation Assays
3.3.1. Ex Vivo Permeation
3.3.2. Permeation Assay in Vivo
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Eming, S.A.; Brachvogel, B.; Odorisio, T.; Koch, M. Regulation of angiogenesis: Wound healing as a model. Prog. Histochem. Cytochem. 2007, 42, 115–170. [Google Scholar] [CrossRef] [PubMed]
- Demidova-Rice, T.N.; Hamblin, M.R.; Herman, I.M. Current Methods for Drug Delivery, Part 1: Normal and Chronic Wounds: Biology, Causes, and Approaches to Care. Adv. Skin Wound Care 2013, 25, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Axibal, E.; Brown, M. Surgical Dressings and Novel Skin Substitutes. Dermatol. Clin. 2019, 37, 349–366. [Google Scholar] [CrossRef] [PubMed]
- Aderibigbe, B.A.; Buyana, B. Alginate in wound dressings. Pharmaceutics 2018, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Morgado, P.I.; Aguiar-Ricardo, A.; Correia, I.J. Asymmetric membranes as ideal wound dressings: An overview on production methods, structure, properties and performance relationship. J. Memb. Sci. 2015, 490, 139–151. [Google Scholar] [CrossRef]
- Mi, F.L.; Wu, Y.B.; Shyu, S.S.; Chao, A.C.; Lai, J.Y.; Su, C.C. Asymmetric chitosan membranes prepared by dry/wet phase separation: A new type of wound dressing for controlled antibacterial release. J. Memb. Sci. 2003, 212, 237–254. [Google Scholar] [CrossRef]
- Priya, S.G.; Gupta, A.; Jain, E.; Sarkar, J.; Damania, A.; Jagdale, P.R.; Chaudhari, B.P.; Gupta, K.C.; Kumar, A. Bilayer Cryogel Wound Dressing and Skin Regeneration Grafts for the Treatment of Acute Skin Wounds. ACS Appl. Mater. Interfaces 2016, 8, 15145–15159. [Google Scholar] [CrossRef]
- Miguel, S.P.; Moreira, A.F.; Correia, I.J. Chitosan based-asymmetric membranes for wound healing: A review. Int. J. Biol. Macromol. 2019, 127, 460–475. [Google Scholar] [CrossRef]
- Sood, A.; Granick, M.S.; Tomaselli, N.L. Wound Dressings and Comparative Effectiveness Data. Adv. Wound Care 2014, 3, 511–529. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, B.B.; Harikumar, K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int. J. Biochem. Cell Biol. 2009, 41, 40–59. [Google Scholar] [CrossRef] [Green Version]
- Augustyniak, A.; Bartosz, G.; Čipak, A.; Duburs, G.; Horáková, L.; Łuczaj, W.; Majekova, M.; Odysseos, A.D.; Rackova, L.; Skrzydlewska, E.; et al. Natural and synthetic antioxidants: An updated overview. Free Radic. Res. 2010, 44, 1216–1262. [Google Scholar] [CrossRef]
- Del Prado-Audelo, M.L.; Caballero-Florán, I.H.; Meza-Toledo, J.A.; Mendoza-Muñoz, N.; González-Torres, M.; Florán, B.; Cortés, H.; Leyva-Gómez, G. Formulations of curcumin nanoparticles for brain diseases. Biomolecules 2019, 9, 56. [Google Scholar] [CrossRef]
- Sidhu, G.S.; Singh, A.K.; Thaloor, D.; Banaudha, K.K.; Patnaik, G.K.; Srimal, R.C.; Maheshwari, R.K. Enhancement of wound healing by curcumin in animals. Wound Repair Regen. 1998, 6, 167–177. [Google Scholar] [CrossRef]
- Thangapazham, R.L.; Sharad, S.; Maheshwari, R.K. Skin regenerative potentials of curcumin. Biofactors 2013, 39, 141–149. [Google Scholar] [CrossRef]
- Yallapu, M.M.; Jaggi, M.; Chauhan, S.C. Curcumin nanoformulations: A future nanomedicine for cancer. Drug Discov. Today 2012, 17, 71–80. [Google Scholar] [CrossRef]
- Quintanar-guerrero, D.; Allémann, E.; Fessi, H.; Doelker, E.; Allémann, E.; Fessi, H.; Doelker, E. Preparation Techniques and Mechanisms of Formation of Biodegradable Nanoparticles from Preformed Polymers. Drug Dev. Ind. Pharm. 1998, 24, 1113–1128. [Google Scholar] [CrossRef]
- Karki, S.; Kim, H.; Na, S.; Shin, D.; Jo, K.; Lee, J. Thin films as an emerging platform for drug delivery. Asian J. Pharm. Sci. 2016, 11, 559–574. [Google Scholar] [CrossRef] [Green Version]
- Golafshan, N.; Rezahasani, R.; Tarkesh Esfahani, M.; Kharaziha, M.; Khorasani, S.N. Nanohybrid hydrogels of laponite: PVA-Alginate as a potential wound healing material. Carbohydr. Polym. 2017, 176, 392–401. [Google Scholar] [CrossRef]
- Song, Y.; Jiang, Z.; Gao, B.; Wang, H.; Wang, M.; He, Z.; Cao, X.; Pan, F. Embedding hydrophobic MoS2nanosheets within hydrophilic sodium alginate membrane for enhanced ethanol dehydration. Chem. Eng. Sci. 2018, 185, 231–242. [Google Scholar] [CrossRef]
- Mao, K.; Fan, Z.; Yuan, J.; Chen, P.; Yang, J.; Xu, J. Skin-penetrating polymeric nanoparticles incorporated in silk fibroin hydrogel for topical delivery of curcumin to improve its therapeutic effect on psoriasis mouse model. Colloids Surf. B Biointerfaces 2017, 160, 704–714. [Google Scholar] [CrossRef]
- Kamar, S.S.; Abdel-Kader, D.H.; Rashed, L.A. Beneficial effect of Curcumin Nanoparticles-Hydrogel on excisional skin wound healing in type-I diabetic rat: Histological and immunohistochemical studies. Ann. Anat. 2019, 222, 94–102. [Google Scholar] [CrossRef]
- Clayton, K.N.; Salameh, J.W.; Wereley, S.T.; Kinzer-Ursem, T.L. Physical characterization of nanoparticle size and surface modification using particle scattering diffusometry. Biomicrofluidics 2016, 10, 1–14. [Google Scholar] [CrossRef]
- Singh, R.; Lillard, J.W., Jr. Nanoparticle-based targeted drug delivery. Exp. Mol. Pathol. 2009, 86, 215–223. [Google Scholar] [CrossRef] [Green Version]
- Moghimi, S.M.; Hunter, A.C. Good Review on Poloxamers and Poloxamines in Pharma. Elsevier 2000, 18, 412–420. [Google Scholar]
- Ramanujam, R.; Sundaram, B.; Janarthanan, G.; Devendran, E.; Venkadasalam, M.; John Milton, M.C. Biodegradable Polycaprolactone Nanoparticles Based Drug Delivery Systems: A Short Review. Biosci. Biotechnol. Res. Asia 2018, 15, 679–685. [Google Scholar] [CrossRef]
- Kraeling, M.E.K.; Topping, V.D.; Keltner, Z.M.; Belgrave, K.R.; Bailey, K.D.; Gao, X.; Yourick, J. In vitro percutaneous penetration of silver nanoparticles in pig and human skin. Regul. Toxicol. Pharmacol. 2018, 95, 314–322. [Google Scholar] [CrossRef]
- Quintanar-Guerrero, D.; de la Luz Zambrano-Zaragoza, M.; Gutierrez-Cortez, E.; Mendoza-Munoz, N. Impact of the emulsification-diffusion method on the development of pharmaceutical nanoparticles. Recent Pat. Drug Deliv. Formul. 2012, 6, 184–194. [Google Scholar] [CrossRef]
- Pinto Reis, C.; Neufeld, R.J.; Ribeiro, A.J.; Veiga, F.; Nanoencapsulation, I. Methods for preparation of drug-loaded polymeric nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2006, 2, 8–21. [Google Scholar] [CrossRef]
- Judefeind, A.; de Villiers, M.M. Nanotechnology in Drug Delivery; de Villiers, M.M., Aramwit, P.S., Kwon, G., Eds.; Springer US: New York, NY, USA, 2009; ISBN 9780387776675. [Google Scholar]
- Singh, B.; Pal, L. Radiation crosslinking polymerization of sterculia polysaccharide-PVA-PVP for making hydrogel wound dressings. Int. J. Biol. Macromol. 2011, 48, 501–510. [Google Scholar] [CrossRef]
- Göpferich, A. Mechanisms of polymer degradation and erosion. Biomaterials 1996, 17, 103–114. [Google Scholar] [CrossRef]
- GD, M. Quantifying wound fluids for the clinician and researcher. Ostomy Wound Manag. 1994, 40, 66–69. [Google Scholar]
- Parikh, D.V.; Fink, T.; Delucca, A.J.; Parikh, A.D. Absorption and swelling characteristics of silver (I) antimicrobial wound dressings. Text. Res. J. 2011, 81, 494–503. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Kenawy, E.S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef]
- Shi, S.; Peng, X.; Liu, T.; Chen, Y.N.; He, C.; Wang, H. Facile preparation of hydrogen-bonded supramolecular polyvinyl alcohol-glycerol gels with excellent thermoplasticity and mechanical properties. Polymer 2017, 111, 168–176. [Google Scholar] [CrossRef]
- Wang, R.-M.; Zheng, S.-R.; Zheng, Y.-P. Matrix materials. In Polymer Matrix Composites and Technology; Elsevier: Amsterdam, The Netherlands, 2011; pp. 101–548. [Google Scholar]
- Sun, B.; Tian, Y.; Chen, L.; Jin, Z. Food Hydrocolloids Linear dextrin as curcumin delivery system: Effect of degree of polymerization on the functional stability of curcumin. Food Hydrocoll. 2018, 77, 911–920. [Google Scholar] [CrossRef]
- Campus, K.; Kanchanaburi, M.L.; Road, S. Nanocomposites Based on Cassava Starch and Chitosan-Modified Clay: Physico-Mechanical Properties and Biodegradability in Simulated Compost Soil. J. Braz. Chem. Soc. 2017, 28, 649–658. [Google Scholar]
- Aghazadeh, M.; Karim, R.; Abdul Rahman, R.; Sultan, M.T.; Johnson, S.K.; Paykary, M. Effect of Glycerol on the Physicochemical Properties of Cereal Starch Films. Food Technol. Econ. Eng. Phys. Prop. 2018, 36, 403–409. [Google Scholar] [CrossRef]
- Shrotriya, S.; Ranpise, N.; Satpute, P.; Vidhate, B. Skin targeting of curcumin solid lipid nanoparticles-engrossed topical gel for the treatment of pigmentation and irritant contact dermatitis. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1471–1482. [Google Scholar] [CrossRef]
- Kossack, W.; Kremer, F. Banded spherulites and twisting lamellae in poly–ε–caprolactone. Colloid Polym. Sci. 2019, 297, 771–779. [Google Scholar] [CrossRef]
- Verhoeven, N.; Neoh, T.L.; Furuta, T.; Yamamoto, C.; Ohashi, T.; Yoshii, H. Characteristics of dehydration kinetics of dihydrate trehalose to its anhydrous form in ethanol by DSC. Food Chem. 2012, 132, 1638–1643. [Google Scholar] [CrossRef]
- Bueno, C.Z.; Moraes, Â.M. Development of Porous Lamellar Chitosa-Alginate Membranes: Effect of Different Surfactants on Biomaterial Properties. J. Appl. Polym. Sci. 2011, 122, 624–631. [Google Scholar] [CrossRef]
- Del Prado-Audelo, M.L.; Magaña, J.J.; Mejía-Contreras, B.A.; Borbolla-Jiménez, F.V.; Giraldo-Gomez, D.M.; Piña-Barba, M.C.; Quintanar-Guerrero, D.; Leyva-Gómez, G. In vitro cell uptake evaluation of curcumin-loaded PCL/F68 nanoparticles for potential application in neuronal diseases. J. Drug Deliv. Sci. Technol. 2019, 52, 905–914. [Google Scholar] [CrossRef]
- Blattner, C.M.; Coman, G.; Blickenstaff, N.R.; Maibach, H.I. Percutaneous absorption of water in skin: A review. Rev. Environ. Health 2014, 29, 175–180. [Google Scholar] [CrossRef]
- Tagami, H.; Kanamaru, Y.; Inoue, K.; Suehisa, S.; Iwatsuki, K.; Yoshikuni, K.; Yamada, M. Water sorption-desorption test of the skin in vivo for functional assessment of the stratum corneum. J. Invest. Dermatol. 1982, 78, 425–428. [Google Scholar] [CrossRef]
- Van der Molen, R.; Spies, F.; van´t Noordende, J.M.; Boelsma, E.; Mommaas, A.M.; Koerten, H.K. Tape stripping of human stratum corneum yields cell layers that originate from various depths because of furrows in the skin. Arch. Dermatol. Res. 1997, 289, 514–518. [Google Scholar] [CrossRef]
- Kucukturmen, B.; Oz, U.C.; Bozkir, A. In Situ Hydrogel Formulation for Intra-Articular Application of Diclofenac Sodium-Loaded Polymeric Nanoparticles. Turk. J. Pharm. Sci. 2017, 14, 56–64. [Google Scholar] [CrossRef]
- Zhang, N.; Said, A.; Wischke, C.; Kral, V.; Brodwolf, R.; Volz, P.; Boreham, A.; Gerecke, C.; Li, W.; Neffe, A.T.; et al. Poly[acrylonitrile-co-(N-vinyl pyrrolidone)] nanoparticles—Composition-dependent skin penetration enhancement of a dye probe and biocompatibility. Eur. J. Pharm. Biopharm. 2017, 116, 66–75. [Google Scholar] [CrossRef]
- Goto, N.; Morita, Y.; Terada, K. Deposits from Creams Containing 20% (w/w) Urea and Suppression of Crystallization (Part 2): Novel Analytical Methods of Urea Accumulated in the Stratum Corneum by Tape stripping and Colorimetry. Chem. Pharm. Bull. 2016, 64, 1092–1098. [Google Scholar] [CrossRef] [Green Version]
- Langer, R. New Methods of Drug Delivery. Science 1990, 249, 1527–1533. [Google Scholar] [CrossRef]
- Urrejola, M.C.; Soto, L.V.; Zumarán, C.; Peñaloza, P.; Álvarez, B.; Fuentevilla, I.; Haidar, Z.S. Sistemas de Nanopartículas Poliméricas II: Estructura, Métodos de Elaboración, Características, Propiedades, Biofuncionalización y Tecnologías de Auto-Ensamblaje Capa por Capa (Layer-by-Layer Self-Assembly). Int. J. Morphol. 2018, 36, 1463–1471. [Google Scholar] [CrossRef]
- Lademann, J.; Richter, H.; Teichmann, A.; Otberg, N.; Blume-Peytavi, U.; Luengo, J.; Weiß, B.; Schaefer, U.F.; Lehr, C.M.; Wepf, R.; et al. Nanoparticles—An efficient carrier for drug delivery into the hair follicles. Eur. J. Pharm. Biopharm. 2007, 66, 159–164. [Google Scholar] [CrossRef]









| Formulation Code | SA (% w/v) | Polymer | Plasticizer | ||
|---|---|---|---|---|---|
| PVA (%w/v) | PVP (%w/v) | Gly (%w/v) | Prop (%v/v) | ||
| 4 | 2 | ---- | 10 | ---- | |
| M2 | 4 | 2 | ---- | 10 | 12 |
| M3 | 4 | ---- | 2 | 10 | ---- |
| M4 | 4 | ---- | 2 | 10 | 12 |
| CNp‒M4 | 4 | ---- | 2 | 10 | 12 |
| Sample | Tm1 (°C) | Tm2 (°C) | Tm3 (°C) |
|---|---|---|---|
| Curcumin | 174 | ---- | ---- |
| CNp | 63.5 | 101 | 212 |
| M4 | 87 | 249 | ---- |
| CNp‒M4 | 87 | 233 | ---- |
| Mathematical Model | Equation | CNp | CNp‒M4 | ||||
|---|---|---|---|---|---|---|---|
| R2 | A | B | R2 | A | B | ||
| Zero-order | 0.842 | 0.5264 | 4.7385 | 0.7358 | 1.2362 | 31.432 | |
| First-order | 0.8436 | −0.0066 | −2.3991 | 0.8453 | −0.0303 | −3.1975 | |
| Higuchi | 0.9551 | 0.0852 | −0.0459 | 0.8939 | 0.1017 | 0.194 | |
| Korsmeyer–Peppas | 0.9037 | 0.5422 | −1.2655 | 0.9536 | 0.3119 | −0.5609 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guadarrama-Acevedo, M.C.; Mendoza-Flores, R.A.; Del Prado-Audelo, M.L.; Urbán-Morlán, Z.; Giraldo-Gomez, D.M.; Magaña, J.J.; González-Torres, M.; Reyes-Hernández, O.D.; Figueroa-González, G.; Caballero-Florán, I.H.; et al. Development and Evaluation of Alginate Membranes with Curcumin-Loaded Nanoparticles for Potential Wound-Healing Applications. Pharmaceutics 2019, 11, 389. https://doi.org/10.3390/pharmaceutics11080389
Guadarrama-Acevedo MC, Mendoza-Flores RA, Del Prado-Audelo ML, Urbán-Morlán Z, Giraldo-Gomez DM, Magaña JJ, González-Torres M, Reyes-Hernández OD, Figueroa-González G, Caballero-Florán IH, et al. Development and Evaluation of Alginate Membranes with Curcumin-Loaded Nanoparticles for Potential Wound-Healing Applications. Pharmaceutics. 2019; 11(8):389. https://doi.org/10.3390/pharmaceutics11080389
Chicago/Turabian StyleGuadarrama-Acevedo, Mónica C., Raisa A. Mendoza-Flores, María L. Del Prado-Audelo, Zaida Urbán-Morlán, David M. Giraldo-Gomez, Jonathan J. Magaña, Maykel González-Torres, Octavio D. Reyes-Hernández, Gabriela Figueroa-González, Isaac H. Caballero-Florán, and et al. 2019. "Development and Evaluation of Alginate Membranes with Curcumin-Loaded Nanoparticles for Potential Wound-Healing Applications" Pharmaceutics 11, no. 8: 389. https://doi.org/10.3390/pharmaceutics11080389