Microfluidic Synthesis of Vinblastine-Loaded Multifunctional Particles for Magnetically Responsive Controlled Drug Release
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
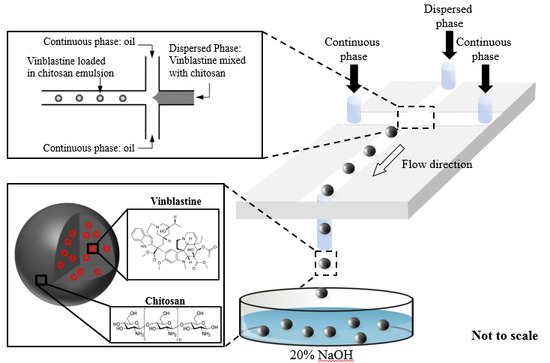
2.2. Design and Fabrication of a Microfluidic Chip
2.3. Synthesis of the Chitosan Particles
2.4. Synthesis of SPIO NPs–Chitosan Composite Particles
2.5. Preparation of VBL-Loaded SPIO NPs–Chitosan Composite Particles
2.6. Cytotoxicity Test
2.7. Application in Magnetically Responsive Drug Release
3. Results
3.1. Morphology
3.2. Characterization
3.3. Biocompatibility Test and In Vitro Release Study
3.4. Application in Controlled Drug Release
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Moudi, M.; Go, R.; Yien, C.Y.; Nazre, M. Vinca alkaloids. Int. J. Prev. Med. 2013, 4, 1231–1235. [Google Scholar] [PubMed]
- Martino, E.; Casamassima, G.; Castiglione, S.; Cellupica, E.; Pantalone, S.; Papagni, F.; Rui, M.; Siciliano, A.M.; Collina, S. Vinca alkaloids and analogues as anti-cancer agents: Looking back, peering ahead. Bioorg. Med. Chem. Lett. 2018, 28, 2816–2826. [Google Scholar] [CrossRef]
- Lee, C.T.; Huang, Y.W.; Yang, C.H.; Huang, K.S. Drug delivery systems and combination therapy by using vinca alkaloids. Curr. Top. Med. Chem. 2015, 15, 1491–1500. [Google Scholar] [CrossRef]
- Samperiz, M.M.; Blumen, G.; Merzel, J. Effect of vinblastine on the cell cycle and migration of ameloblasts of mouse incisors as shown by autoradiography using 3H-thymidine. Cell Tissue. Kinet. 1985, 18, 493–503. [Google Scholar]
- Osswald, H. Potentiation of the chemotherapeutic effect of vinblastine by thymidine. Arzneimittelforschung 1972, 22, 1421. [Google Scholar]
- Lin, W. Introduction: Nanoparticles in medicine. Chem. Rev. 2015, 115, 10407–10409. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wu, F.; Lin, X.; Shen, L.; Feng, Y. Developments in drug delivery of bioactive alkaloids derived from traditional Chinese medicine. Drug Deliv. 2018, 25, 398–416. [Google Scholar] [CrossRef]
- Aspland, S.E.; Ballatore, C.; Castillo, R.; Desharnais, J.; Eustaquio, T.; Goelet, P.; Guo, Z.; Li, Q.; Nelson, D.; Sun, C.; et al. Kinase-mediated trapping of bi-functional conjugates of paclitaxel or vinblastine with thymidine in cancer cells. Bioorg. Med. Chem. Lett. 2006, 16, 5194–5198. [Google Scholar] [CrossRef]
- Dhawan, D.; Ramos-Vara, J.A.; Naughton, J.F.; Cheng, L.; Low, P.S.; Rothenbuhler, R.; Leamon, C.P.; Parker, N.; Klein, P.J.; Vlahov, I.R.; et al. Targeting folate receptors to treat invasive urinary bladder cancer. Cancer Res. 2013, 73, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Diop-Bove, N.; Goldman, I.D. Enhanced receptor-mediated endocytosis and cytotoxicity of a folic acid-desacetylvinblastine monohydrazide conjugate in a pemetrexed-resistant cell line lacking folate-specific facilitative carriers but with increased folate receptor expression. Mol. Pharmacol. 2014, 85, 310–321. [Google Scholar] [CrossRef]
- Zu, Y.; Zhang, Y.; Zhao, X.; Zhang, Q.; Liu, Y.; Jiang, R. Optimization of the preparation process of vinblastine sulfate (VBLS)-loaded folate-conjugated bovine serum albumin (BSA) nanoparticles for tumor-targeted drug delivery using response surface methodology (RSM). Int. J. Nanomed. 2009, 4, 321–333. [Google Scholar] [CrossRef]
- Marinina, J.; Shenderova, A.; Mallery, S.R.; Schwendeman, S.P. Stabilization of vinca alkaloids encapsulated in poly(lactide-co-glycolide) microspheres. Pharm. Res. 2000, 17, 677–683. [Google Scholar] [CrossRef]
- Li, X.T.; He, M.L.; Zhou, Z.Y.; Jiang, Y.; Cheng, L. The antitumor activity of PNA modified vinblastine cationic liposomes on Lewis lung tumor cells: In vitro and in vivo evaluation. Int. J. Pharm. 2015, 487, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Maswadeh, H.; Demetzos, C.; Dimas, K.; Loukas, Y.L.; Georgopoulos, A.; Mavromoustakos, T.; Papaioannou, G.T. In-vitro cytotoxic/cytostatic activity of anionic liposomes containing vinblastine against leukaemic human cell lines. J. Pharm. Pharmacol. 2002, 54, 189–196. [Google Scholar] [CrossRef]
- Seid, C.A.; Fidler, I.J.; Clyne, R.K.; Earnest, L.E.; Fan, D. Overcoming murine tumor cell resistance to vinblastine by presentation of the drug in multilamellar liposomes consisting of phosphatidylcholine and phosphatidylserine. Sel. Cancer Ther. 1991, 7, 103–112. [Google Scholar] [CrossRef]
- Yang, F.; Wang, H.; Hu, P.; Jiang, J. Validation of an UPLC-MS-MS method for quantitative analysis of vincristine in human urine after intravenous administration of vincristine sulfate liposome injection. J. Chromatogr. Sci. 2015, 53, 974–978. [Google Scholar] [CrossRef]
- Dandamudi, S.; Patil, V.; Fowle, W.; Khaw, B.A.; Campbell, R.B. External magnet improves antitumor effect of vinblastine and the suppression of metastasis. Cancer Sci. 2009, 100, 1537–1543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Zhang, G.; Wu, Z. Nanostructures for pH-sensitive Drug Delivery and Magnetic Resonance Contrast Enhancement Systems. Curr. Med. Chem. 2018, 25, 3036–3057. [Google Scholar] [CrossRef]
- Pon-On, W.; Tithito, T.; Maneeprakorn, W.; Phenrat, T.; Tang, I.-M. Investigation of magnetic silica with thermoresponsive chitosan coating for drug controlled release and magnetic hyperthermia application. Mater. Sci. Eng. C 2019, 97, 23–30. [Google Scholar] [CrossRef]
- Chen, H.; Liu, L. One-step synthesis of polyethylenimine-coated Fe3O4 superparamagnetic nanoparticles for latent fingermark enhancement. Bull. Chem. Soc. Jpn. 2018, 91, 1319–1324. [Google Scholar] [CrossRef]
- German, S.V.; Navolokin, N.A.; Kuznetsova, N.R.; Zuev, V.V.; Inozemtseva, O.A.; Anis’kov, A.A.; Volkova, E.K.; Bucharskaya, A.B.; Maslyakova, G.N.; Fakhrullin, R.F.; et al. Liposomes loaded with hydrophilic magnetite nanoparticles: Preparation and application as contrast agents for magnetic resonance imaging. Colloids Surf. B 2015, 135, 109–115. [Google Scholar] [CrossRef]
- Zeleňák, V.; Zeleňáková, A.; Kapusta, O.; Hrubovčák, P.; Girman, V.; Bednarčík, J. Fe2O3 and Gd2O3 nanoparticles loaded in mesoporous silica: Insights into influence of NPs concentration and silica dimensionality. RSC Adv. 2019, 9, 3679–3687. [Google Scholar] [CrossRef]
- Dandamudi, S.; Campbell, R.B. The drug loading, cytotoxicty and tumor vascular targeting characteristics of magnetite in magnetic drug targeting. Biomaterials 2007, 28, 4673–4683. [Google Scholar] [CrossRef]
- Deng, L.; Ren, J.; Li, J.; Leng, J.; Qu, Y.; Lin, C.; Shi, D. Magnetothermally responsive star-block copolymeric micelles for controlled drug delivery and enhanced thermo-chemotherapy. Nanoscale 2015, 7, 9655–9663. [Google Scholar] [CrossRef]
- Nagura, K.; Takemoto, Y.; Yoshino, F.; Bogdanov, A.; Chumakova, N.; Vorobiev, A.K.; Imai, H.; Matsuda, T.; Shimono, S.; Kato, T.; et al. Magnetic Mixed Micelles Composed of a Non-Ionic Surfactant and Nitroxide Radicals Containing a d-Glucosamine Unit: Preparation, Stability, and Biomedical Application. Pharmaceutics 2019, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- Veloso, S.; Ferreira, P.; Martins, J.; Coutinho, P.; Castanheira, E. Magnetogels: Prospects and Main Challenges in Biomedical Applications. Pharmaceutics 2018, 10, 145. [Google Scholar] [CrossRef] [PubMed]
- Estelrich, J.; Escribano, E.; Queralt, J.; Busquets, M.A. Iron oxide nanoparticles for magnetically-guided and magnetically-responsive drug delivery. Int. J. Mol. Sci. 2015, 16, 8070–8101. [Google Scholar] [CrossRef] [PubMed]
- Kost, J.; Wolfrum, J.; Langer, R. Magnetically enhanced insulin release in diabetic rats. J. Biomed. Mater. Res. 1987, 21, 1367–1373. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, H.; Li, H.; Zhang, D. Magnetic albumin immuno-nanospheres as an efficient gene delivery system for a potential use in lung cancer: preparation, in vitro targeting and biological effect analysis. J. Drug Target. 2016, 24, 247–256. [Google Scholar] [CrossRef]
- Lin, Y.S.; Huang, K.S.; Yang, C.H.; Wang, C.Y.; Yang, Y.S.; Hsu, H.C.; Liao, Y.J.; Tsai, C.W. Microfluidic synthesis of microfibers for magnetic-responsive controlled drug release and cell culture. PLoS ONE 2012, 7, e33184. [Google Scholar] [CrossRef]
- Yang, C.H.; Wang, L.S.; Chen, S.Y.; Huang, M.C.; Li, Y.H.; Lin, Y.C.; Chen, P.F.; Shaw, J.F.; Huang, K.S. Microfluidic assisted synthesis of silver nanoparticle-chitosan composite microparticles for antibacterial applications. Int. J. Pharm. 2016, 510, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Guo, F.; Kiraly, B.; Mao, X.; Lu, M.; Leong, K.W.; Huang, T.J. Microfluidic synthesis of multifunctional Janus particles for biomedical applications. Lab Chip 2012, 12, 2097–2102. [Google Scholar] [CrossRef] [PubMed]
- Bruinsmann, F.A.; Pigana, S.; Aguirre, T.; Souto, G.D.; Pereira, G.G.; Bianchera, A.; Fasiolo, L.T.; Colombo, G.; Marques, M.; Pohlmann, A.R. Chitosan-Coated Nanoparticles: Effect of Chitosan Molecular Weight on Nasal Transmucosal Delivery. Pharmaceutics 2019, 11, 86. [Google Scholar] [CrossRef]
- Yang, C.H.; Wang, C.Y.; Grumezescu, A.M.; Wang, A.H.; Hsiao, C.J.; Chen, Z.Y.; Huang, K.S. Core-shell structure microcapsules with dual pH-responsive drug release function. Electrophoresis 2014, 35, 2673–2680. [Google Scholar] [CrossRef]
- Yang, C.H.; Wang, C.Y.; Huang, K.S.; Kung, C.P.; Chang, Y.C.; Shaw, J.F. Microfluidic one-step synthesis of Fe3O4-chitosan composite particles and their applications. Int. J. Pharm. 2014, 463, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; Huang, K.S.; Grumezescu, A.M.; Wang, C.Y.; Tzeng, S.C.; Chen, S.Y.; Lin, Y.H.; Lin, Y.S. Synthesis of uniform poly(d,l-lactide) and poly(d,l-lactide-co-glycolide) microspheres using a microfluidic chip for comparison. Electrophoresis 2014, 35, 316–322. [Google Scholar] [CrossRef]
- Zheng, G.X.; Zhang, X.M.; Yang, Y.S.; Zeng, S.R.; Wei, J.F.; Wang, Y.H.; Li, Y.J. An integrated microfludic device for culturing and screening of Giardia lamblia. Exp. Parasitol. 2014, 137, 1–7. [Google Scholar] [CrossRef]
- Huang, K.S.; Shieh, D.B.; Yeh, C.S.; Wu, P.C.; Cheng, F.Y. Antimicrobial applications of water-dispersible magnetic nanoparticles in biomedicine. Curr. Med. Chem. 2014, 21, 3312–3322. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; Yen, C.C.; Jheng, J.J.; Wang, C.Y.; Chen, S.S.; Huang, P.Y.; Huang, K.S.; Shaw, J.F. Immobilization of Brassica oleracea chlorophyllase 1 (BoCLH1) and Candida rugosa lipase (CRL) in magnetic alginate beads: an enzymatic evaluation in the corresponding proteins. Molecules 2014, 19, 11800–11815. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.S.; Lin, Y.S.; Yang, C.H.; Tsai, C.W.; Hsu, M.Y. In situ synthesis of twin monodispersed alginate microparticles. Soft Matter 2011, 7, 6713–6718. [Google Scholar] [CrossRef]
- Huang, K.S.; Yang, C.H.; Kung, C.P.; Grumezescu, A.M.; Ker, M.D.; Lin, Y.S.; Wang, C.Y. Synthesis of uniform core-shell gelatin-alginate microparticles as intestine-released oral delivery drug carrier. Electrophoresis 2014, 35, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; Huang, K.S.; Wang, C.Y.; Hsu, Y.Y.; Chang, F.R.; Lin, Y.S. Microfluidic-assisted synthesis of hemispherical and discoidal chitosan microparticles at an oil/water interface. Electrophoresis 2012, 33, 3173–3180. [Google Scholar] [CrossRef]
- Huang, K.S.; Yang, C.H.; Lin, Y.S.; Wang, C.Y.; Lu, K.; Chang, Y.F.; Wang, Y.L. Electrostatic droplets assisted synthesis of alginate microcapsules. Drug Deliv. Transl. Res. 2011, 1, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, X.; Zu, Y.; Chen, X.; Lu, Q.; Ma, Y.; Yang, L. Preparation and physicochemical properties of vinblastine microparticles by supercritical antisolvent process. Int. J. Mol. Sci. 2012, 13, 12598–12607. [Google Scholar] [CrossRef]
- Yang, C.H.; Wang, C.Y.; Huang, K.S.; Yeh, C.S.; Wang, A.H.; Wang, W.T.; Lin, M.Y. Facile synthesis of radial-like macroporous superparamagnetic chitosan spheres with in-situ co-precipitation and gelation of ferro-gels. PLoS ONE 2012, 7, e49329. [Google Scholar] [CrossRef]
- Zhao, C.X. Multiphase flow microfluidics for the production of single or multiple emulsions for drug delivery. Adv. Drug Deliv. Rev. 2013, 65, 1420–1446. [Google Scholar] [CrossRef]
- Yang, C.H.; Huang, K.S.; Lin, Y.S.; Lu, K.; Tzeng, C.C.; Wang, E.C.; Lin, C.H.; Hsu, W.Y.; Chang, J.Y. Microfluidic assisted synthesis of multi-functional polycaprolactone microcapsules: incorporation of CdTe quantum dots, Fe3O4 superparamagnetic nanoparticles and tamoxifen anticancer drugs. Lab Chip 2009, 9, 961–965. [Google Scholar] [CrossRef] [PubMed]








| Flow Rate of Continuous Phase (mL/min) | Flow Rate of Dispersed Phase (mL/min) | Emulsions | Particles | Shrinking (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Average Size (µm) | S.D. a (µm) | R.S.D. b (%) | Average Size (µm) | S.D. a (µm) | R.S.D. b (%) | |||
| 0.8 | 0.008 | 550.3 | 10.8 | 1.6 | 390.3 | 24.7 | 6.2 | 27.4 |
| 0.006 | 544.9 | 8.6 | 1.3 | 385 | 20.8 | 5.3 | 28.9 | |
| 0.004 | 531.7 | 11.6 | 1.7 | 372.5 | 20.1 | 5.6 | 29.9 | |
| 0.002 | 504.2 | 11.1 | 1.7 | 359.4 | 12.8 | 3.6 | 28.7 | |
| 0.7 | 0.008 | 553.5 | 6 | 0.9 | 397 | 17.5 | 4.4 | 28.3 |
| 0.006 | 546.2 | 5.4 | 0.8 | 389.3 | 17 | 4.4 | 30.4 | |
| 0.004 | 534 | 10.4 | 1.5 | 376.1 | 13.8 | 3.7 | 29.6 | |
| 0.002 | 508 | 10.2 | 1.6 | 375 | 17.2 | 4.6 | 25.7 | |
| 0.6 | 0.008 | 574 | 10.8 | 1.4 | 408.6 | 18.9 | 4.7 | 29.5 |
| 0.006 | 561.6 | 3.1 | 0.4 | 403.5 | 21 | 5.2 | 28.2 | |
| 0.004 | 552 | 7.7 | 1.1 | 398.8 | 19.8 | 5 | 27.8 | |
| 0.002 | 547.8 | 13.8 | 2 | 389.3 | 22.1 | 5.7 | 28.9 | |
| 0.5 | 0.008 | 585 | 17.9 | 2.4 | 423.1 | 11.9 | 2.8 | 27.7 |
| 0.006 | 578.5 | 20.7 | 2.8 | 421.6 | 14.3 | 3.4 | 27.1 | |
| 0.004 | 560.6 | 27.1 | 3.8 | 416.9 | 13.1 | 3.2 | 25.6 | |
| 0.002 | 554.3 | 21.5 | 3.1 | 406.4 | 20.9 | 5.1 | 26.7 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, K.-S.; Yang, C.-H.; Wang, Y.-C.; Wang, W.-T.; Lu, Y.-Y. Microfluidic Synthesis of Vinblastine-Loaded Multifunctional Particles for Magnetically Responsive Controlled Drug Release. Pharmaceutics 2019, 11, 212. https://doi.org/10.3390/pharmaceutics11050212
Huang K-S, Yang C-H, Wang Y-C, Wang W-T, Lu Y-Y. Microfluidic Synthesis of Vinblastine-Loaded Multifunctional Particles for Magnetically Responsive Controlled Drug Release. Pharmaceutics. 2019; 11(5):212. https://doi.org/10.3390/pharmaceutics11050212
Chicago/Turabian StyleHuang, Keng-Shiang, Chih-Hui Yang, Ya-Chin Wang, Wei-Ting Wang, and Yen-Yi Lu. 2019. "Microfluidic Synthesis of Vinblastine-Loaded Multifunctional Particles for Magnetically Responsive Controlled Drug Release" Pharmaceutics 11, no. 5: 212. https://doi.org/10.3390/pharmaceutics11050212