Antifungal Properties of Chenopodium ambrosioides Essential Oil Against Candida Species
Abstract
:1. Introduction
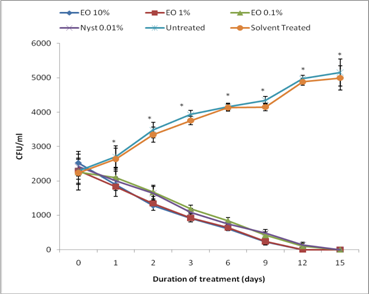
2. Results and Discussion
2.1. Chemical composition of the essential oil
| N° | Constituents | RI | % |
|---|---|---|---|
| 1 | α-Pinene | 936 | 0.1 |
| 2 | α-Terpinene | 1028 | 51,3 |
| 3 | p-Cymene | 1035 | 23,4 |
| 4 | Limonene | 1036 | 0,9 |
| 5 | β-Phellandrene | 1038 | 0,2 |
| 6 | γ-Terpinene | 1063 | 0,7 |
| 7 | Dehydro-p-cymene | 1096 | 0,1 |
| 8 | L-Carvacrol | 1128 | 0,1 |
| 9 | p-Mentha-1,8-diene | 1291 | 15,3 |
| 10 | Oxyde de piperitone | 1302 | 0,4 |
| 11 | Ascaridole | 1305 | 0,7 |
| 12 | Thymol | 1332 | 0,2 |
| 13 | Carvacrol | 1340 | 0,3 |
| 14 | Isoascaridole | 1347 | 5,1 |
2.2. Antifungal properties
| Microorganism | MIC | MFC | ||
|---|---|---|---|---|
| Essential oil (mg/mL) | Nystatin (µg/mL) | Essential oil (mg/mL) | Nystatin (µg/mL) | |
| C. albicans ATCC 9002 | 1.00 | 1.00 | 2.00 | 2.00 |
| C. albicans ATCC 2091 | 2.00 | 1.00 | 2.00 | 2.00 |
| C. albicans ATCC 1663 | 1.00 | 1.00 | 2.00 | 4.00 |
| C. glabrata | 0.25 | 1.00 | 0.25 | 1.00 |
| C. guilliermondi | 0.25 | 1.00 | 0.25 | 2.00 |
| C. krusei | 1.00 | 1.00 | 2.00 | 4.00 |
| C. lusitaneae | 1.00 | 2.00 | 1.00 | 4.00 |
| C. parapsilosis | 0.50 | 0.50 | 1.00 | 1.00 |
| C. tropicalis | 1.00 | 0.50 | 1.00 | 1.00 |


3. Experimental
3.1. Plant material, oil extraction and chemical composition
3.2. Antifungal assay
3.2.1. Fungi strains and culture media
3.2.2. Determination of minimum inhibitory concentrations and minimum fungicidal concentrations
3.2.3. Effect of C. ambrosioides essential oil on C. albicans fatty acid profiles
3.2.3.1. Fungal culture and fatty acid extraction
3.2.3.2. Fatty acid profile analysis
3.2.4. Evaluation of therapeutic effect of C. ambrosioides essential oil
- Group 1 received nystatin at 0.01% (positive control),
- Group 2 was infected and not treated (negative control),
- Group 3 was infected and received 10 µL of Tween 80 solution 1%,
- Group 4 was not infected and did not receive any substance,
- Group 5, 6 and 7 were infected and treated with 0.1, 1 and 10% of essential oil solutions (test groups).
3.3. Statistical analysis
4. Conclusions
References
- Klepser, M.E. Antifungal resistance among Candida species. Pharmacotherapy 2001, 21, 124s–132s. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, A.H.; Shigefumi, M.; Kotaro, M.; Hiroshi, K.; Eisuke, S.; Kazunori, T.; Takayoshi, T.; Shigera, K. In vitro and in vivo activities of SCH56592 against Cryptococcus neoformans. J. Antimicrob. Chemother. 1999, 44, 827–829. [Google Scholar] [CrossRef] [PubMed]
- Sean, O.H.; Renee, N.M. Candidiasis. Emerg. Med. 2006, 19, 1–11. [Google Scholar]
- Fidel, P.; Lynch, M.E.; Sobel, J.D. Candida-specific Th1-type responsiveness in mice with experimental vaginal candidiasis. Infect. Immunol. 1993, 61, 4201–4207. [Google Scholar]
- Goff, D.A.; Koletar, S.L.; Buesching, W.J.; Banishan, J.; Fass, R.J. Isolation of fluconazole-resitant Candida albicans from human immunodeficiency virus-negative patients never treated with azole. Clin. Infect. Dis. 1995, 20, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Dupont, B.F.; Dromer, F.; Improvisi, L. The problem of resistance to azoles in Candida. J. Med. Mycol. 1996, 6, 12–19. [Google Scholar]
- Heeres, J.; Meerpoel, L.; Lewi, P. Conazoles. Molecules 2010, 15, 4129–4188. [Google Scholar]
- Ellepolla, A.N.B.; Samaranayake, L.P. Oral candidal infections and antimycotics. Rev. Oral Biol. Med. 2000, 11, 172–198. [Google Scholar]
- Georgopadakou, N.A.; Dix, B.A.; Smith, S.A.; Freudenberger, J. Effect of antifungal agents on lipid biosynthesis and membrane integrity in Candida albicans. Antimicrob. Agents Chemother. 1987, 31, 46–51. [Google Scholar] [PubMed]
- Kauffman, C.A.; Carver, P.L. Antifungal agents in the 1990s. Current status and future developments. Drugs. 1997, 53, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Mondello, F.; De Bernardis, F.; Girolamo, A.; Cassone, A.; Salvatore, G. In vivo activity of terpinen-4-ol, the main bioactive component of Melaleuca alternifolia Cheel (tea tree) oil against azole-susceptible and resistant human pathogenic Candida species. BMC Infect Dis. 2006, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kishore, N.; Chansouria, J.P.N.; Dubey, N.K. Antidermatophytic action of the essential oil of Chenopodium ambrosioides and an ointment prepared from it. India Phytother. Res. 1999, 10, 453–455. [Google Scholar]
- Jardim, C.M.; Jham, G.N.; Dhingra, O.D.; Freire, M.M. Composition and antifungal activity of the essential oil of the Brazilian Chenopodium ambrosioides L. J. Chem. Ecol. 2008, 34, 1213–1218. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Kumar, M.A.; Dubey, N.K.; Tripathi, Y.B. Evaluation of Chenopodium ambroisioides oil as a potential source of antifungal, antiaflatoxigenic and antioxidant activity. Int. J. Food Microbiol. 2007, 115, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Potawale, S.E.; Luniya, K.P.; Mantri, R.A.; Mehta, U.K.; Waseem, M.D.; Sadiq, M.D.; Vetal, Y.D.; Deshmukh, R.S. Chenopodium ambrosioides: An ethnopharmacological review. Pharmacologyonline 2008, 2, 272–286. [Google Scholar]
- Tapondjou, A.L.; Adler, C.; Bouda, H.; Fontem, A.D. Bioefficacy of powders and essential oil from leaves of C. ambrosoides and E. saligna to the cowpea bruchid Collosobruchus maculatus Fab. (coleoptera. Bruchidae). Cahiers d’études et de Recherches Francophones/Agricultures 2003, 12, 401–408. [Google Scholar]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J.C. Factors affecting secondary metabolite production in plants: volatile components and essential oils. Flavor Frag. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Onocha, P.A.; Ekundayo, O.; Eramo, T.; Laakso, I. Essential oil constituents of Chenopodium ambrosioides L. leaves from Nigeria. J. Essent. Oil Res. 1999, 11, 220–222. [Google Scholar]
- Gupta, D.; Charles, R.; Mehta, V.K.; Garg, S.N.; Kumar, S. Chemical examination of the essential oil of Chenopodium ambrosioides L. from the southern hills of India. J. Essent. Oil Res. 2002, 14, 93–94. [Google Scholar]
- Koba, K.; Catherine, G.; Raynaud, C.; Chaumont, J.P.; Sanda, K.; Laurence, N. Chemical composition and cytotoxic activity of Chenopodium ambrosioides L. essential oil from Togo. Bangladesh J. Sci. Ind. Res. 2009, 44, 435–440. [Google Scholar]
- Cavalli, J.F.; Tomi, F.; Bermadini, A.F.; Casanova, J. Combined Analysis of the Essential oil of Chenopodium ambrosioides by GC, GC-MS and 13C NMR Spectroscopy: Quantitative Determination of Ascaridole, a Heat Sensitive Compound. Phytochem. Anal. 2004, 15, 275–279. [Google Scholar] [PubMed]
- Mondello, F.; De Bernardis, F.; Girolamo, A.; Salvatore, G.; Cassone, A. In vitro and in vivo activity of tea tree oil against azole-susceptible and resistant human pathogenic yeasts. J. Antimicrob. Chemother. 2003, 51, 1223–1229. [Google Scholar] [PubMed]
- Giorgione, J.; Epand, R.M.; Buda, C.; Farkas, T. Role of phospholipids containing docohexaenoyl chains in modulating the activity of protein kinase C. Proc. Natl. Acad. Sci. USA 1995, 92, 9767–9770. [Google Scholar]
- Ntambi, J.M.; Bene, H. Polyunsaturated fatty acid regulation of gene expression. J. Mol. Neurosci. 2001, 16, 273–278. [Google Scholar]
- Ells, R.; Kock, J.L.F.; Van Wyk, P.W.J.; Botes, P.J.; Pohl, C.H. Arachidonic acid increases antifungal susceptibility of Candida albicans and Candida dubliniensis. J. Antimicrob. Chemother. 2009, 63, 124–128. [Google Scholar] [PubMed]
- Shigeharu, I.; Miki, T.; Shigeru, A. Inhibitory activity of hydrosols, herbal teas and related essential oils against filament formation and growth of Candida albicans. Jpn. J. Med. Mycol. 2009, 50, 243–251. [Google Scholar] [CrossRef]
- National Committee for Clinical Laboratory Standards, Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts.; National Committee for Clinical Laboratory Standards: Wayne, PA, US, 2002; Approved Standard M27-A2.
- Tamokou, J.D.; Tala, F.M.; Wabo, H.K.; Kuiate, J.R.; Tane, P. Antimicrobial activities of methanol extract and compounds from stem bark of Vismia rubescens. J. Ethnopharmacol. 2009, 124, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, M.; Manfio, G.P.; Canhos, V.P. Characterization of selected strain of Mucorales using fatty acid profiles. Rev. Microbiol. 1998, 29, 1–8. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chekem, M.S.G.; Lunga, P.K.; Tamokou, J.D.D.; Kuiate, J.R.; Tane, P.; Vilarem, G.; Cerny, M. Antifungal Properties of Chenopodium ambrosioides Essential Oil Against Candida Species. Pharmaceuticals 2010, 3, 2900-2909. https://doi.org/10.3390/ph3092900
Chekem MSG, Lunga PK, Tamokou JDD, Kuiate JR, Tane P, Vilarem G, Cerny M. Antifungal Properties of Chenopodium ambrosioides Essential Oil Against Candida Species. Pharmaceuticals. 2010; 3(9):2900-2909. https://doi.org/10.3390/ph3092900
Chicago/Turabian StyleChekem, Marie Stéphanie Goka, Paul Keilah Lunga, Jean De Dieu Tamokou, Jules Roger Kuiate, Pierre Tane, Gerard Vilarem, and Muriel Cerny. 2010. "Antifungal Properties of Chenopodium ambrosioides Essential Oil Against Candida Species" Pharmaceuticals 3, no. 9: 2900-2909. https://doi.org/10.3390/ph3092900