Lactoferrin and Its Potential Impact for the Relief of Pain: A Preclinical Approach
Abstract
:1. Introduction
2. Lactoferrin Overview
Lactoferrin: Modulatory Properties on the Inflammatory Response
3. Lactoferrin: Animal Models to Study Anti-Nociceptive Properties
3.1. Anti-Nociceptive Effect Induced by Lactoferrin in Animal Models
3.2. Anti-Allodynic and Anti-Hyperalgesic Effects Induced by Lactoferrin in Animal Models
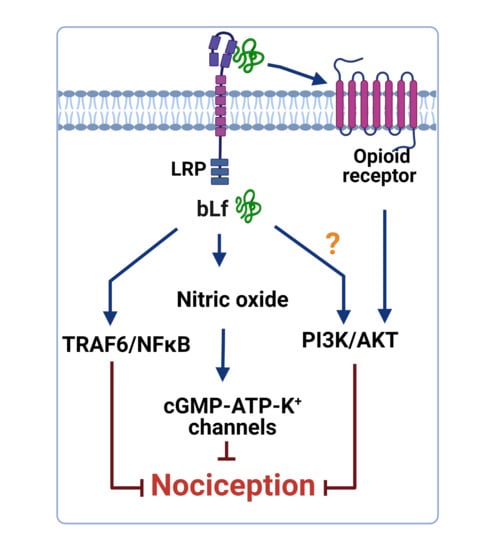
4. Mechanisms of Action Underlying the Anti-Nociceptive Activity of Lactoferrin
4.1. Role of the TRAF6–NFκB Signaling Pathway
4.2. Role of the NO–cGMP–ATP-Sensitive K+ Channel Signaling Pathway
4.3. Role of the Opioidergic System
4.4. Potentiation of Peripheral and Spinal µ Opioid Receptor-Mediated Anti-Nociception by Bovine Lactoferrin
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cervero, F. Pain: Friend or foe? A neurobiologic perspective: The 2008 Bonica Award lecture. Reg. Anesth. Pain Med. 2009, 34, 569–574. [Google Scholar] [CrossRef]
- IASP Terminology: Washington, DC, USA: International Association for the Study of Pain. Available online: https://www.iasp-pain.org/terminology?%0A (accessed on 9 July 2018).
- Freynhagen, R.; Parada, H.A.; Calderon-Ospina, C.-A.; Chen, J.; Emril, D.R.; Fernandez-Villacorta, F.J.; Franco, H.; Ho, K.-Y.; Lara-Solares, A.; Li, C.C.-F.; et al. Current understanding of the mixed pain concept: A brief narrative review. Curr. Med. Res. Opin. 2019, 35, 1011–1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colloca, L.; Ludman, T.; Bouhassira, D.; Baron, R.; Dickenson, A.H.; Yarnitsky, D.; Freeman, R.; Truini, A.; Attal, N.; Finnerup, N.B.; et al. Neuropathic Pain. Nat. Rev. Dis. Prim. 2017, 16, 749–757. [Google Scholar] [CrossRef] [Green Version]
- Cavalli, E.; Mammana, S.; Nicoletti, F.; Bramanti, P.; Mazzon, E. The neuropathic pain: An overview of the current treatment and future therapeutic approaches. Int. J. Immunopathol. Pharmacol. 2019, 33, 2058738419838383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitzcharles, M.A.; Cohen, S.P.; Clauw, D.J.; Littlejohn, G.; Usui, C.; Häuser, W. Nociplastic pain: Towards an understanding of prevalent pain conditions. Lancet 2021, 397, 2098–2110. [Google Scholar] [CrossRef]
- Kosek, E.; Clauw, D.; Nijs, J.; Baron, R.; Gilron, I.; Harris, R.E.; Mico, J.-A.; Rice, A.S.C.; Sterling, M. Chronic nociplastic pain affecting the musculoskeletal system. Pain 2021. [Google Scholar] [CrossRef] [PubMed]
- Ahlbeck, K. Opioids: A two-faced Janus. Curr. Med. Res. Opin. 2011, 27, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Remesic, M.; Hruby, V.J.; Porreca, F.; Lee, Y.S. Recent Advances in the Realm of Allosteric Modulators for Opioid Receptors for Future Therapeutics Michael. ACS Chem. Neurosci. 2017, 8, 1147–1158. [Google Scholar] [CrossRef]
- Cervero, F. Undersanding Pain; The MIT Press: Cambridge, MA, USA, 2014; ISBN 9780262526067. [Google Scholar]
- Atkinson, T.J.; Fudin, J. Nonsteroidal Antiinflammatory Drugs for Acute and Chronic Pain. Phys. Med. Rehabil. Clin. N. Am. 2020, 31, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacol. 2020, 180, 114–147. [Google Scholar] [CrossRef] [PubMed]
- Finnerup, N.B.; Haroutounian, S.; Kamerman, P.; Baron, R.; Bennett, D.L.H.; Bouhassira, D.; Cruccu, G.; Freeman, R.; Hansson, P.; Nurmikko, T.; et al. Neuropathic pain: An updated grading system for research and clinical practice. Pain 2016, 157, 1599–1606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghayur, M.N. Case Report Potential Adverse Consequences of Combination Therapy with Gabapentin and Pregabalin. Case Rep. Med. 2021, 2, 5559981. [Google Scholar] [CrossRef]
- Goldstein, F.J. Adjuncts to opioid therapy. J. Am. Osteopath. Assoc. 2002, 102, 15–20. [Google Scholar]
- Hayashida, K.; Takeuchi, T.; Shimizu, H.; Ando, K.; Harada, E. Novel function of bovine milk-derived lactoferrin on antinociception mediated by μ-opioid receptor in the rat spinal cord. Brain Res. 2003, 965, 239–245. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Yoshida, K.; Uchida, M. Novel functions of bovine milk-derived α-lactalbumin: Anti-nociceptive and anti-inflammatory activity caused by inhibiting cyclooxygenase-2 and phospholipase A2. Biol. Pharm. Bull. 2009, 32, 366–371. [Google Scholar] [CrossRef] [Green Version]
- Rosa, L.; Cutone, A.; Lepanto, M.S.; Paesano, R.; Valenti, P. Lactoferrin: A natural glycoprotein involved in iron and inflammatory homeostasis. Int. J. Mol. Sci. 2017, 18, 1985. [Google Scholar] [CrossRef]
- Yamauchi, K.; Toida, T.; Nishimura, S.; Nagano, E.; Kusuoka, O.; Teraguchi, S.; Hayasawa, H.; Shimamura, S.; Tomita, M. 13-Week oral repeated administration toxicity study of bovine lactoferrin in rats. Food Chem. Toxicol. 2000, 38, 503–512. [Google Scholar] [CrossRef]
- Okada, S.; Tanaka, K.; Sato, T.; Ueno, H.; Saito, S.; Okusaka, T.; Sato, K.; Yamamoto, S.; Kakizoe, T. Dose-response Trial of Lactoferrin in Patients with Chronic Hepatitis C. Jpn. J. Cancer Res. 2002, 93, 1063–1069. [Google Scholar] [CrossRef]
- Giunta, G.; Giuffrida, L.; Mangano, K.; Fagone, P.; Cianci, A. Influence of lactoferrin in preventing preterm delivery: A pilot study. Mol. Med. Rep. 2012, 5, 162–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paesano, R.; Torcia, F.; Berlutti, F.; Pacifici, E.; Ebano, V.; Moscarini, M.; Valenti, P. Oral administration of lactoferrin increases hemoglobin and total serum iron in pregnant women. Biochem. Cell Biol. 2006, 84, 377–380. [Google Scholar] [CrossRef]
- Manzoni, P.; Rinaldi, M.; Cattani, S.; Pugni, L.; Romeo, M.G.; Messner, H.; Stolfi, I.; Decembrino, L.; Laforgia, N.; Vagnarelli, F.; et al. Bovine Lactoferrin Supplementation for Prevention of Late-Onset Sepsis in Very Low-Birth-Weight Neonates A Randomized Trial. JAMA 2009, 302, 1421–1428. [Google Scholar] [CrossRef] [PubMed]
- King, J.C.; Cummings, G.E.; Guo, N.; Trivedi, L.; Readmond, B.X.; Keane, V.; Feigelman, S.; De Waard, R. A double-blind, placebo-controlled, pilot study of bovine lactoferrin supplementation in bottle-fed infants. J. Pediatric Gastroenterol. Nutr. 2007, 44, 245–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miranda, M.; Saccone, G.; Ammendola, A.; Salzano, E.; Iannicelli, M.; De Rosa, R.; Nazzaro, G.; Locci, M. Vaginal lactoferrin in prevention of preterm birth in women with bacterial vaginosis. J. Matern.-Fetal Neonatal Med. 2019, 2019, 1–5. [Google Scholar] [CrossRef]
- Nakano, M.; Yoshida, A.; Wakabayashi, H.; Tanaka, M.; Yamauchi, K.; Abe, F.; Masuda, Y. Effect of tablets containing lactoferrin and lactoperoxidase on gingival health in adults: A randomized, double-blind, placebo-controlled clinical trial. J. Periodontal Res. 2019, 54, 702–708. [Google Scholar] [CrossRef]
- Pammi, M.; Gautham, S. Enteral lactoferrin supplementation for prevention of sepsis and necrotizing enterocolitis in preterm infants. Cochrane Data Base Syst. Rev. 2017, 6, CD007137. [Google Scholar] [CrossRef] [PubMed]
- Lönnerdal, B.; Du, X.; Jiang, R. Biological activities of commercial bovine lactoferrin sources. Biochem. Cell Biol. 2021, 99, 35–46. [Google Scholar] [CrossRef]
- Horie, K.; Watanabe, M.; Chanbora, C.; Awada, T.; Kunimatsu, R.; Uchida, T.; Takata, T.; Tanimoto, K. Bovine lactoferrin reduces extra-territorial facial allodynia/hyperalgesia following a trigeminal nerve injury in the rat. Brain Res. 2017, 1669, 89–96. [Google Scholar] [CrossRef]
- Lee, A.S.; Ellman, M.B.; Yan, D.; Kroin, J.S.; Cole, B.J.; van Wijnen, A.J.; Im, H. A current review of molecular mechanisms regarding osteoarthritis and pain. Gene 2013, 25, 440–447. [Google Scholar] [CrossRef] [Green Version]
- Hao, L.; Shan, Q.; Wei, J.; Ma, F.; Sun, P. Lactoferrin: Major Physiological Functions and Applications. Curr. Protein Pept. Sci. 2019, 20, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Karav, S.; German, J.B.; Rouquié, C.; Le Parc, A.; Barile, D. Studying lactoferrin N-glycosylation. Int. J. Mol. Sci. 2017, 18, 870. [Google Scholar] [CrossRef] [Green Version]
- Kell, D.B.; Heyden, E.L.; Pretorius, E. The Biology of Lactoferrin, an Iron-Binding Protein That Can Help Defend Against Viruses and Bacteria. Front. Immunol. 2020, 11, 1221. [Google Scholar] [CrossRef]
- Sinha, M.; Kaushik, S.; Kaur, P.; Sharma, S.; Singh, T.P. Antimicrobial lactoferrin peptides: The hidden players in the protective function of a multifunctional protein. Int. J. Pept. 2013, 2013, 390230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayeur, S.; Spahis, S.; Pouliot, Y.; Levy, E. Lactoferrin, a Pleiotropic Protein in Health and Disease. Antioxid. Redox Signal. 2016, 24, 813–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piccinini, A.M.; Midwood, M.S. DAMPening inflammation by modulating TLR signalling. Mediat. Inflamm. 2010, 2010, 672395. [Google Scholar] [CrossRef] [Green Version]
- Drago-Serrano, M.E.; De La Garza-Amaya, M.; Luna, J.S.; Campos-Rodríguez, R. Lactoferrin-lipopolysaccharide (LPS) binding as key to antibacterial and antiendotoxic effects. Int. Immunopharmacol. 2012, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Latorre, D.; Puddu, P.; Valenti, P.; Gessani, S. Reciprocal interactions between lactoferrin and bacterial endotoxins and their role in the regulation of the immune response. Toxins 2010, 2, 54–68. [Google Scholar] [CrossRef] [Green Version]
- Takayama, Y.; Aoki, R.; Uchida, R.; Tajima, A.; Aoki-Yoshida, A. Role of CXC chemokine receptor type 4 as a lactoferrin receptor 2. Biochem. Cell Biol. 2017, 95, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Fillebeen, C.; Dehouck, B.; Benaïssa, M.; Dhennin-Duthille, I.; Cecchelli, R.; Pierce, A. Tumor necrosis factor-α increases lactoferrin transcytosis through the blood-brain barrier. J. Neurochem. 1999, 73, 2491–2500. [Google Scholar] [CrossRef]
- Qiu, Z.; Hyman, B.T.; Rebeck, G.W. Apolipoprotein E receptors mediate neurite outgrowth through activation of p44/42 mitogen-activated protein kinase in primary neurons. J. Biol. Chem. 2004, 279, 34948–34956. [Google Scholar] [CrossRef] [Green Version]
- Guzmán-Mejía, F.; Godínez-Victoria, M.; Vega-Bautista, A.; Pacheco-Yépez, J.; Drago-Serrano, M.E. Intestinal homeostasis under stress siege. Int. J. Mol. Sci. 2021, 22, 5095. [Google Scholar] [CrossRef]
- Bertuccini, L.; Costanzo, M.; Iosi, F.; Tinari, A.; Terruzzi, F.; Stronati, L.; Aloi, M.; Cucchiara, S.; Superti, F. Lactoferrin prevents invasion and inflammatory response following E. coli strain LF82 infection in experimental model of Crohn’s disease. Dig. Liver Dis. 2014, 4646, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, H.; Dosako, S.; Lonnerdal, B. Iron uptake from transferrin and lactoferrin by rat intestinal brush-border membrane vesicles. Am. J. Physiol. 1990, 258, G535–G541. [Google Scholar] [CrossRef] [PubMed]
- Safaeian, L.; Zabolian, H. Antioxidant Effects of Bovine Lactoferrin on Dexamethasone-Induced Hypertension in Rat. ISRN Pharmacol. 2014, 22, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talukder, M.J.R.; Harada, E. Bovine lactoferrin protects lipopolysaccharide-induced diarrhea modulating nitric oxide and prostaglandin E2 in mice. Can. J. Physiol. Pharmacol. 2007, 85, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Önal, A.; Kayalioǧlu, G.; Parlar, A.; Keser, A.; Ülker, S. Effect of prolonged administration of bovine lactoferrin in neuropathic pain: Involvement of opioid receptors, nitric oxide and TNF-α. Life Sci. 2010, 86, 251–259. [Google Scholar] [CrossRef] [PubMed]
- El-Zayat, S.R.; Sibaii, H.; Mannaa, F.A. Toll-like receptors activation, signaling, and targeting: An overview. Bull. Natl. Res. Cent. 2019, 43, 187. [Google Scholar] [CrossRef] [Green Version]
- Rasheed, N.; Alghasham, A.; Rasheed, Z. Lactoferrin from Camelus dromedarius inhibits nuclear transcription Factor-kappa B activation, cyclooxygenase-2 expression and prostaglandin E2 production in stimulated human chondrocytes. Pharmacogn. Res. 2016, 8, 135–141. [Google Scholar] [CrossRef] [Green Version]
- Salvi, V.; Vaira, X.; Gianello, V.; Vermi, W.; Bugatti, M.; Sozzani, S.; Bosisio, D. TLR Signalling Pathways Diverge in Their Ability to Induce PGE2. Mediat. Inflamm. 2016, 2016, 5678046. [Google Scholar] [CrossRef] [Green Version]
- Hu, P.; Zhao, F.; Wang, J.; Zhu, W. Lactoferrin attenuates lipopolysaccharide-stimulated inflammatory responses and barrier impairment through the modulation of NF-κB/MAPK/Nrf2 pathways in IPEC-J2 cells. Food Funct. 2020, 11, 8516–8526. [Google Scholar] [CrossRef]
- Kong, X.; Yang, M.; Guo, J.; Feng, Z. Effects of Bovine Lactoferrin on Rat Intestinal Epithelial Cells. J. Pediatric Gastroenterol. Nutr. 2020, 70, 645–651. [Google Scholar] [CrossRef]
- Wisgrill, L.; Wessely, I.; Spittler, A.; Förster-Waldl, E.; Berger, A.; Sadeghi, K. Human lactoferrin attenuates the proinflammatory response of neonatal monocyte-derived macrophages. Clin. Exp. Immunol. 2018, 192, 315–324. [Google Scholar] [CrossRef] [Green Version]
- Kruzel, M.L.; Harari, Y.; Mailman, D.; Actor, J.K.; Zimecki, M. Differential effects of prophylactic, concurrent and therapeutic lactoferrin treatment on LPS-induced inflammatory responses in mice. Clin. Exp. Immunol. 2002, 130, 25–31. [Google Scholar] [CrossRef]
- Zemankova, N.; Chlebova, K.; Matiasovic, J.; Prodelalova, J.; Gebauer, J.; Faldyna, M. Bovine lactoferrin free of lipopolysaccharide can induce a proinflammatory response of macrophages. BMC Vet. Res. 2016, 12, 251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baveye, S.; Elass, E.; Fernig, D.G.; Blanquart, C.; Mazurier, J.; Legrand, D. Human lactoferrin interacts with soluble CD14 and inhibits expression of endothelial adhesion molecules, E-selectin and ICAM-1, induced by the CD14-lipopolysaccharide complex. Infect. Immun. 2000, 68, 6519–6525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeida, T.F.; Roizenblatt, S.; Tufik, S. Afferent pain pathways: A neuroanatomical review. Brain Res. 2004, 1000, 40–56. [Google Scholar] [CrossRef] [PubMed]
- Sneddon, L.U. Comparative physiology of nociception and pain. Physiology 2018, 33, 63–73. [Google Scholar] [CrossRef]
- Hayashida, K.; Takeuchi, T.; Shimizu, H.; Ando, K.; Harada, E. Lactoferrin enhances opioid-mediated analgesia via nitric oxide in the rat spinal cord. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 285, 306–312. [Google Scholar] [CrossRef] [Green Version]
- Hayashida, K.I.; Kaneko, T.; Takeuchi, T.; Shimizu, H.; Ando, K.; Harada, E. Oral administration of lactoferrin inhibits inflammation and nociception in rat adjuvant-induced arthritis. J. Vet. Med. Sci. 2004, 66, 149–154. [Google Scholar] [CrossRef] [Green Version]
- Harada, E.; Sugiyama, A.; Takeuchi, T.; Sitizyo, K.; Syuto, B.; Yajima, T.; Kuwata, T. Characteristic transfer of colostral components into cerebrospinal fluid via serum in neonatal pigs. Biol. Neonate 1999, 76, 33–43. [Google Scholar] [CrossRef]
- Hayashida, K.; Takeuchi, T.; Harada, E. Lactoferrin enhances peripheral opioid-mediated antinociception via nitric oxide in rats. Eur. J. Pharmacol. 2004, 484, 175–181. [Google Scholar] [CrossRef]
- Faucheux, B.; Nillesse, N.; Damier, P.; Spik, G.; Mouatt-Prigent, A.; Pierce, A.; Leveugle, B.; Kubis, N.; Hauw, J.-J.; Agid, Y. Expression of lactoferrin receptors in increased in the mesencephalon of patients with Parkinson disease. Proc. Natl. Acad. Sci. USA 1995, 92, 9603–9607. [Google Scholar] [CrossRef] [Green Version]
- Leveugle, B.; Faucheux, B.; Bouras, C.; Nillesse, N.; Spik, G.; Hirsch, E.C.; Agid, Y.; Hof, P.R. Cellular distribution of the iron-binding protein lactotrasferrin in the mesencephalon of Parkinson´s disease cases. Acta Neurophathol. 1996, 91, 566–572. [Google Scholar] [CrossRef]
- Huang, R.Q.; Ke, W.L.; Qu, Y.H.; Zhu, J.H.; Pei, Y.Y.; Jiang, C. Characterization of lactoferrin receptor in brain endothelial capillary cells and mouse brain. J. Biomed. Sci. 2007, 14, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.A.; Lopez, V.; Lönnerdal, B. Mammalian lactoferrin receptors: Structure and function. Cell. Mol. Life Sci. 2005, 62, 2560–2575. [Google Scholar] [CrossRef] [PubMed]
- Bennett, G.J.; Chung, J.M.; Honore, M.; Seltzer, Z. Models of Neuropathic Pain in the Rat. Curr. Protoc. Neurosci. 2003, 22, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Watanabe, M.; Suekawa, Y.; Ito, G.; Inubushi, T.; Hirose, N.; Murasaki, K.; Hiyama, S.; Uchida, T.; Tanne, K. IL-1beta in the trigeminal subnucleus caudalis contributes to extra-territorial allodynia/hyperalgesia following a trigeminal nerve injury. Eur. J. Pain 2011, 15, 467.e1–467.e14. [Google Scholar] [CrossRef]
- Sasaki, N.; Sekiguchi, M.; Kikuchi, S.I.; Konno, S.I. Anti-nociceptive effect of bovine milk-derived lactoferrin in a rat lumbar disc herniation model. Spine 2010, 35, 1663–1667. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, M.; Egashira, N.; Kawashiri, T.; Yano, T.; Ikesue, H.; Oishi, R. Oxaliplatin-induced neuropathy in the rat: Involvement of oxalate in cold hyperalgesia but not mechanical allodynia. Pain 2009, 147, 165–174. [Google Scholar] [CrossRef]
- Cersosimo, R.J. Oxaliplatin-associated neuropathy: A review. Ann. Pharmacother. 2005, 39, 128–135. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.C.; Lv, Y.W.; Ji, Y.; Yan, X.J.; Xue, J.P. Involvement of the nitric oxide-cyclic GMP-protein kinase G-K+ channel pathway in the antihyperalgesic effects of bovine lactoferrin in a model of neuropathic pain. Brain Res. 2008, 1209, 1–7. [Google Scholar] [CrossRef]
- Fujimura, T.; Iguchi, A.; Sato, A.; Kagaya, S.; Hoshino, T.; Takeuchi, T. The pain-relieving effects of lactoferrin on oxaliplatin-induced neuropathic pain. J. Vet. Med. Sci. 2020, 82, 1648–1654. [Google Scholar] [CrossRef]
- Ma, W.; Bisby, M.A. Increased activation of nuclear factor kappa B in rat lumbar dorsal root ganglion neurons following partial sciatic nerve injuries. Brain Res. 1998, 797, 243–254. [Google Scholar] [CrossRef]
- Chan, C.F.; Sun, W.Z.; Lin, J.K.; Lin-Shiau, S.Y. Activation of transcription factors of nuclear factor kappa B, activator protein-1 and octamer factors in hyperalgesia. Eur. J. Pharmacol. 2000, 402, 61–68. [Google Scholar] [CrossRef]
- Sakaue, G.; Shimaoka, M.; Fukuoka, T.; Hiroi, T.; Inoue, T.; Hashimoto, N.; Sakaguchi, T.; Sawa, Y.; Morishita, R.; Kiyono, H.; et al. NF-κB decoy suppresses cytokine expression and thermal hyperalgesia in a rat neuropathic pain model. Neuroreport 2001, 12, 2079–2084. [Google Scholar] [CrossRef]
- Lee, K.M.; Kang, B.S.; Lee, H.L.; Son, S.J.; Hwang, S.H.; Kim, D.S.; Park, J.S.; Cho, H.J. Spinal NF-kB activation induces COX-2 upregulation and contributes to inflammatory pain hypersensitivity. Eur. J. Neurosci. 2004, 19, 3375–3381. [Google Scholar] [CrossRef] [PubMed]
- Haversen, L.; Ohlsson, B.G.; Hahn-Zoric, M.; Hanson, L.Å.; Mattsby-Baltzer, I. Lactoferrin down-regulates the LPS-induced cytokine production in monocytic cells via NF-κB. Cell. Immunol. 2002, 220, 83–95. [Google Scholar] [CrossRef]
- Inubushi, T.; Kawazoe, A.; Miyauchi, M.; Kudo, Y.; Ao, M.; Ishikado, A.; Makino, T.; Takata, T. Molecular mechanisms of the inhibitory effects of bovine lactoferrin on lipopolysaccharide-mediated osteoclastogenesis. J. Biol. Chem. 2012, 287, 23527–23536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zong, X.; Song, D.; Wang, T.; Xia, X.; Hu, W.; Han, F.; Wang, Y. LFP-20, a porcine lactoferrin peptide, ameliorates LPS-induced inflammation via the MyD88/NF-κB and MyD88/MAPK signaling pathways. Dev. Comp. Immunol. 2015, 52, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Cury, Y.; Picolo, G.; Gutierrez, V.P.; Ferreira, S.H. Pain and analgesia: The dual effect of nitric oxide in the nociceptive system. Nitric Oxide 2011, 25, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Meller, S.T.; Gebhart, G.F. Nitric oxide (NO) and nociceptive processing in the spinal cord. Pain 1993, 52, 127–136. [Google Scholar] [CrossRef]
- Kusuda, R.; Carreira, E.U.; Ulloa, L.; Cunha, F.Q.; Kanashiro, A.; Cunha, T.M. Choline attenuates inflammatory hyperalgesia activating nitric oxide/cGMP/ATP-sensitive potassium channels pathway. Brain Res. 2020, 15, 146567. [Google Scholar] [CrossRef]
- Mata-Bermudez, A.; Izquierdo, T.; de los Monteros-Zuñiga, E.; Coen, A.; Godínez-Chaparro, B. Antiallodynic effect induced by [6]-gingerol in neuropathic rats is mediated by activation of the serotoninergic system and the nitric oxide–cyclic guanosine monophosphate–adenosine triphosphate-sensitive K+ channel pathway. Phyther. Res. 2018, 32, 2520–2530. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez-Ocaña, D.Y.; Ambriz-Tututi, M.; Pérez-Severiano, F.; Granados-Soto, V. Pharmacological evidence for the participation of NO-cyclic GMP-PKG-K+ channel pathway in the antiallodynic action of resveratrol. Pharmacol. Biochem. Behav. 2006, 84, 535–542. [Google Scholar] [CrossRef]
- Duarte, I.D.G.; Ferreira, S.H. The molecular mechanism of central analgesia induced by morphine or carbachol and the L-arginine-nitric oxide-cGMP pathway. Eur. J. Pharmacol. 1992, 221, 171–174. [Google Scholar] [CrossRef]
- Levy, D.; Strassman, A.M. Modulation of dural nociceptor mechanosensitivity by the nitric oxide-cyclic GMP signaling cascade. J. Neurophysiol. 2004, 92, 766–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mercadante, S.; Arcuri, E.; Santoni, A. Opioid-Induced Tolerance and Hyperalgesia. CNS Drugs 2019, 33, 943–955. [Google Scholar] [CrossRef]
- Hong, Y.; Abbott, F.V. Peripheral opioid modulation of pain and inflammation in the formalin test. Eur. J. Pharmacol. 1995, 277, 21–28. [Google Scholar] [CrossRef]
- Mousa, S.A.; Zhang, Q.; Sitte, N.; Ji, R.R.; Stein, C. β-endorphin-containing memory-cells and μ-opioid receptors undergo transport to peripheral inflamed tissue. J. Neuroimmunol. 2001, 115, 71–78. [Google Scholar] [CrossRef]
- Wenk, H.N.; Honda, C.N. Immunohistochemical localization of delta opioid receptors in peripheral tissues. J. Comp. Neurol. 1999, 408, 567–579. [Google Scholar] [CrossRef]
- Ferreira, S.H.; Duarte, I.D.; Lorenzetti, B.B. The molecular mechanism of action of peripheral morphine analgesia: Stimulation of the cGMP system via nitric oxide release. Eur. J. Pharmacol. 1991, 201, 121–122. [Google Scholar] [CrossRef]
- Cunha, T.M.; Souza, G.R.; Domingues, A.C.; Carreira, E.U.; Lotufo, C.M.; Funez, M.I.; Verri, W.A.; Cunha, F.Q.; Ferreira, S.H. Stimulation of Peripheral Kappa Opioid Receptors Inhibits Inflammatory Hyperalgesia via Activation of the PI3Kγ/AKT/nNOS/NO Signaling Pathway. Mol. Pain 2012, 8, 1744–8069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunha, T.M.; Roman-Campos, D.; Lotufo, C.M.; Duarte, H.L.; Souza, G.R.; Verri, W.A.; Funez, M.I.; Dias, Q.M.; Schivo, I.R.; Domingues, A.C.; et al. Morphine peripheral analgesia depends on activation of the PI3K /AKT/nNOS/NO/KATP signaling pathway. Proc. Natl. Acad. Sci. USA 2010, 107, 4442–4447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuchiya, T.; Takeuchi, T.; Hayashida, K.I.; Shimizu, H.; Ando, K.; Harada, E. Milk-derived lactoferrin may block tolerance to morphine analgesia. Brain Res. 2006, 1068, 102–108. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Yang, H.; Liu, L.; Wang, J.; Zheng, N. Lactoferrin Induces the Synthesis of Vitamin B6 and Protects HUVEC Functions by Activating PDXP and the PI3K/AKT/ERK1/2 Pathway. Int. J. Mol. Sci. 2019, 20, 587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.H.; Pyo, C.W.; Hahm, D.H.; Kim, J.; Choi, S.Y. Iron-saturated lactoferrin stimulates cell cycle progression through PI3K/Akt pathway. Mol. Cells 2009, 28, 37–42. [Google Scholar] [CrossRef] [PubMed]



| Nociceptive Pain | ||
|---|---|---|
| Animal Model | Lactoferrin Treatment | Effect |
| Formalin test | bLf (30–300 mg/kg, i.p.) bLf (1 g/kg each day for 4 weeks, i.p.) bLf (100 µg/rat, i.t.) bLf (0.385–3.85 nmol/paw, i.p.) rhLf (1.25 nmol/paw, i.p.) | Anti-nociception in phases 1 and 2 [16,59,62] |
| Hot plate test | bLf (100 mg/kg, i.p.) | Thermal anti-nociception [16] |
| Acetic acid writhing test | bLf (1 or 3 mg/kg, i.p.) | Visceral anti-nociception [16] |
| CFA | bLf (100 mg/kg, p.o.) | Anti-inflammatory and anti-hyperalgesia [60] |
| Neuropathic Pain | ||
|---|---|---|
| Animal Model | Lactoferrin Treatment | Effect |
| CCI | bLf (100 µg/rat, i.t.) | Thermal anti-hyperalgesia [72] |
| bLf (100 and 200 mg/kg/ daily for 15 days, i.p.) | Mechanical and thermal anti-hyperalgesia Tactile anti-allodynia [47] | |
| MNT | bLf (200 µg/rat, i.t.) | Tactile anti-allodynia Mechanical anti-hyperalgesia [29] |
| Herniation | bLf (100 mg/kg, i.p.) | Anti-allodynia [69] |
| Oxaliplatin | rhLf (100 mg/kg, i.p., 3 doses (once per week for 3 weeks)); rhLf-Fc (100 mg/kg, i.p., 3 doses (once per week for 3 weeks); continuous infusion of rhLf or rhLf-IgGFc (10 g/kg/ at days 0, 4, 7, 11, 14, 17, 21, 24 and 28) | Mechanical anti-allodynia [73] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Godínez-Chaparro, B.; Guzmán-Mejía, F.; Drago-Serrano, M.E. Lactoferrin and Its Potential Impact for the Relief of Pain: A Preclinical Approach. Pharmaceuticals 2021, 14, 868. https://doi.org/10.3390/ph14090868
Godínez-Chaparro B, Guzmán-Mejía F, Drago-Serrano ME. Lactoferrin and Its Potential Impact for the Relief of Pain: A Preclinical Approach. Pharmaceuticals. 2021; 14(9):868. https://doi.org/10.3390/ph14090868
Chicago/Turabian StyleGodínez-Chaparro, Beatriz, Fabiola Guzmán-Mejía, and Maria Elisa Drago-Serrano. 2021. "Lactoferrin and Its Potential Impact for the Relief of Pain: A Preclinical Approach" Pharmaceuticals 14, no. 9: 868. https://doi.org/10.3390/ph14090868