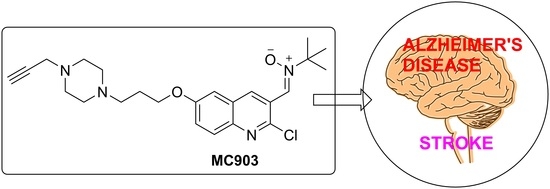
Privileged Quinolylnitrones for the Combined Therapy of Ischemic Stroke and Alzheimer’s Disease
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
2.2. Evaluation of Neuroprotection in an Experimental Model of Ischemia in Primary Neuronal Cultures
2.3. Evaluation of Neuroprotection in an Experimental Model of AD in Neuronal Cell Line
2.4. Virtual Absorption, Distribution, Metabolism, and Excretion (ADME) Properties of Compound MC903
3. Materials and Methods
3.1. Synthesis
3.2. Neuroprotection Analysis in an Ischemia Experimental Model
3.2.1. Primary Neuronal Cultures
3.2.2. Experimental Ischemia in Neuronal Cultures and Treatments
3.2.3. Cell Viability Assay
3.3. Neuroprotection Analysis in AD Experimental Models
3.3.1. SHSY-5Y Cell Culture
3.3.2. Neuroprotection Studies
3.4. Statistical Analysis
3.5. ADME Analysis
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carney, J.M.; Starke-Reed, P.E.; Oliver, C.N.; Landum, R.W.; Cheng, M.S.; Wu, J.F.; Floyd, R.A. Reversal of age-related increase in brain protein oxidation, decrease in enzyme activity, and loss in temporal and spatial memory by chronic administration of the spin-trapping compound N-tert-butyl-alpha-phenylnitrone. Proc. Natl. Acad. Sci. USA 1991, 88, 3633–3636. [Google Scholar] [CrossRef] [Green Version]
- Maples, K.R.; Green, A.R.; Floyd, R.A. Nitrone-Related Therapeutics. CNS Drugs 2004, 18, 1071–1084. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Gao, T.; Luo, Y.; Chen, X.; Gao, G.; Gao, X.; Zhou, Y.; Dai, J. Transient focal cerebral ischemia/reperfusion induces early and chronic axonal changes in rats: Its importance for the risk of Alzheimer’s disease. PLoS ONE 2012, 7, e33722. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.A.; Miller, A.A.; Drummond, G.R.; Amanda, G.; Thrift, A.G.; Arumugam, T.V.; Phan, T.G.; Srikanth, V.K.; Sobey, C.G. Vascular cognitive impairment and Alzheimer’s disease: Role of cerebral hypoperfusion and oxidative stress. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2012, 385, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Zhen, G.; Kim, Y.T.; Li, R.C.; Yocum, J.; Kapoor, N.; Langer, J.; Dobrowolski, P.; Maruyama, T.; Narumiya, S.; Doré, S. PGE2 EP1 receptor exacerbated neurotoxicity in a mouse model of cerebral ischemia and Alzheimer’s disease. Neurobiol. Aging 2012, 33, 2215–2219. [Google Scholar] [CrossRef] [Green Version]
- Macrae, M.I.; Allan, S.M. Stroke: The past, present and future. Brain Neurosci. Adv. 2018, 2, 1–5. [Google Scholar] [CrossRef]
- Chioua, M.; Martìnez-Alonso, E.; Gonzalo-Gobernado, R.; Ayuso, M.I.; Escobar-Peso, A.; Infantes, L.; Hadjipavlou-Litina, D.; Montoya, J.J.; Montaner, J.; Alcázar, A.; et al. New quinolylnitrones for stroke therapy: Antioxidant and neuroprotective (Z)-N-tert-butyl-1-(2-chloro-6-methoxyquinolin-3-yl)methanimine oxide (as a new lead-compound for ischemic stroke treatment. J. Med. Chem. 2019, 62, 2184–2201. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Aguilera, O.M.; Hagenow, S.; Palomino-Antolìn, A.; Farré-Alìns, V.; Ismaili, L.; Joffrin, P.-L.; Jimeno, M.L.; Soukup, O.; Janockova, J.; Kalinowsky, L.; et al. Multitarget-directed ligands combining cholinesterase and monoamine oxidase inhibition with histamine H3R antagonism for neurodegenerative diseases. Angew. Chem. Int. Ed. 2017, 56, 12765–12769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bautista-Aguilera, O.M.; Budni, J.; Mina, F.; Medeiros, E.B.; Deuther-Conrad, W.; Entrena, J.M.; Moraleda, I.; Iriepa, I.; Lòpez-Munoz, F.; Marco-Contelles, J. Contilisant, a tetratarget small molecule for Alzheimer’s disease therapy combining cholinesterase, monoamine oxidase inhibition, and H3R antagonism with S1R agonism profile. J. Med. Chem. 2018, 61, 6937–6943. [Google Scholar] [CrossRef] [PubMed]
- Bolea, I.; Juàrez-Jiménez, J.; de los Rìos, C.; Chioua, M.; Pouplana, R.; Luque, F.J.; Unzeta, M.; Marco-Contelles, J.; Samadi, A. Synthesis, biological evaluation, and molecular modeling of donepezil and n-[(5-(benzyloxy)-1-methyl-1h-indol-2-yl)methyl]-nmethylprop-2-yn-1-amine hybrids as new multipotent cholinesterase/monoamine oxidase inhibitors for the treatment of Alzheimer’s disease. J. Med. Chem. 2011, 54, 8251–8270. [Google Scholar] [CrossRef] [PubMed]
- Brouns, R.; De Deyn, P.P. The complexity of neurobiological processes in acute ischemic stroke. Clin. Neurol. Neurosurg. 2009, 111, 483–495. [Google Scholar] [CrossRef]
- Marco-Contelles, J. A Tau, lobby or religion? ACS Med. Chem. Lett. 2019, 10, 1361–1362. [Google Scholar] [CrossRef] [PubMed]
- Oset-Gasque, M.J.; Marco-Contelles, J. Alzheimer’s disease, the “one-molecule, one-target” paradigm, and the multi-target directed ligand approach. ACS Chem. Neurosci. 2018, 9, 401–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, P.; Zhou, W.; Yue, H.; Zhang, C.; Ou, Y.; Yang, Z.; Hu, W. Compound AD110 acts as therapeutic management for Alzheimer’s disease and stroke in mouse and rat models. ACS Chem. Neurosci. 2020, 11, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Cai, W.; Lang, M.; Yan, R.; Li, Z.; Zhang, G.; Yu, P.; Wang, Y.; Sun, Y.; Zhang, Z. Neuroprotective effects and mechanisms of action of multifunctional agents targeting free radicals, monoamine oxidase B and cholinesterase in Parkinson’s disease model. J. Mol. Neurosci. 2017, 61, 498–510. [Google Scholar] [CrossRef] [PubMed]
- Quevedo, C.; Salinas, M.; Alcázar, A. Initiation factor 2b activity is regulated by protein phosphatase 1, which is activated by the mitogen-activated protein kinase (mapk)-dependent pathway in insulin-like growth factor 1-stimulated neuronal cells. J. Biol. Chem. 2003, 278, 16579–16586. [Google Scholar] [CrossRef] [Green Version]
- Cid, C.; Alcázar, A.; Regidor, I.; Masjuán, J.; Salinas, M.; Álvarez-Cermeño, J. Neuronal apoptosis induced by cerebrospinal fluid from multiple sclerosis patients correlates with hypointense lesions on t1 magnetic resonance imaging. J. Neurol. Sci. 2002, 193, 103–109. [Google Scholar] [CrossRef]
- Chioua, M.; Salgado-Ramos, M.; Diez-Iriepa, D.; Escobar-Peso, A.; Iriepa, I.; Hadjipavlou-Litina, D.; Martínez-Alonso, E.; Alcázar, A.; Marco-Contelles, J. Novel quinolylnitrones combining neuroprotective and antioxidant properties. ACS Chem. Neurosci. 2019, 10, 2703–2706. [Google Scholar] [CrossRef] [PubMed]
- Malek, R.; Arribas, R.L.; Palomino-Antolín, A.; Totoson, P.; Demougeot, C.; Kobrlova, T.; Soukup, O.; Iriepa, I.; Moraleda, I.; Diez-Iriepa, D.; et al. New dual small molecules for alzheimer’s disease therapy combining histamine h 3 receptor (h3r) antagonism and calcium channels blockade with additional cholinesterase inhibition. J. Med. Chem. 2019, 26, 11416–11422. [Google Scholar] [CrossRef]
- Buendía, I.; Egea, J.; Parada, E.; Navarro, E.; León, R.; Rodríguez-Franco, M.I.; López, M.G. The melatonin-N,N-dibenzyl(N-methyl)amine hybrid ITH91/IQM157 affords neuroprotection in an in vitro Alzheimer’s model via hemo-oxygenase-1 induction. ACS Chem. Neurosci. 2015, 18, 288–296. [Google Scholar] [CrossRef] [Green Version]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Duffy, E.M.; Jorgensen, W.L. Prediction of properties from simulations: Free energies of solvation in hexadecane, octanol, and wáter. J. Am. Chem. Soc. 2000, 122, 2878–2888. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Duffy, E.M. Prediction of drug solubility from monte carlo simulations. Bioorg. Med. Chem. Lett. 2000, 10, 1155–1158. [Google Scholar] [CrossRef]


| QN | Concentration (μM) | Neuroprotection (%) |
|---|---|---|
| NXY-059 | 100 | 42.11 ± 2.27 |
| 250 | 51.41 ± 2.13 | |
| 500 | 45.81 ± 1.56 | |
| QN23 | 10 | 49.16 ± 0.90 |
| 100 | 78.11 ± 1.18 ** | |
| 250 | 60.80 ± 1.29 ** | |
| 500 | 17.86 ± 0.78 | |
| JMA101A | 1 | 35.98 ± 0.64 |
| 10 | 54.19 ± 1.24 | |
| 100 | 57.23 ± 0.46 * | |
| 250 | 9.22 ± 0.32 | |
| JMA12A | 1 | 10.32 ± 0.27 |
| 10 | 38.89 ± 1.51 | |
| 100 | 31.79 ± 0.80 | |
| 250 | <0 | |
| JMA98C | 0.1 | 42.81 ± 1.02 |
| 1 | 46.57 ± 0.60 | |
| 10 | 63.82 ± 0.81 ** | |
| 100 | <0 | |
| MC902 | 0.1 | 24.99 ± 0.51 |
| 1 | 30.34 ± 0.59 | |
| 10 | 45.56 ± 0.54 | |
| 100 | 46.97 ± 0.34 | |
| MC903 | 1 | 23.40 ± 0.54 |
| 10 | 72.45 ± 1.64 ** | |
| 100 | 72.67 ± 1.34 ** | |
| 250 | 13.75 ± 0.46 | |
| JMA11A | 0.1 | 28.66 ± 0.36 |
| 1 | 30.12 ± 0.51 | |
| 10 | 44.01 ± 0.71 | |
| 100 | <0 | |
| DDI88 | 0.1 | 31.67 ± 0.44 |
| 1 | 40.91 ± 0.87 | |
| 10 | 39.54 ± 0.54 | |
| 100 | 48.39 ± 1.70 | |
| DDI89 | 1 | 13.49 ± 0.66 |
| 10 | 39.63 ± 1.06 | |
| 100 | 44.49 ± 2.25 | |
| 250 | <0 | |
| Contilisant | 0.1 | 5.81 ± 0.10 |
| 1 | 41.27 ± 1.35 | |
| 10 | 34.62 ± 0.46 | |
| 20 | 47.56 ± 0.36 | |
| 50 | 50.24 ± 1.11 | |
| 100 | 15.22 ± 0.43 |
| QN (μM) | R/O | OA | Aβ25–35 | |
|---|---|---|---|---|
| JMA98C | 0.3 | 26.0 ± 24.5 | 37.5 ± 9.5 | 14.5 ± 7.9 |
| 1 | 40.6 ± 1.5 * | 52.9 ± 8.8 * | 14.2 ± 13.7 | |
| 3 | 70.5 ± 9.7 *** | 78.3 ± 16.2 *** | 27.8 ± 9.6 | |
| 10 | 57.2 ± 16.3 ** | 20.8 ± 12.8 | 3.3 ± 15.2 | |
| JMA101A | 0.3 | 5.7 ± 27.3 | 35.3 ± 14.4 | n.d. |
| 1 | 26.9 ± 28.3 | 37.0 ± 21.1 | n.d. | |
| 3 | 22.0 ± 29.2 | 38.3 ± 4.9 | n.d. | |
| 10 | 24.9 ± 29.1 | 17.5 ± 23.0 | n.d. | |
| JMA11A | 0.3 | 52.8 ± 37.9 | 21.1 ± 12.8 | n.d. |
| 1 | 16.7 ± 29.2 | 29.7 ±10.7 | n.d. | |
| 3 | 37.9 ± 8.8 | 8.9 ±20.0 | n.d. | |
| 10 | 31.5 ± 30.1 | 21.5 ± 17.4 | n.d. | |
| JMA12A | 0.3 | 11.1 ± 30.0 | 28.8 ± 26.4 | n.d. |
| 1 | 39.23± 17.7 | 20.8 ±13.0 | n.d. | |
| 3 | 11.0± 30.3 | n.p. | n.d. | |
| 10 | 36.0 ± 21.2 | 22.0 ± 23.2 | n.d. | |
| MC902 | 0.3 | 10.3 ± 13.3 | n.p. | n.d. |
| 1 | 32.3 ± 20.3 | n.p. | n.d. | |
| 3 | n.p. | n.p. | n.d. | |
| 10 | n.p. | n.p. | n.d. | |
| MC903 | 0.3 | 44.1 ± 9.2 *** | 33.3 ± 10.4 | 29.7 ± 3.6 * |
| 1 | 40.4 ± 10.8 *** | 46.7 ± 13.4 * | 28.9 ± 6.8 * | |
| 3 | 74.9 ± 5.3 *** | 56.2 ± 11.1 ** | 47.9 ± 8.9 *** | |
| 10 | 14.4 ± 7.0 | n.p. | 27.7 ± 8.0 * | |
| DDI88 | 0.3 | n.p. | n.p. | n.d. |
| 1 | n.p. | n.p. | n.d. | |
| 3 | n.p. | n.p. | n.d. | |
| 10 | n.p. | n.p. | n.d. | |
| DDI89 | 0.3 | n.p. | n.p. | n.d. |
| 1 | n.p. | n.p. | n.d. | |
| 3 | n.p. | n.p. | n.d. | |
| 10 | n.p. | 37.2 ± 27.2 | n.d. | |
| Melatonin | 10 nM | 53.7 ± 10.6 ** | 53.9 ± 9.5 ** | 65.3 ± 4.3 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alonso, J.M.; Escobar-Peso, A.; Palomino-Antolín, A.; Diez-Iriepa, D.; Chioua, M.; Martínez-Alonso, E.; Iriepa, I.; Egea, J.; Alcázar, A.; Marco-Contelles, J. Privileged Quinolylnitrones for the Combined Therapy of Ischemic Stroke and Alzheimer’s Disease. Pharmaceuticals 2021, 14, 861. https://doi.org/10.3390/ph14090861
Alonso JM, Escobar-Peso A, Palomino-Antolín A, Diez-Iriepa D, Chioua M, Martínez-Alonso E, Iriepa I, Egea J, Alcázar A, Marco-Contelles J. Privileged Quinolylnitrones for the Combined Therapy of Ischemic Stroke and Alzheimer’s Disease. Pharmaceuticals. 2021; 14(9):861. https://doi.org/10.3390/ph14090861
Chicago/Turabian StyleAlonso, José M., Alejandro Escobar-Peso, Alejandra Palomino-Antolín, Daniel Diez-Iriepa, Mourad Chioua, Emma Martínez-Alonso, Isabel Iriepa, Javier Egea, Alberto Alcázar, and José Marco-Contelles. 2021. "Privileged Quinolylnitrones for the Combined Therapy of Ischemic Stroke and Alzheimer’s Disease" Pharmaceuticals 14, no. 9: 861. https://doi.org/10.3390/ph14090861