Protective Effect of the Total Saponins from Rosa laevigata Michx Fruit against Carbon Tetrachloride-Induced Liver Fibrosis in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of the Crude Extract
2.3. Chemical Determination
2.4. Animals and Experimental Design
2.5. Pathological Examination
2.6. Transmission Electron Microscopy (TEM)
2.7. Biochemical Analysis
2.8. Immunofluorescence and Immunohistochemical Assays for Alpha-Smooth Muscle Actin (α-SMA) and Transforming Growth Factor-β1 (TGF-β1)
2.9. Quantitative Real-Time PCR Assay
| Gene | Full Name of the Gene | Sequence (5′→3′) a | GenBank b |
|---|---|---|---|
| GAPDH | Glyceraldehyde 3-phosphate | GGCACAGTCAAGGCTGAGAATG | NM_017008.3 |
| dehydrogenase | ATGGTGGTGAAGACGCCAGTA | ||
| Col 1A1 | Collagen I | GACATGTTCAGCTTTGTGGACCC | NM_053304 |
| AGGGACCCTTAGGCCATTGTGTA | |||
| Col 3A1 | Collagen III | TTTGGCACAGCAGTCCAATGTA | NM_032085 |
| GACAGATCCCGAGTCGCAGA | |||
| TNF-α | Tumor necrosis factor α | TCAGTTCCATGGCCCAGAC | NM_012675.3 |
| GTTGTCTTTGAGATCCATGCCATT | |||
| IL-1β | Interleukin 1β | CCCTGAACTCAACTGTGAAATAGCA | NM_031512.2 |
| CCCAAGTCAAGGGCTTGGAA | |||
| IL-6 | Interleukin 6 | ATTGTATGAACAGCGATGATGCAC | NM_012589.1 |
| CCAGGTAGAAACGGAACTCCAGA |
2.10. Western Blotting Assay
| Antibody | Full Name | Source | Dilution | Company |
|---|---|---|---|---|
| Fibronectin | Fibronectin | rabbit | 1:500 | Proteintech Group, Chicago, IL, USA |
| Col 1A1 | Collagen I | rabbit | 1:500 | Bioworld Technology, St. Louis Park, MN, USA |
| Col 3A1 | Collagen III | rabbit | 1:500 | Bioworld Technology, St. Louis Park, MN, USA |
| MMP-2 | Matrix metalloproteinase 2 | rabbit | 1:500 | Proteintech Group, Chicago, IL, USA |
| MMP-9 | Matrix metalloproteinase 9 | rabbit | 1:500 | Proteintech Group, Chicago, IL, USA |
| TIMP1 | Tissue inhibitors of metalloproteinases 1 | rabbit | 1:1000 | Proteintech Group, Chicago, IL, USA |
| p-ERK | Phosphorylated-Extracellular regulated kinase | rabbit | 1:500 | Bioworld Technology, St. Louis Park, MN, USA |
| ERK | Extracellular regulated kinase | rabbit | 1:500 | Bioworld Technology, St. Louis Park, MN, USA |
| p-p38 | Phosphorylated-p38 mitogen-activated protein kinase | rabbit | 1:500 | Bioworld Technology, St. Louis Park, MN, USA |
| p38 | p38 mitogen-activated protein kinase | rabbit | 1:500 | Bioworld Technology, St. Louis Park, MN, USA |
| p-JNK | Phosphorylated-c-Jun N-terminal kinase | rabbit | 1:500 | Bioworld Technology, St. Louis Park, MN, USA |
| JNK | c-Jun N-terminal kinase | rabbit | 1:500 | Bioworld Technology, St. Louis Park, MN, USA |
| HO-1 | Heme oxygenase-1 | rabbit | 1:1000 | Bioworld Technology, St. Louis Park, MN, USA |
| SOD2 | Superoxide dismutase 2 | rabbit | 1:1000 | Proteintech Group, Chicago, IL, USA |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 | rabbit | 1:1000 | Bioworld Technology, St. Louis Park, MN, USA |
| CYP2E1 | Cytochrome P450 2E1 | rabbit | 1:1000 | Proteintech Group, Chicago, IL, USA |
| p-Smad2/3 | Phosphorylated-Smad 2/3 | rabbit | 1:1000 | Bioworld Technology, St. Louis Park, MN, USA |
| Smad2/3 | Smad 2/3 | rabbit | 1:1000 | Proteintech Group, Chicago, IL, USA |
| Smad7 | Smad 7 | rabbit | 1:1000 | Abcam, Cambridge, MA, USA |
| PDGF-β | Platelet derived growth factor-β | rabbit | 1:1000 | Proteintech Group, Chicago, IL, USA |
| p-Akt | Phosphorylated-amino kinase terminal | rabbit | 1:500 | Proteintech Group, Chicago, IL, USA |
| Akt | Amino kinase terminal | rabbit | 1:500 | Proteintech Group, Chicago, IL, USA |
| p-p70S6K | Phosphorylated-70-kDa ribosomal S6 Kinase | rabbit | 1:1000 | Santa Cruz Biotechnology, Santa Cruz, CA, USA |
| p70S6K | 70-kDa ribosomal S6 Kinase | rabbit | 1:1000 | Santa Cruz Biotechnology, Santa Cruz, CA, USA |
| TLR4 | Toll-like receptor 4 | rabbit | 1:500 | Proteintech Group, Chicago, IL, USA |
| MyD88 | Myeloid differentiation factor 88 | rabbit | 1:1000 | Santa Cruz Biotechnology, Santa Cruz, CA, USA |
| NF-κB | Nuclear factor kappa B | rabbit | 1:1000 | Proteintech Group, Chicago, IL, USA |
| iNOS | Inducible nitric oxide synthase | rabbit | 1:1000 | Proteintech Group, Chicago, IL, USA |
| COX-2 | Cyclooxygenase-2 | rabbit | 1:1000 | Proteintech Group, Chicago, IL, USA |
| IL-10 | Interleukin-10 | rabbit | 1:400 | Boster Biological Technology, Wuhan, China |
| TNF-α | Tumor necrosis factor α | rabbit | 1:400 | Proteintech Group, Chicago, IL, USA |
| IL-1β | Interleukin-1β | rabbit | 1:500 | Bioworld Technology, St. Louis Park, MN, USA |
| IL-6 | Interleukin-6 | rabbit | 1:500 | Bioworld Technology, St. Louis Park, MN, USA |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase | rabbit | 1:1000 | Proteintech Group, Chicago, IL, USA |
2.11. Statistical Analysis
3. Results
3.1. Contents of the Chemicals in the Extract
3.2. RLTS Improved Liver Injury Caused by CCl4

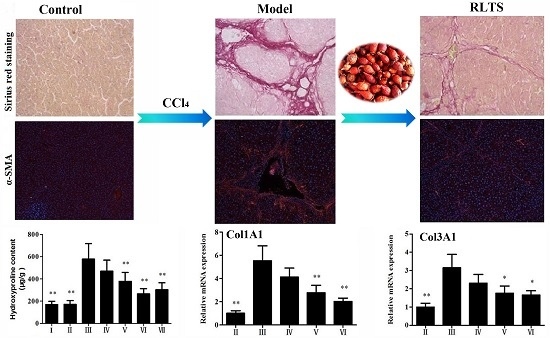
3.3. Effects of RLTS on Liver Fibrosis Caused by CCl4 in Rats


3.4. RLTS Decreased the Levels of Pro-Fibrotic Factors Related to ECM
3.5. Effect of RLTS on MAPK Signal Pathway

3.6. RLTS Reduced Lipid Peroxidation and Oxidative Stress

3.7. RLTS Decreased Activation, Proliferation and Migration of HSCs

3.8. Effects of RLTS on the Protein Expressions Related to Inflammatory Mediators

4. Discussion
5. Conclusions

Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shaker, M.E.; Zalata, K.R.; Mehal, W.Z.; Shiha, G.E.; Ibrahim, T.M. Comparison of imatinib, nilotinib and silymarin in the treatment of carbon tetrachloride-induced hepatic oxidative stress, injury and fibrosis. Toxicol. Appl. Pharmacol. 2011, 252, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Tipoe, G.L.; Leung, T.M.; Liong, E.C.; Lau, T.Y.; Fung, M.L.; Nanji, A.A. Epigallocatechin-3-gallate (EGCG) reduces liver inflammation, oxidative stress and fibrosis in carbon tetrachloride (CCl4)-induced liver injury in mice. Toxicology 2010, 273, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Mohamed Suhaimi, N.A.; Zhuo, L. Imidazolium salt attenuates thioacetamide-induced liver fibrosis in mice by modulating inflammation and oxidative stress. Dig. Liver Dis. 2012, 44, 665–673. [Google Scholar] [CrossRef]
- Mannaerts, I.; Nuytten, N.R.; Rogiers, V.; Vanderkerken, K.; van Grunsven, L.A.; Geerts, A. Chronic administration of valproic acid inhibits activation of mouse hepatic stellate cells in vitro and in vivo. Hepatology 2010, 51, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Van Hul, N.K.; Abarca-Quinones, J.; Sempoux, C.; Horsmans, Y.; Leclercq, I.A. Relation between liver progenitor cell expansion and extracellular matrix deposition in a CDE-induced murine model of chronic liver injury. Hepatology 2009, 49, 1625–1635. [Google Scholar] [CrossRef]
- Rashed, K.; Potočnjak, I.; Giacometti, J.; Škoda, M.; Domitrović, R. Terminalia bellericaaerial parts ethyl acetate extract exhibits antioxidant, anti-inflammatory and antifibrotic activity in carbon tetrachloride-intoxicated mice. J. Funct. Foods 2014, 8, 319–330. [Google Scholar] [CrossRef]
- Weerawatanakorn, M.; Hsieh, S.C.; Tsai, M.L.; Lai, C.S.; Wu, L.M.; Badmaev, V.; Ho, C.T.; Pan, M.H. Inhibitory effect of tetrahydrocurcumin on dimethylnitrosamine-induced liver fibrosis in rats. J. Funct. Foods 2014, 7, 305–317. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Lin, J.T.; Liu, S.C.; Hu, C.C.; Shyu, Y.S.; Yang, D.J. Protective role of litchi (Litchi chinensis Sonn.) flower extract against cadmium- and lead-induced cytotoxicity and transforming growth factor β1-stimulated expression of smooth muscle α-actin estimated with rat liver cell lines. J. Funct. Foods 2013, 5, 698–705. [Google Scholar] [CrossRef]
- Takahashi, Y.; Soejima, Y.; Kumagai, A.; Watanabe, M.; Uozaki, H.; Fukusato, T. Inhibitory effects of Japanese herbal medicines sho-saiko-to and juzen-taiho-to on nonalcoholic steatohepatitis in mice. PLoS ONE 2014, 9, e87279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Shi, M.Q.; He, H.B.; Bai, C.H.; Wang, J.Z.; Cheng, F.; Liu, G.Y.; Deng, W.; Zou, K.; Chen, Z.F. Hepatoprotective effects of saponins from Rhizoma panacis Majorison hepatic fibrosis induced by carbon tetrachloride in rats. Appl. Mech. Mater. 2014, 568–570, 1915–1920. [Google Scholar]
- Peng, X.D.; Dai, L.L.; Huang, C.Q.; He, C.M.; Yang, B.; Chen, L.J. Relationship between anti-fibrotic effect of Panax notoginseng saponins and serum cytokines in rat hepatic fibrosis. Biochem. Biophys. Res. Commun. 2009, 388, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.Y.; Nie, L.W.; Wu, B.J.; Yang, Y.; Zhao, S.S.; Jin, T. Hypolipedemic activity of the polysaccharose from Rosa laevigata Michx fruit. Chin. J. Public Health 2004, 20, 829–830. [Google Scholar]
- Gao, P.Y.; Li, L.Z.; Peng, Y.; Piao, S.J.; Zeng, N.; Lin, H.W.; Song, S.J. Triterpenes from fruits of Rosa Laevigata. Biochem. Syst. Ecol. 2010, 38, 457–459. [Google Scholar] [CrossRef]
- Zhao, Y.T.; Guo, X.M.; Li, F.Z. Antioxidative activity of polysaccharide from Rosa laevigata Mickx. J. Biol. 2003, 20, 23–24. [Google Scholar]
- Dong, D.S.; Zhang, S.; Yin, L.H.; Tang, X.Q.; Xu, Y.W.; Han, X.; Qi, Y.; Peng, J.Y. Protective effects of the total saponins from Rosa laevigata Michx fruit against carbon tetrachloride-induced acute liver injury in mice. Food Chem. Toxicol. 2013, 62, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.S.; Xu, L.N.; Han, X.; Qi, Y.; Xu, Y.W.; Yin, L.H.; Liu, K.X.; Peng, J.Y. Effects of the total saponins from Rosa laevigata Michx fruit against acetaminophen-induced liver damage in mice via induction of autophagy and suppression of inflammation and apoptosis. Molecules 2014, 19, 7189–7206. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.S.; Qi, Y.; Xu, L.N.; Yin, L.H.; Xu, Y.W.; Han, X.; Zhao, Y.Y.; Peng, J.Y. Total saponins from Rosa laevigata Michx fruit attenuates hepatic steatosis induced by high-fat diet in rats. Food Funct. 2014, 5, 3065–3075. [Google Scholar] [CrossRef] [PubMed]
- Li, K.P.; Pan, T.L.; Bi, Y.G.; Lin, R.Q. Study on quantitative analysis and extraction of total saponins from Schefflera arboricola Hayata. Chem. Ind. For. Prod. 2008, 28, 99–102. [Google Scholar]
- China Pharmacopoeia Committee. Pharmacopoeia of the People’s Republic of China, the First Division of 2010 Edition; China Chemical Industry Press: Beijing, China, 2010. [Google Scholar]
- Zhang, S.; Zheng, L.L.; Dong, D.S.; Xu, L.N.; Yin, L.H.; Qi, Y.; Han, X.; Lin, Y.; Liu, K.X.; Peng, J.Y. Effects of flavonoids from Rosa laevigata Michx fruit against high-fat diet-induced non-alcoholic fatty liver disease in rats. Food Chem. 2013, 141, 2108–2116. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Diao, Y.; Zhang, H.; Wang, S.; Zhang, Z.; Yu, B.; Huang, S.; Yang, H. Tannin extracts from immature fruits of Terminalia chebula Fructus Retz. Promote cutaneous wound healing in rats. BMC Complement. Altern. Med. 2011, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.N.; Ji, L.; Zhang, S.; Xu, L.N.; Yin, L.H.; Li, L.; Zhao, Y.Y.; Peng, J.Y. Total flavonoids from Rosa Laevigata Michx fruit attenuates hydrogen peroxide induced injury in human umbilical vein endothelial cells. Food Chem. Toxicol. 2012, 50, 3133–3141. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.T.; Zheng, L.L.; Xu, L.N.; Yin, L.H.; Qi, Y.; Xu, Y.W.; Han, X.; Peng, J.Y. Protective effects of dioscin against alcohol-induced liver injury. Arch. Toxicol. 2014, 88, 739–753. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Zhang, G.L.; Cheng, D.L.; Wu, F.E. The chemical constitute from Rosa laevigata Michx. Nat. Prod. Res. Dev. 2000, 13, 21–23. [Google Scholar]
- Wang, J.H.; Choi, M.K.; Shin, J.W.; Hwang, S.Y.; Son, C.G. Antifibrotic effects of Artemisia capillaris and Artemisia iwayomogi in a carbon tetrachloride-induced chronic hepatic fibrosis animal model. J. Ethnopharmacol. 2012, 140, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Leeming, D.J.; Karsdal, M.A.; Byrjalsen, I.; Bendtsen, F.; Trebicka, J.; Nielsen, M.J.; Christiansen, C.; Møller, S.; Krag, A. Novel serological neo-epitope markers of extracellular matrix proteins for the detection of portal hypertension. Aliment. Pharmacol. Ther. 2013, 38, 1086–1096. [Google Scholar] [PubMed]
- Sun, K.; Tordjman, J.; Clément, K.; Scherer, P.E. Fibrosis and adipose tissue dysfunction. Cell Metab. 2013, 18, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Pellicoro, A.; Ramachandran, P.; Iredale, J.P. Reversibility of liver fibrosis. Fibrogenesis Tissue Repair 2012, 5 (Suppl. 1). [Google Scholar] [CrossRef]
- Recknagel, R.O.; Glende, E.A., Jr.; Dolak, J.A.; Waller, R.L. Mechanisms of carbon tetrachloride toxicity. Pharmacol. Ther. 1989, 43, 139–154. [Google Scholar] [CrossRef]
- Tsukamoto, H.; Matsuoka, M.; French, S.W. Experimental models of hepatic fibrosis: A review. Semin. Liver Dis. 1990, 10, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Kong, B.H.; Li, G.X.; Liu, N.; Xia, X.F. Hepatoprotective and antioxidant effects of porcine plasma protein hydrolysates on carbon tetrachloride-induced liver damage in rats. Food Chem. Toxicol. 2011, 49, 1316–1321. [Google Scholar] [CrossRef] [PubMed]
- Bataller, R.; Brenner, D.A. Liver fibrosis. J. Clin. Investig. 2005, 115, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.F.; Huang, R.B.; Zhang, S.J.; Lin, J.; Wei, L.; He, M.; Zhuo, L.; Lin, X. Protective effect of genistein isolated from Hydrocotyle sibthorpioides on hepatic injury and fibrosis induced by chronic alcohol in rats. Toxicol. Lett. 2013, 217, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Tsukada, S.; Parsons, C.J.; Rippe, R.A. Mechanisms of liver fibrosis. Clin. Chim. Acta 2006, 364, 33–60. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Y.; Li, C.Z.; Wu, Y.T.; Lan, X.L.; Cao, L.; Hong, L.C.; Wang, H.Y.; Wu, Y.G.; Wang, F.; Zhang, Y.Z. The expression of TGF-β1, Smad3, phospho-Smad3 and Smad7 is correlated with the development and invasion of nonfunctioning pituitary adenomas. J. Transl. Med. 2014, 12. [Google Scholar] [CrossRef]
- Jiang, H.Q.; Zhang, X.L.; Liu, L.; Yang, C.C. Relationship between focal adhesion kinase and hepatic stellate cell proliferation during rat hepatic fibrogenesis. World J. Gastroenterol. 2004, 10, 3001–3005. [Google Scholar] [PubMed]
- Robinson, M.J.; Cobb, M.H. Mitogen-activated protein kinase pathways. Curr. Opin. Cell Biol. 1997, 9, 180–186. [Google Scholar] [CrossRef]
- Johnson, G.L.; Lapadat, R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 2002, 298, 1911–1912. [Google Scholar] [CrossRef] [PubMed]
- Szuster-Ciesielska, A.; Plewka, K.; Daniluk, J.; Kandefer-Szerszeń, M. Betulin and betulinic acid attenuate ethanol-induced liver stellate cell activation by inhibiting reactive oxygen species (ROS), cytokine (TNF-α, TGF-β) production and by influencing intracellular signaling. Toxicology 2011, 280, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Henderson, N.C.; Iredale, J.P. Liver fibrosis: Cellular mechanisms of progression and resolution. Clin. Sci. 2007, 112, 265–280. [Google Scholar] [PubMed]
- Wiest, R.; Garcia-Tsao, G. Bacterial translocation (BT) in cirrhosis. Hepatology 2005, 41, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, S.; Nobili, V.; Alisi, A. Toll-like receptor-mediated signaling cascade as a regulator of the inflammation network during alcoholic liver disease. World J. Gastroenterol. 2014, 20, 16443–16451. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.P.; Xie, D.Y.; Liang, X.J.; Peng, L.; Zhang, G.L.; Ye, Y.N.; Xie, C.; Gao, Z.L. HGF and direct mesenchymal stem cells contact synergize to inhibit hepatic stellate cells activation through TLR4/NF-κB pathway. PLoS ONE 2012, 7, e43408. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Loke, J.; Zheng, F.; Hong, F.; Fukata, M.; Tarocchi, M.; Abar, O.T.; Huang, H.; Sninsky, J.J.; Yea, S.; Friedman, S.L. Functional linkage of cirrhosis-predictive single nucleotide polymorphisms of Toll-like receptor 4 to hepatic stellate cell responses. Hepatology 2009, 49, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Adachi, O.; Ogawa, T.; Takeda, K.; Akira, S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity 1999, 11, 115–122. [Google Scholar] [CrossRef]
- Paik, Y.H.; Schwabe, R.F.; Bataller, R.; Russo, M.P.; Jobin, C.; Brenner, D.A. Toll-like receptor 4 mediates inflammatory signaling by bacterial lipopolysaccharide in human hepatic stellate cells. Hepatology 2003, 37, 1043–1055. [Google Scholar] [CrossRef] [PubMed]
- Taki-Eldin, A.; Zhou, L.; Xie, H.Y.; Chen, K.J.; Yong He, D.Y.; Zheng, S.S. Triiodothyronine attenuates hepatic ischemia/reperfusion injury in a partial heaptectomy model through inhibition of proinflammatory cytokines, transcription factors, and adhesion molecules. J. Surg. Res. 2012, 178, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, A.; Yokoyama, Y.; Umemoto, M.; Futagami, M.; Sakamoto, T.; Bing, X.; Mizunuma, H. Clinical implication of expression of cyclooxygenase-2 and peroxisome proliferator activated-receptor gamma in epithelial ovarian tumours. Br. J. Cancer 2004, 91, 633–638. [Google Scholar] [PubMed]
- Xu, W.; Liu, L.Z.; Loizidou, M.; Ahmed, M.; Charles, I.G. The role of nitric oxide in cancer. Cell Res. 2002, 12, 311–320. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, D.; Yin, L.; Qi, Y.; Xu, L.; Peng, J. Protective Effect of the Total Saponins from Rosa laevigata Michx Fruit against Carbon Tetrachloride-Induced Liver Fibrosis in Rats. Nutrients 2015, 7, 4829-4850. https://doi.org/10.3390/nu7064829
Dong D, Yin L, Qi Y, Xu L, Peng J. Protective Effect of the Total Saponins from Rosa laevigata Michx Fruit against Carbon Tetrachloride-Induced Liver Fibrosis in Rats. Nutrients. 2015; 7(6):4829-4850. https://doi.org/10.3390/nu7064829
Chicago/Turabian StyleDong, Deshi, Lianhong Yin, Yan Qi, Lina Xu, and Jinyong Peng. 2015. "Protective Effect of the Total Saponins from Rosa laevigata Michx Fruit against Carbon Tetrachloride-Induced Liver Fibrosis in Rats" Nutrients 7, no. 6: 4829-4850. https://doi.org/10.3390/nu7064829