Flavonoids: Antioxidants Against Atherosclerosis
Abstract
:1. Introduction
2. Oxidative Stress and Endothelial Dysfunction
3. Dietary Strategies Against Oxidative Stress
4. Polyphenols: Structure and Classification

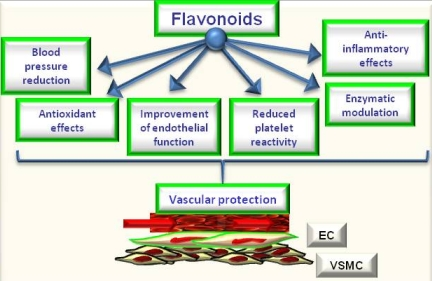
5. Flavonoids, Oxidative Stress and Endothelial Effects

6. Conclusions
References
- Grassi, D.; Desideri, G.; Tiberti, S.; Ferri, C. Oxidative stress, endothelial dysfunction and prevention of cardiovascular diseases. AgroFOOD Industry Ii-tech. 2009, 20, 76–79. [Google Scholar]
- Stocker, R.; Keaney, J.F., Jr. Role of oxidative modifications in atherosclerosis. Physiol. Rev. 2004, 84, 1381–1478. [Google Scholar]
- Madamanchi, N.R.; Vendrov, A.; Runge, M.S. Oxidative stress and vascular disease. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 29–38. [Google Scholar]
- Grassi, D.; Desideri, G.; Croce, G.; Tiberti, S.; Aggio, A.; Ferri, C. Flavonoids, vascular function and cardiovascular protection. Curr. Pharm. Des. 2009, 15, 1072–1084. [Google Scholar]
- Grassi, D.; Aggio, A.; Onori, L.; Croce, G.; Tiberti, S.; Ferri, C.; Ferri, L.; Desideri, G. Tea, flavonoids and NO-mediated vascular reactivity. J. Nutr. 2008, 138, 1554S–1560S. [Google Scholar]
- Ferri, C.; Grassi, D. Antioxidants and beneficial microvascular effects. is this the remedy? Hypertension 2010, 55, 1310–1311. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar]
- Sies, H.; Stahl, W.; Sevanian, A. Nutritional, dietary and postprandial oxidative stress. J. Nutr. 2005, 135, 969–972. [Google Scholar]
- Keaney, J.F., Jr. Oxidative stress and the vascular wall: NADPH oxidases take center stage. Circulation. 2005, 112, 2585–2588. [Google Scholar]
- Higashi, Y.; Noma, K.; Yoshizumi, M.; Kihara, Y. Endothelial Function and Oxidative Stress in Cardiovascular Diseases. Circ. J. 2009, 73, 411–418. [Google Scholar]
- Bonetti, P.O.; Lerman, L.O.; Lerman, A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 168–175. [Google Scholar]
- Desideri, G.; Ferri, C. Endothelial activation. Sliding door to atherosclerosis. Curr. Pharm. Des. 2005, 11, 2163–2175. [Google Scholar] [CrossRef] [PubMed]
- Forstermann, U.; Munzel, T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation 2006, 113, 1708–1714. [Google Scholar] [CrossRef] [PubMed]
- Landmesser, U.; Dikalov, S.; Price, S.R.; McCann, L.; Fukai, T.; Holland, S.M.; Mitch, W.E.; Harrison, D.G. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J. Clin. Invest. 2003, 111, 1201–1209. [Google Scholar]
- Okumura, K.; Yasue, H.; Matsuyama, K.; Ogawa, H.; Morikami, Y.; Obata, K.; Sakaino, N. Effect of acetylcholine on the highly stenotic coronary artery: difference between the constrictor response of the infarct-related coronary artery and that of the noninfarct-related artery. J. Am. Coll. Cardiol. 1992, 19, 752–758. [Google Scholar]
- van Boven, A.J.; Jukema, J.W.; Zwinderman, A.H.; Crijns, H.J.; Lie, K.I.; Bruschke, A.V. Reduction of transient myocardial ischemia with pravastatin in addition to the conventional treatment in patients with angina pectoris. REGRESS Study Group. Circulation 1996, 94, 1503–1505. [Google Scholar] [PubMed]
- John, E.; Deanfield, J.; Halcox, P.; Rabelink, T.J. Endothelial function and dysfunction: testing and clinical relevance. Circulation 2007, 115, 1285–1295. [Google Scholar]
- Crisby, M.; Nordin-Fredriksson, G.; Shah, P.K.; Yano, J.; Zhu, J.; Nilsson, J. Pravastatin treatment increases collagen content and decreases lipid content, inflammation, metalloproteinases, and cell death in human carotid plaques: implications for plaque stabilization. Circulation 2001, 103, 926–933. [Google Scholar] [PubMed]
- Libby, P. Molecular bases of the acute coronary syndromes. Circulation 1995, 91, 2844–2850. [Google Scholar]
- Halcox, J.P.; Schenke, W.H.; Zalos, G.; Mincemoyer, R.; Prasad, A.; Waclawiw, M.A.; Nour, K.R.; Quyyumi, A.A. Prognostic value of coronary vascular endothelial dysfunction. Circulation 2002, 106, 653–658. [Google Scholar]
- Schachinger, V.; Britten, M.B.; Zeiher, A.M. Prognostic impact of coronary vasodilator dysfunction and adverse long-term outcome of coronary heart disease. Circulation 2000, 101, 1899–1906. [Google Scholar]
- Heitzer, T.; Schlinzig, T.; Krohn, K.; Meinertz, T.; Münzel, T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation 2001, 104, 2673–2678. [Google Scholar] [CrossRef] [PubMed]
- Vogel, R.A.; Corretti, M.C.; Plotnick, G.D. The postprandial effect of components of the Mediterranean diet on endothelial function. JACC 2000, 36, 1455–1460. [Google Scholar]
- Rimm, E.B.; Ascherio, A.; Giovannucci, E.; Spiegelman, D.; Stampfer, M.J.; Willett, W.C. Vegetable, fruit, and cereal fiber intake and risk of coronary heart disease among men. JAMA 1996, 275, 447–451. [Google Scholar] [PubMed]
- Hertog, M.G.; Kromhout, D.; Aravanis, C.; Blackburn, H.; Buzina, R.; Fidanza, F.; Giampaoli, S.; Jansen, A.; Menotti, A.; Nedeljkovic, S.; et al. Flavonoid intake and long-term risk of coronary heart disease and cancer in the seven countries study. Arch. Intern. Med. 1995, 155, 381–386. [Google Scholar] [PubMed]
- Scalbert, A.; Manach, C.; Morand, C.; Remesy, C.; Jimenez, L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar]
- Scalbert, A.; Johnson, I.T.; Saltmarsh, M. Polyphenols: antioxidants and beyond. Am. J. Clin. Nutr. 2005, 81, 215S–217S. [Google Scholar]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar]
- Masella, R.; Giovannini, C.; Vari, R.; Di Benedetto, R.; Coni, E.; Volpe, R.; Fraone, N.; Bucci, A. Effects of dietary virgin olive oil phenols on low density lipoprotein oxidation in hyperlipidemic patients. Lipids. 2001, 36, 1195–1202. [Google Scholar] [PubMed]
- Williams, R.J.; Spencer, J.P.; Rice-Evans, C. Flavonoids: antioxidants or signalling molecules? Free Radic. Biol. Med. 2004, 36, 838–849. [Google Scholar] [PubMed]
- Schewe, T.; Steffen, Y.; Sies, H. How do dietary flavanols improve vascular function? A position paper. Arch. Biochem. Biophys. 2008, 476, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Croft, K.D. The chemistry and biological effects of flavonoids and phenolic acids. Ann. N. Y. Acad. Sci. 1998, 854, 435–442. [Google Scholar]
- Elbling, L.; Weiss, R.M.; Teufelhofer, O.; Uhl, M.; Knasmueller, S.; Schulte-Hermann, R.; Berger, W.; Micksche, M. Green tea extract and (-)-epigallocatechin-3-gallate, the major tea catechin, exert oxidant but lack antioxidant activities. FASEB J. 2005, 19, 807–809. [Google Scholar] [PubMed]
- Lambert, J.D.; Hong, J.; Yang, G.Y.; Liao, J.; Yang, C.S. Inhibition of carcinogenesis by polyphenols: evidence from laboratori investigations. Am. J. Clin. Nutr. 2005, 81, 284S–291S. [Google Scholar]
- Wiseman, S.; Mulder, T.; Rietveld, A. Tea flavonoids: bioavailability in vivo and effects on cell signaling pathways in vitro. Antioxid. Redox Signal. 2001, 3, 1009–1021. [Google Scholar] [CrossRef] [PubMed]
- Rezzani, R.; Porteri, E.; De Ciuceis, C.; Bonomini, F.; Rodella, L.F.; Paiardi, S.; Boari, G.; Platto, C.; Pilu, A.; Avanzi, D.; Rizzoni, D.; Agabiti, R.E. Effects of melatonin and pycnogenol on small artery structure and function in spontaneously hypertensive rats. Hypertension 2010, 55, 1373–1380. [Google Scholar]
- Nijveldt, R.J.; van Nood, E.; van Hoorn, D.E.; Boelens, P.G.; van Norren, K.; van Leeuwen, P.A. Flavonoids: a review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar]
- Heiss, C.; Dejam, A.; Kleinbongard, P.; Schewe, T.; Sies, H.; Kelm, M. Vascular effects of cocoa rich in flavan-3-ols. JAMA 2003, 290, 1030–1031. [Google Scholar]
- Davison, K.; Coates, A.M.; Buckley, J.D.; Howe, P.R. Effect of cocoa flavanols and exercise on cardiometabolic risk factors in overweight and obese subjects. Int. J. Obes. (Lond). 2008, 32, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Grassi, D.; Necozione, S.; Lippi, C.; Croce, G.; Valeri, L.; Pasqualetti, P.; Desideri, G.; Blumberg, J.B.; Ferri, C. Cocoa reduces blood pressure and insulin resistance and improves endothelium-dependent vasodilation in hypertensives. Hypertension 2005, 46, 398–405. [Google Scholar]
- Grassi, D.; Desideri, G.; Necozione, S.; Lippi, C.; Casale, R.; Properzi, G.; Blumberg, J.B.; Ferri, C. Blood pressure is reduced and insulin sensitivity increased in glucose-intolerant, hypertensive subjects after 15 days of consuming high-polyphenol dark chocolate. J. Nutr. 2008, 138, 1671–1676. [Google Scholar]
- Duffy, S.J.; Keaney, J.F., Jr.; Holbrook, M.; Gokce, N.; Swerdloff, P.L.; Frei, B.; Vita, J.A. Short- and long-term black tea consumption reverses endothelial dysfunction in patients with coronary artery disease. Circulation 2001, 104, 151–156. [Google Scholar] [PubMed]
- Grassi, D.; Mulder, T.P.J.; Draijer, R.; Desideri, G.; Molhuizen, H.O.F.; Ferri, C. Black tea consumption dose-dependently improve flow-mediated dilation in healthy males. J. Hypertens. 2009, 27, 774–781. [Google Scholar]
- Schini-Kerth, V.B.; Auger, C.; Kim, J.H.; Etienne-Selloum, N.; Chataigneau, T. Nutritional improvement of the endothelial control of vascular tone by polyphenols: role of NO and EDHF. Pflugers Arch. 2010, 459, 853–862. [Google Scholar]
- Si, H.; Liu, D. Genistein, a soy phytoestrogen, upregulates the expression of human endothelial nitric oxide synthase and lowers blood pressure in spontaneously hypertensive rats. J. Nutr. 2008, 138, 297–304. [Google Scholar] [PubMed]
- Xu, J.W.; Ikeda, K.; Yamori, Y. Cyanidin-3-glucoside regulates phosphorylation of endothelial nitric oxide synthase. FEBS Lett. 2004, 574, 176–180. [Google Scholar]
- Hooper, L.; Kroon, P.A.; Rimm, E.B.; Cohn, J.S.; Harvey, I.; Le Cornu, K.A.; Ryder, J.J.; Hall, W.L.; Cassidy, A. Flavonoids, flavonoid-rich foods, and cardiovascular risk: a meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2008, 88, 38–50. [Google Scholar] [PubMed]
- Sies, H. Polyphenols and health: Update and perspectives. Arch. Biochem. Biophys. 2010, in press. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Grassi, D.; Desideri, G.; Ferri, C. Flavonoids: Antioxidants Against Atherosclerosis. Nutrients 2010, 2, 889-902. https://doi.org/10.3390/nu2080889
Grassi D, Desideri G, Ferri C. Flavonoids: Antioxidants Against Atherosclerosis. Nutrients. 2010; 2(8):889-902. https://doi.org/10.3390/nu2080889
Chicago/Turabian StyleGrassi, Davide, Giovambattista Desideri, and Claudio Ferri. 2010. "Flavonoids: Antioxidants Against Atherosclerosis" Nutrients 2, no. 8: 889-902. https://doi.org/10.3390/nu2080889