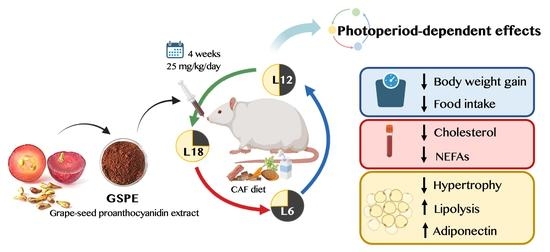
Grape-Seed Proanthocyanidins Modulate Adipose Tissue Adaptations to Obesity in a Photoperiod-Dependent Manner in Fischer 344 Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Grape-Seed Proanthocyanidin Extract
2.2. Animal Experimental Procedure
2.3. Serum Analysis
2.4. Histology of Adipose Tissues
2.5. Gene Expression Analysis
2.6. Statistical Analysis
3. Results
3.1. GSPE Consumption Restored Cholesterol and NEFAs Serum Concentrations in a Photoperiod-Dependent Manner in Obese Animals
3.2. GSPE Consumption Restored Adipocyte Morphology in iWAT in a Photoperiod-Independent Manner
3.3. Gene Expression of iWAT Was Affected by GSPE in a Photoperiod-Dependent Manner
3.4. GSPE Consumption Restored Adipocyte Morphology in eWAT in a Photoperiod-Independent Manner
3.5. GSPE Consumption Influenced the Expression of Adipogenic Genes in eWAT in a Photoperiod-Dependent Manner
3.6. GSPE Consumption Reverted the Obesity-Induced Downregulation of Pgc1α in BAT in a Photoperiod-Dependent Manner
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abarca-Gómez, L.; Abdeen, Z.A.; Hamid, Z.A.; Abu-Rmeileh, N.M.; Acosta-Cazares, B.; Acuin, C.; Adams, R.J.; Aekplakorn, W.; Afsana, K.; Aguilar-Salinas, C.A.; et al. Worldwide Trends in Body-Mass Index, Underweight, Overweight, and Obesity from 1975 to 2016: A Pooled Analysis of 2416 Population-Based Measurement Studies in 128.9 Million Children, Adolescents, and Adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization (WHO). Global Health Observatory Data. In Overweight and Obesity; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Nicolaidis, S. Environment and Obesity. Metabolism 2019, 100, 153942. [Google Scholar] [CrossRef] [PubMed]
- Rohde, K.; Keller, M.; la Cour Poulsen, L.; Blüher, M.; Kovacs, P.; Böttcher, Y. Genetics and Epigenetics in Obesity. Metabolism 2019, 92, 37–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshimura, E.; Tajiri, E.; Hatamoto, Y.; Tanaka, S. Changes in Season Affect Body Weight, Physical Activity, Food Intake, and Sleep in Female College Students: A Preliminary Study. Int. J. Environ. Res. Public Health 2020, 17, 8713. [Google Scholar] [CrossRef]
- Au-Yong, I.T.H.; Thorn, N.; Ganatra, R.; Perkins, A.C.; Symonds, M.E. Brown Adipose Tissue and Seasonal Variation in Humans. Diabetes 2009, 58, 2583–2587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turicchi, J.; O’Driscoll, R.; Horgan, G.; Duarte, C.; Palmeira, A.L.; Larsen, S.C.; Heitmann, B.L.; Stubbs, J. Weekly, Seasonal and Holiday Body Weight Fluctuation Patterns among Individuals Engaged in a European Multi-Centre Behavioural Weight Loss Maintenance Intervention. PLoS ONE 2020, 15, e0232152. [Google Scholar] [CrossRef]
- Yokoya, M.; Higuchi, Y. Day Length May Make Geographical Difference in Body Size and Proportions: An Ecological Analysis of Japanese Children and Adolescents. PLoS ONE 2019, 14, e0210265. [Google Scholar] [CrossRef]
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar] [CrossRef] [Green Version]
- Frigolet, M.E.; Gutiérrez-Aguilar, R. The Colors of Adipose Tissue. Gac. Med. Mex. 2020, 156, 142–149. [Google Scholar] [CrossRef] [Green Version]
- Pellegrinelli, V.; Carobbio, S.; Vidal-Puig, A. Adipose Tissue Plasticity: How Fat Depots Respond Differently to Pathophysiological Cues. Diabetologia 2016, 59, 1075–1088. [Google Scholar] [CrossRef] [Green Version]
- Bargut, T.C.L.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. Brown Adipose Tissue: Updates in Cellular and Molecular Biology. Tissue Cell 2016, 48, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of Polyphenols on Gut Microbiota and Implications in Human Health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mezhibovsky, E.; Knowles, K.A.; He, Q.; Sui, K.; Tveter, K.M.; Duran, R.M.; Roopchand, D.E. Grape Polyphenols Attenuate Diet-Induced Obesity and Hepatic Steatosis in Mice in Association With Reduced Butyrate and Increased Markers of Intestinal Carbohydrate Oxidation. Front. Nutr. 2021, 8, 675267. [Google Scholar] [CrossRef] [PubMed]
- Tresserra-Rimbau, A.; Medina-Remón, A.; Pérez-Jiménez, J.; Martínez-González, M.A.; Covas, M.I.; Corella, D.; Salas-Salvadó, J.; Gómez-Gracia, E.; Lapetra, J.; Arós, F.; et al. Dietary Intake and Major Food Sources of Polyphenols in a Spanish Population at High Cardiovascular Risk: The PREDIMED Study. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Ávila-Román, J.; Soliz-Rueda, J.R.; Bravo, F.I.; Aragonès, G.; Suárez, M.; Arola-Arnal, A.; Mulero, M.; Salvadó, M.J.; Arola, L.; Torres-Fuentes, C.; et al. Phenolic Compounds and Biological Rhythms: Who Takes the Lead? Trends Food Sci. Technol. 2021, 113, 77–85. [Google Scholar] [CrossRef]
- Arola-Arnal, A.; Cruz-Carrión, Á.; Torres-Fuentes, C.; Ávila-Román, J.; Aragonès, G.; Mulero, M.; Bravo, F.I.; Muguerza, B.; Arola, L.; Suárez, M. Chrononutrition and Polyphenols: Roles and Diseases. Nutrients 2019, 11, 2602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez, R.M.; Colom-Pellicer, M.; Blanco, J.; Calvo, E.; Aragonès, G.; Mulero, M. Grape-Seed Procyanidin Extract (GSPE) Seasonal-Dependent Modulation of Glucose and Lipid Metabolism in the Liver of Healthy F344 Rats. Biomolecules 2022, 12, 839. [Google Scholar] [CrossRef]
- Arreaza-Gil, V.; Escobar-Martínez, I.; Muguerza, B.; Aragonès, G.; Suárez, M.; Torres-Fuentes, C.; Arola-Arnal, A. The Effects of Grape Seed Proanthocyanidins in Cafeteria Diet-Induced Obese Fischer 344 Rats Are Influenced by Faecal Microbiota in a Photoperiod Dependent Manner. Food Funct. 2022, 13, 8363–8374. [Google Scholar] [CrossRef]
- Soliz-Rueda, J.R.; López-Fernández-sobrino, R.; Bravo, F.I.; Aragonès, G.; Suarez, M.; Muguerza, B. Grape Seed Proanthocyanidins Mitigate the Disturbances Caused by an Abrupt Photoperiod Change in Healthy and Obese Rats. Nutrients 2022, 14, 1834. [Google Scholar] [CrossRef]
- Serra, A.; MacI, A.; Romero, M.P.; Valls, J.; Bladé, C.; Arola, L.; Motilva, M.J. Bioavailability of Procyanidin Dimers and Trimers and Matrix Food Effects in in Vitro and in Vivo Models. Br. J. Nutr. 2010, 103, 944–952. [Google Scholar] [CrossRef] [Green Version]
- Quiñones, M.; Guerrero, L.; Suarez, M.; Pons, Z.; Aleixandre, A.; Arola, L.; Muguerza, B. Low-Molecular Procyanidin Rich Grape Seed Extract Exerts Antihypertensive Effect in Males Spontaneously Hypertensive Rats. Food Res. Int. 2013, 51, 587–595. [Google Scholar] [CrossRef]
- Pfaffl, M.W. Relative Quantification. In Real-Time PCR; Dorak, R., Ed.; International University Line: La Jolla, CA, USA, 2004; pp. 64–82. ISBN 9780203967317. [Google Scholar]
- Sachdeva, V.; Roy, A.; Bharadvaja, N. Current Prospects of Nutraceuticals: A Review. Curr. Pharm. Biotechnol. 2020, 21, 884–896. [Google Scholar] [CrossRef]
- Colom-Pellicer, M.; Rodríguez, R.M.; Navarro-Masip, È.; Bravo, F.I.; Mulero, M.; Arola, L.; Aragonès, G. Time-of-Day Dependent Effect of Proanthocyanidins on Adipose Tissue Metabolism in Rats with Diet-Induced Obesity. Int. J. Obes. 2022, 46, 1394–1402. [Google Scholar] [CrossRef] [PubMed]
- Helfer, G.; Barrett, P.; Morgan, P.J. A Unifying Hypothesis for Control of Body Weight and Reproduction in Seasonally Breeding Mammals. J. Neuroendocrinol. 2019, 31, e12680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pajuelo, D.; Quesada, H.; Díaz, S.; Fernández-Iglesias, A.; Arola-Arnal, A.; Bladé, C.; Salvadó, J.; Arola, L. Chronic Dietary Supplementation of Proanthocyanidins Corrects the Mitochondrial Dysfunction of Brown Adipose Tissue Caused by Diet-Induced Obesity in Wistar Rats. Br. J. Nutr. 2012, 107, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Ebling, F.J.P. On the Value of Seasonal Mammals for Identifying Mechanisms Underlying the Control of Food Intake and Body Weight. Horm. Behav. 2014, 66, 56–65. [Google Scholar] [CrossRef] [Green Version]
- Serrano, J.; Casanova-Martí, À.; Gual, A.; Pérez-Vendrell, A.M.; Blay, M.T.; Terra, X.; Ardévol, A.; Pinent, M. A Specific Dose of Grape Seed-Derived Proanthocyanidins to Inhibit Body Weight Gain Limits Food Intake and Increases Energy Expenditure in Rats. Eur. J. Nutr. 2017, 56, 1629–1636. [Google Scholar] [CrossRef]
- Soares, S.; Brandão, E.; Mateus, N.; de Freitas, V. Sensorial Properties of Red Wine Polyphenols: Astringency and Bitterness. Crit. Rev. Food Sci. Nutr. 2017, 57, 937–948. [Google Scholar] [CrossRef] [PubMed]
- Ibars, M.; Ardid-Ruiz, A.; Suárez, M.; Muguerza, B.; Bladé, C.; Aragonès, G. Proanthocyanidins Potentiate Hypothalamic Leptin/STAT3 Signalling and Pomc Gene Expression in Rats with Diet-Induced Obesity. Int. J. Obes. 2017, 41, 129–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serrano, J.; Casanova-Martí, À.; Blay, M.; Terra, X.; Ardévol, A.; Pinent, M. Defining Conditions for Optimal Inhibition of Food Intake in Rats by a Grape-Seed Derived Proanthocyanidin Extract. Nutrients 2016, 8, 652. [Google Scholar] [CrossRef] [Green Version]
- Togo, Y.; Otsuka, T.; Goto, M.; Furuse, M.; Yasuo, S. Photoperiod Regulates Dietary Preferences and Energy Metabolism in Young Developing Fischer 344 Rats but Not in Same-Age Wistar Rats. Am. J. Physiol. Endocrinol. Metab. 2012, 303, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Ibars, M.; Aragonès, G.; Ardid-Ruiz, A.; Gibert-Ramos, A.; Arola-Arnal, A.; Suárez, M.; Bladé, C. Seasonal Consumption of Polyphenol-Rich Fruits Affects the Hypothalamic Leptin Signaling System in a Photoperiod-Dependent Mode. Sci. Rep. 2018, 8, 13572. [Google Scholar] [CrossRef] [Green Version]
- Mariné-Casadó, R.; Domenech-Coca, C.; del Bas, J.M.; Bladé, C.; Arola, L.; Caimari, A. The Exposure to Different Photoperiods Strongly Modulates the Glucose and Lipid Metabolisms of Normoweight Fischer 344 Rats. Front. Physiol. 2018, 9, 416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, H.; Wang, Q.; Li, T.; Ren, D.; Yang, X. Grape Seed Proanthocyanidins Reduced the Overweight of C57BL/6J Mice through Modulating Adipose Thermogenesis and Gut Microbiota. Food Funct. 2021, 12, 8467–8477. [Google Scholar] [CrossRef]
- Yilmazer-Musa, M.; Griffith, A.M.; Michels, A.J.; Schneider, E.; Frei, B. Grape Seed and Tea Extracts and Catechin 3-Gallates Are Potent Inhibitors of α-Amylase and α-Glucosidase Activity. J. Agric. Food Chem. 2012, 60, 8924–8929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ursula, W.; Eric, R. Dynamics of Adipose Tissue Turnover in Human Metabolic Health and Disease HHS Public Access. Physiol. Behav. 2019, 176, 139–148. [Google Scholar] [CrossRef]
- Despres, J.P.; Lemieux, I. Abdominal Obesity and Metabolic Syndrome. Nature 2006, 444, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Drolet, R.; Richard, C.; Sniderman, A.D.; Mailloux, J.; Fortier, M.; Huot, C.; Rhéaume, C.; Tchernof, A. Hypertrophy and Hyperplasia of Abdominal Adipose Tissues in Women. Int. J. Obes. 2008, 32, 283–291. [Google Scholar] [CrossRef] [Green Version]
- Jiao, X.; Wang, Y.; Lin, Y.; Lang, Y.; Li, E.; Zhang, X.; Zhang, Q.; Feng, Y.; Meng, X.; Li, B. Blueberry Polyphenols Extract as a Potential Prebiotic with Anti-Obesity Effects on C57BL/6 J Mice by Modulating the Gut Microbiota. J. Nutr. Biochem. 2019, 64, 88–100. [Google Scholar] [CrossRef]
- Ali, A.T.; Hochfeld, W.E.; Myburgh, R.; Pepper, M.S. Adipocyte and Adipogenesis. Eur. J. Cell Biol. 2013, 92, 229–236. [Google Scholar] [CrossRef]
- Matsukawa, T.; Inaguma, T.; Han, J.; Villareal, M.O.; Isoda, H. Cyanidin-3-Glucoside Derived from Black Soybeans Ameliorate Type 2 Diabetes through the Induction of Differentiation of Preadipocytes into Smaller and Insulin-Sensitive Adipocytes. J. Nutr. Biochem. 2015, 26, 860–867. [Google Scholar] [CrossRef]
- Wang, S.; Moustaid-Moussa, N.; Chen, L.; Mo, H.; Shastri, A.; Su, R.; Bapat, P.; Kwun, I.S.; Shen, C.L. Novel Insights of Dietary Polyphenols and Obesity. J. Nutr. Biochem. 2014, 25, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalilpourfarshbafi, M.; Gholami, K.; Murugan, D.D.; Abdul Sattar, M.Z.; Abdullah, N.A. Differential Effects of Dietary Flavonoids on Adipogenesis. Eur. J. Nutr. 2019, 58, 5–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Most, J.; Warnke, I.; Boekschoten, M.v.; Jocken, J.W.E.; de Groot, P.; Friedel, A.; Bendik, I.; Goossens, G.H.; Blaak, E.E. The Effects of Polyphenol Supplementation on Adipose Tissue Morphology and Gene Expression in Overweight and Obese Humans. Adipocyte 2018, 7, 190–196. [Google Scholar] [CrossRef]
- de Lima, L.P.; de Paula Barbosa, A. A Review of the Lipolytic Effects and the Reduction of Abdominal Fat from Bioactive Compounds and Moro Orange Extracts. Heliyon 2021, 7, e07695. [Google Scholar] [CrossRef]
- Fang, H.; Judd, R.L. Adiponectin Regulation and Function. Compr. Physiol. 2018, 8, 1031–1063. [Google Scholar] [CrossRef] [PubMed]
- Terra, X.; Montagut, G.; Bustos, M.; Llopiz, N.; Ardèvol, A.; Bladé, C.; Fernández-Larrea, J.; Pujadas, G.; Salvadó, J.; Arola, L.; et al. Grape-Seed Procyanidins Prevent Low-Grade Inflammation by Modulating Cytokine Expression in Rats Fed a High-Fat Diet. J. Nutr. Biochem. 2009, 20, 210–218. [Google Scholar] [CrossRef]





| P | STD-VH | CAF-VH | CAF-GSPE | p Diet 1 | p Diet × P 1 | p GSPE 1 | p GSPE × P 1 | |
|---|---|---|---|---|---|---|---|---|
| Body weight (g) | L12 | 479.8 ± 12.1 a | 584.5 ± 11.9 b | 555.5 ± 14.2 b | <0.0001 | ns | ns | ns |
| L18 | 491.9 ± 12.9 a | 598.3 ± 11.4 b | 559.1 ± 15.9 b | |||||
| L6 | 491.4 ± 12.0 a | 554.9 ± 19.9 b | 554.8 ± 18.5 b | |||||
| Body weight gain (g) | L12 | 96.5 ± 8.9 a | 180. ± 10.5 b | 156.5 ± 9.7 b | <0.0001 | ns | 0.0403 | ns |
| L18 | 104.8 ± 9.1 a | 197.3 ± 8.2 c | 159.4 ± 12.8 b | |||||
| L6 | 106.1 ± 8.1 a | 158.3 ± 14.9 b | 155.8 ± 16.1 b | |||||
| Food intake (g) | L12 | 201.3 ± 3.4 a | 308.9 ± 14.2 c | 256.8 ± 16.4 b | <0.0001 | ns | 0.0015 | ns |
| L18 | 212.1 ± 6.9 a | 301.8 ± 13.4 b | 292.1 ± 12.8 b | |||||
| L6 | 219.3 ± 2.2 a | 311.9 ± 7.8 c | 264.3 ± 12.7 b | |||||
| Fat mass (g) | L12 | 35.3 ± 3.4 a | 83.5 ± 5.3 b | 82.2 ± 4.8 b | <0.0001 | ns | ns | ns |
| L18 | 46.5 ± 3.5 a | 95.1 ± 5.4 b | 82.5 ± 6.8 b | |||||
| L6 | 40.1 ± 3.7 a | 74.3 ± 9.4 b | 76.1 ± 5.9 b | |||||
| iWAT mass (g) | L12 | 4.1 ± 0.3 a | 8.6 ± 0.7 b | 9.4 ± 1.1 b | <0.0001 | ns | ns | ns |
| L18 | 4.8 ± 0.1 a | 9.2 ± 1.3 b | 7.1 ± 1.1 ab | |||||
| L6 | 4.2 ± 0.6 a | 8.2 ± 1.5 b | 7.2 ± 0.8 ab | |||||
| eWAT mass (g) | L12 | 7.4 ± 0.8 a | 18.9 ± 1.1 b | 18.1 ± 1.1 b | <0.0001 | ns | ns | ns |
| L18 | 10.9 ± 0.5 a | 19.6 ± 1.5 b | 16.4 ± 1.7 b | |||||
| L6 | 9.3 ± 0.9 a | 16.0 ± 1.7 b | 18.6 ± 1.1 b | |||||
| BAT mass (g) | L12 | 0.54 ± 0.05 a | 0.96 ± 0.07 b | 0.94 ± 0.09 b | <0.0001 | ns | ns | ns |
| L18 | 0.49 ± 0.05 a | 0.92 ± 0.16 ab | 0.99 ± 0.14 b | |||||
| L6 | 0.64 ± 0.06 a | 0.93 ± 0.12 ab | 1.01 ± 0.10 b | |||||
| Glucose (mmol/L) | L12 | 5.7 ± 0.3 a | 8.1 ± 0.3 b | 7.1 ± 0.4 ab | <0.0001 | ns | ns | ns |
| L18 | 5.7 ± 0.2 a | 7.1 ± 0.2 b | 7.6 ± 0.1 b | |||||
| L6 | 6.3 ± 0.1 | 7.4 ± 0.4 | 6.9 ± 0.4 | |||||
| Cholesterol (mmol/L) | L12 | 1.23 ± 0.07 a | 1.95 ± 0.28 b | 1.23 ± 0.09 a | ns | ns | ns | 0.0056 |
| L18 | 1.28 ± 0.11 ab | 1.18 ± 0.10 a | 1.57 ± 0.08 b | |||||
| L6 | 1.20 ± 0.06 | 1.36 ± 0.19 | 1.15 ± 0.14 | |||||
| NEFAs (mmol/L) | L12 | 0.57 ± 0.06 a | 1.17 ± 0.12 b | 0.82 ± 0.01 a | 0.0003 | 0.0024 | ns | 0.0005 |
| L18 | 0.69 ± 0.07 a | 0.64 ± 0.09 a | 1.03 ± 0.10 b | |||||
| L6 | 0.60 ± 0.06 a | 0.93 ± 0.10 b | 0.68 ± 0.05 ab | |||||
| TG (mmol/L) | L12 | 0.79 ± 0.04 a | 2.79 ± 0.36 c | 1.82 ± 0.16 b | <0.0001 | ns | ns | ns |
| L18 | 0.97 ± 0.13 a | 1.88 ± 0.25 b | 2.18 ± 0.23 b | |||||
| L6 | 0.88 ± 0.11 a | 2.12 ± 0.39 b | 1.49 ± 0.20 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro-Masip, È.; Colom-Pellicer, M.; Manocchio, F.; Arola-Arnal, A.; Bravo, F.I.; Muguerza, B.; Aragonès, G. Grape-Seed Proanthocyanidins Modulate Adipose Tissue Adaptations to Obesity in a Photoperiod-Dependent Manner in Fischer 344 Rats. Nutrients 2023, 15, 1037. https://doi.org/10.3390/nu15041037
Navarro-Masip È, Colom-Pellicer M, Manocchio F, Arola-Arnal A, Bravo FI, Muguerza B, Aragonès G. Grape-Seed Proanthocyanidins Modulate Adipose Tissue Adaptations to Obesity in a Photoperiod-Dependent Manner in Fischer 344 Rats. Nutrients. 2023; 15(4):1037. https://doi.org/10.3390/nu15041037
Chicago/Turabian StyleNavarro-Masip, Èlia, Marina Colom-Pellicer, Francesca Manocchio, Anna Arola-Arnal, Francisca Isabel Bravo, Begoña Muguerza, and Gerard Aragonès. 2023. "Grape-Seed Proanthocyanidins Modulate Adipose Tissue Adaptations to Obesity in a Photoperiod-Dependent Manner in Fischer 344 Rats" Nutrients 15, no. 4: 1037. https://doi.org/10.3390/nu15041037