The Association between Non-Alcoholic Fatty Liver Disease (NAFLD) and Advanced Fibrosis with Serological Vitamin B12 Markers: Results from the NHANES 1999–2004
Abstract
:1. Introduction
2. Materials and Methods
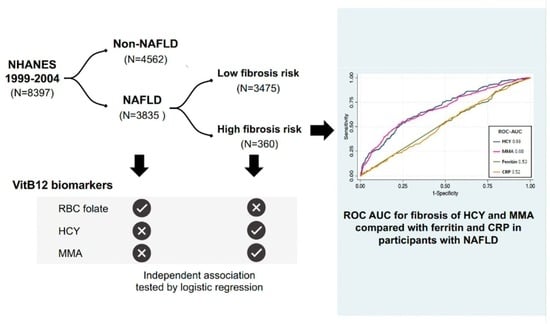
2.1. Study Design and Participants
2.2. Questionnaire and Lab Tests
2.3. Definitions and Index Calculation
2.4. Statistical Analysis
3. Results
3.1. Characteristics of Participants with NAFLD and Advanced Fibrosis
3.2. Association between Vitamin B12 Markers and NAFLD or Advanced Fibrosis
3.3. Diagnostic Performance of MMA and HCY for Advanced Fibrosis in NAFLD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, Predictions, Risk Factors and Prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Díaz, J.; Solis-Urra, P.; Aragón-Vela, J.; Rodríguez-Rodríguez, F.; Olivares-Arancibia, J.; Álvarez-Mercado, A.I. Insights into the Impact of Microbiota in the Treatment of NAFLD/NASH and Its Potential as a Biomarker for Prognosis and Diagnosis. Biomedicines 2021, 9, 145. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Mitnala, S.; Vishnubhotla, R.K.; Mukherjee, R.; Reddy, D.N.; Rao, P.N. The Riddle of Nonalcoholic Fatty Liver Disease: Progression From Nonalcoholic Fatty Liver to Nonalcoholic Steatohepatitis. J. Clin. Exp. Hepatol. 2015, 5, 147–158. [Google Scholar] [CrossRef] [Green Version]
- Byrne, C.D.; Targher, G. NAFLD: A Multisystem Disease. J. Hepatol. 2015, 62, S47–S64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eslam, M.; Sarin, S.K.; Wong, V.W.; Fan, J.G.; Kawaguchi, T.; Ahn, S.H.; Zheng, M.H.; Shiha, G.; Yilmaz, Y.; Gani, R.; et al. The Asian Pacific Association for the Study of the Liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hepatol. Int. 2020, 14, 889–919. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.S.; Guahnon, M.P.; Seganfredo, F.B.; Pinto, L.P.; Tovo, C.V.; Fernandes, S.A. Vitamin B12 and Homocysteine Levels in patients with NAFLD: A Systemic Review and Metanalysis. Arq. Gastroenterol. 2021, 58, 234–239. [Google Scholar] [CrossRef]
- Mason, J.B. Biomarkers of nutrient exposure and status in one-carbon (methyl) metabolism. J. Nutr. 2003, 941S–947S. [Google Scholar] [CrossRef]
- Xu, Y.; Guan, Y.; Yang, X.; Xia, Z.; Wu, J. Association of Serum Homocysteine Levels with Histological Severity of NAFLD. J. Gastrointestin. Liver Dis. 2020, 29, 51–58. [Google Scholar] [CrossRef]
- Gulsen, M.; Yesilova, Z.; Bagci, S.; Uygun, A.; Ozcan, A.; Ercin, C.N.; Erdil, A.; Sanisoglu, S.Y.; Cakir, E.; Ates, Y.; et al. Elevated plasma homocysteine concentrations as a predictor of steatohepatitis in patients with non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2005, 20, 1448–1455. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Kountouras, J.; Patsiaoura, K.; Katsiki, E.; Zafeiriadou, E.; Deretzi, G.; Zavos, C.; Gavalas, E.; Katsinelos, P.; Mane, V.; et al. Serum homocysteine levels in patients with nonalcoholic fatty liver disease. Ann. Hepatol. 2012, 11, 68–76. [Google Scholar] [CrossRef]
- Angulo, P.; Hui, J.M.; Marchesini, G.; Bugianesi, E.; George, J.; Farrell, G.C.; Enders, F.; Saksena, S.; Burt, A.D.; Bida, J.P.; et al. The NAFLD fibrosis score: A Noninvasive System that Identifies Liver Fibrosis in Patients with NAFLD. Hepatology 2007, 45, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.K.; Treeprasertsuk, S.; Goh, G.B.; Fan, J.G.; Song, M.J.; Charatcharoenwitthaya, P.; Duseja, A.; Dan, Y.Y.; Imajo, K.; Nakajima, A.; et al. Optimizing Use of Nonalcoholic Fatty Liver Disease Fibrosis Score, Fibrosis-4 Score, and Liver Stiffness Measurement to Identify Patients with Advanced Fibrosis. Clin. Gastroenterol. Hepatol. 2019, 17, 2570–2580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The Fatty Liver Index: A Simple and Accurate Predictor of Hepatic Steatosis in the General Population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef] [Green Version]
- Ruhl, C.E.; Everhart, J.E. Fatty liver indices in the multiethnic United States National Health and Nutrition Examination Survey. Aliment. Pharmacol. Ther. 2015, 41, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Golabi, P.; Gerber, L.; Paik, J.M.; Deshpande, R.; de Avila, L.; Younossi, Z.M. Contribution of sarcopenia and physical inactivity to mortality in people with non-alcoholic fatty liver disease. JHEP Rep. 2020, 2, 171. [Google Scholar] [CrossRef] [PubMed]
- Rehkopf, D.H.; Needham, B.L.; Lin, J.; Blackburn, E.H.; Zota, A.R.; Wojcicki, J.M.; Epel, E.S. Leukocyte Telomere Length in Relation to 17 Biomarkers of Cardiovascular Disease Risk: A Cross-Sectional Study of US Adults. PLoS Med. 2016, 13, e1002188. [Google Scholar] [CrossRef]
- Zou, B.; Yeo, Y.H.; Nguyen, V.H.; Cheung, R.; Ingelsson, E.; Nguyen, M.H. Prevalence, characteristics and mortality outcomes of obese, nonobese and lean NAFLD in the United States, 1999–2016. J. Intern. Med. 2020, 288, 139–151. [Google Scholar] [CrossRef]
- Adams, P.C.; Reboussin, D.M.; Barton, J.C.; McLaren, C.E.; Eckfeldt, J.H.; McLaren, G.D.; Dawkins, F.W.; Acton, R.T.; Harris, E.L.; Gordeuk, V.R.; et al. Hemochromatosis and Iron-Overload Screening in a Racially Diverse Population. N. Engl. J. Med. 2005, 352, 1769–1778. [Google Scholar] [CrossRef] [Green Version]
- Lazo, M.; Hernaez, R.; Bonekamp, S.; Kamel, I.R.; Brancati, F.L.; Guallar, E.; Clark, J.M. Non-alcoholic fatty liver disease and mortality among US adults: Prospective Cohort Study. BMJ 2011, 343, d6891. [Google Scholar] [CrossRef] [Green Version]
- Udompap, P.; Mannalithara, A.; Heo, N.Y.; Kim, D.; Kim, W.R. Increasing prevalence of cirrhosis among U.S. adults aware or unaware of their chronic hepatitis C virus infection. J. Hepatol. 2016, 64, 1027–1032. [Google Scholar] [CrossRef] [Green Version]
- Cheah, M.C.; McCullough, A.J.; Goh, G.B.-B. Current Modalities of Fibrosis Assessment in Non-alcoholic Fatty Liver Disease. J. Clin. Transl. Hepatol. 2017, 5, 261–271. [Google Scholar] [CrossRef] [Green Version]
- Inker, L.A.; Astor, B.C.; Fox, C.H.; Isakova, T.; Lash, J.P.; Peralta, C.A.; Kurella Tamura, M.; Feldman, H.I. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am. J. Kidney Dis. 2014, 63, 713–735. [Google Scholar] [CrossRef] [Green Version]
- Arshad, T.; Golabi, P.; Paik, J.; Mishra, A.; Younossi, Z.M. Prevalence of Nonalcoholic Fatty Liver Disease in the Female Population. Hepatol. Commun. 2019, 3, 74–83. [Google Scholar] [CrossRef]
- Li, Z.; Gueant-Rodriguez, R.M.; Quilliot, D.; Sirveaux, M.A.; Meyre, D.; Gueant, J.L.; Brunaud, L. Folate and vitamin B12 status is associated with insulin resistance and metabolic syndrome in morbid obesity. Clin. Nutr. 2018, 37, 1700–1706. [Google Scholar] [CrossRef]
- Dai, H.; Wang, W.; Tang, X.; Chen, R.; Chen, Z.; Lu, Y.; Yuan, H. Association between homocysteine and non-alcoholic fatty liver disease in Chinese adults: A Cross-Sectional Study. Nutr. J. 2016, 15, 102. [Google Scholar] [CrossRef] [Green Version]
- Leach, N.V.; Dronca, E.; Vesa, S.C.; Sampelean, D.P.; Craciun, E.C.; Lupsor, M.; Crisan, D.; Tarau, R.; Rusu, R.; Para, I.; et al. Serum homocysteine levels, oxidative stress and cardiovascular risk in non-alcoholic steatohepatitis. Eur. J. Intern. Med. 2014, 25, 762–767. [Google Scholar] [CrossRef]
- Green, R.; Allen, L.H.; Bjørke-Monsen, A.L.; Brito, A.; Guéant, J.L.; Miller, J.W.; Molloy, A.M.; Nexo, E.; Stabler, S.; Toh, B.H.; et al. Vitamin B(12) deficiency. Nat. Rev. Dis. Primers 2017, 3, 17040. [Google Scholar] [CrossRef]
- Chandler, R.J.; Zerfas, P.M.; Shanske, S.; Sloan, J.; Hoffmann, V.; DiMauro, S.; Venditti, C.P. Mitochondrial dysfunction in mut methylmalonic acidemia. FASEB J. 2009, 23, 1252–1261. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Liu, Y.; Liu, J.; Tian, W.; Zhang, X.; Cai, H.; Fang, S.; Yu, B. Mitochondria-derived methylmalonic acid, a surrogate biomarker of mitochondrial dysfunction and oxidative stress, predicts all-cause and cardiovascular mortality in the general population. Redox. Biol. 2020, 37, 101741. [Google Scholar] [CrossRef]
- Liu, X.; Gao, X.; Zhang, R.; Liu, Z.; Shen, N.; Di, Y.; Fang, T.; Li, H.; Tian, F. Discovery and comparison of serum biomarkers for diabetes mellitus and metabolic syndrome based on UPLC-Q-TOF/MS. Clin. Biochem. 2020, 82, 40–50. [Google Scholar] [CrossRef]
- Fling, R.R.; Doskey, C.M.; Fader, K.A.; Nault, R.; Zacharewski, T.R. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) dysregulates hepatic one carbon metabolism during the progression of steatosis to steatohepatitis with fibrosis in mice. Sci. Rep. 2020, 10, 14831. [Google Scholar] [CrossRef]
- Zhang, W.; He, H.; Wang, H.; Wang, S.; Li, X.; Liu, Y.; Jiang, H.; Jiang, H.; Yan, Y.; Wang, Y.; et al. Activation of transsulfuration pathway by salvianolic acid a treatment: A Homocysteine-Lowering Approach with Beneficial Effects on Redox Homeostasis in High-Fat Diet-Induced Hyperlipidemic Rats. Nutr. Metab. 2013, 10, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, C.G.; Banerjee, R. Homocysteine and redox signaling. Antioxid. Redox. Signal 2005, 7, 547–559. [Google Scholar] [CrossRef]
- Casas, J.P.; Bautista, L.E.; Smeeth, L.; Sharma, P.; Hingorani, A.D. Homocysteine and stroke: Evidence on a Causal Link from Mendelian Randomisation. Lancet 2005, 365, 224–232. [Google Scholar] [CrossRef]
- An, P.; Wei, L.L.; Zhao, S.; Sverdlov, D.Y.; Vaid, K.A.; Miyamoto, M.; Kuramitsu, K.; Lai, M.; Popov, Y.V. Hepatocyte mitochondria-derived danger signals directly activate hepatic stellate cells and drive progression of liver fibrosis. Nat. Commun. 2020, 11, 2362. [Google Scholar] [CrossRef]
- Berge, R.K.; Bjorndal, B.; Strand, E.; Bohov, P.; Lindquist, C.; Nordrehaug, J.E.; Svardal, A.; Skorve, J.; Nygard, O. Tetradecylthiopropionic acid induces hepatic mitochondrial dysfunction and steatosis, accompanied by increased plasma homocysteine in mice. Lipids Health Dis. 2016, 15, 24. [Google Scholar] [CrossRef] [Green Version]
- Ai, Y.; Sun, Z.; Peng, C.; Liu, L.; Xiao, X.; Li, J. Homocysteine Induces Hepatic Steatosis Involving ER Stress Response in High Methionine Diet-Fed Mice. Nutrients 2017, 9, 346. [Google Scholar] [CrossRef] [Green Version]
- Bjune, M.S.; Lindquist, C.; Hallvardsdotter Stafsnes, M.; Bjorndal, B.; Bruheim, P.; Aloysius, T.A.; Nygard, O.; Skorve, J.; Madsen, L.; Dankel, S.N.; et al. Plasma 3-hydroxyisobutyrate (3-HIB) and methylmalonic acid (MMA) are markers of hepatic mitochondrial fatty acid oxidation in male Wistar rats. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 158887. [Google Scholar] [CrossRef]
- Luciani, A.; Schumann, A.; Berquez, M.; Chen, Z.; Nieri, D.; Failli, M.; Debaix, H.; Festa, B.P.; Tokonami, N.; Raimondi, A.; et al. Impaired mitophagy links mitochondrial disease to epithelial stress in methylmalonyl-CoA mutase deficiency. Nat. Commun. 2020, 11, 970. [Google Scholar] [CrossRef] [Green Version]
- Kowdley, K.V.; Belt, P.; Wilson, L.A.; Yeh, M.M.; Neuschwander-Tetri, B.A.; Chalasani, N.; Sanyal, A.J.; Nelson, J.E. Serum ferritin is an independent predictor of histologic severity and advanced fibrosis in patients with nonalcoholic fatty liver disease. Hepatology 2012, 55, 77–85. [Google Scholar] [CrossRef] [Green Version]
- Yoneda, M.; Mawatari, H.; Fujita, K.; Iida, H.; Yonemitsu, K.; Kato, S.; Takahashi, H.; Kirikoshi, H.; Inamori, M.; Nozaki, Y.; et al. High-sensitivity C-reactive protein is an independent clinical feature of nonalcoholic steatohepatitis (NASH) and also of the severity of fibrosis in NASH. J. Gastroenterol. 2007, 42, 573–582. [Google Scholar] [CrossRef]
- Dahlhoff, C.; Worsch, S.; Sailer, M.; Hummel, B.A.; Fiamoncini, J.; Uebel, K.; Obeid, R.; Scherling, C.; Geisel, J.; Bader, B.L.; et al. Methyl-donor supplementation in obese mice prevents the progression of NAFLD, activates AMPK and decreases acyl-carnitine levels. Mol. Metab. 2014, 3, 565–580. [Google Scholar] [CrossRef]
- Kim, H.; Min, H. Folic acid supplementation prevents high fructose-induced non-alcoholic fatty liver disease by activating the AMPK and LKB1 signaling pathways. Nutr. Res. Pract. 2020, 14, 309–321. [Google Scholar] [CrossRef]
- Barb, D.; Repetto, E.M.; Stokes, M.E.; Shankar, S.S.; Cusi, K. Type 2 diabetes mellitus increases the risk of hepatic fibrosis in individuals with obesity and nonalcoholic fatty liver disease. Obesity 2021, 29, 1950–1960. [Google Scholar] [CrossRef]
- McPherson, S.; Stewart, S.F.; Henderson, E.; Burt, A.D.; Day, C.P. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut 2010, 59, 1265–1269. [Google Scholar] [CrossRef] [Green Version]
- Shah, A.G.; Lydecker, A.; Murray, K.; Tetri, B.N.; Contos, M.J.; Sanyal, A.J. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 2009, 7, 1104–1112. [Google Scholar] [CrossRef] [Green Version]


| Overall | p | Non-NAFLD | NAFLD | |||||
|---|---|---|---|---|---|---|---|---|
| (NAFLD vs. Non-NAFLD) | Total | Low Fibrosis Risk | High Fibrosis Risk | p (High vs. Low Fibrosis Risk) | ||||
| n (%) | 8397 (100%) | 4562 (55.8) | 3835 (44.2) | 3475 (93.0) | 360 (7.0) | |||
| NAFLD and fibrosis scores | FLI $ | 50.2 (49.0, 51.4) | <0.001 | 26.3 (25.8, 26.8) | 81.5 (80.7, 82.4) | 80.7 (80.0, 81.4) | 88.8 (86.3, 91.4) | <0.001 |
| USFLI $ | 23.6 (22.4, 24.9) | <0.001 | 10.9 (10.5, 11.3) | 40.5 (38.9, 42.1) | 39.6 (38.1, 41.1) | 54.9 (50.2, 59.6) | <0.001 | |
| NFS $ | −2.24 (−2.29, −2.19) | <0.001 | −2.35 (−2.39, −2.32) | −1.67 (−1.73, −1.61) | −1.72 (−1.77, −1.67) | 0.40 (0.24, 0.56) | <0.001 | |
| FIB-4 $ | 1.02 (1.00, 1.04) | <0.001 | 1.17 (1.16, 1.19) | 1.04 (1.02, 1.05) | 1.03 (1.02, 1.05) | 1.84 (1.72, 1.96) | <0.001 | |
| APRI $ | 0.287 (0.283, 0.291) | 0.3080 | 0.293 (0.287, 0.299) | 0.288 (0.283, 0.294) | 0.271 (0.265, 0.276) | 0.507 (0.462, 0.552) | <0.001 | |
| Demographic data | Ethnicity (%) | <0.001 | <0.001 | |||||
| Non-Hispanic Black | 10.8 | 11.0 | 10.6 | 10.6 | 10.5 | |||
| Mexican American | 6.3 | 6.4 | 6.1 | 6.3 | 4.4 | |||
| Other race | 9.9 | 11.0 | 8.7 | 8.8 | 6.9 | |||
| Income level $, % | 0.4469 | <0.001 | ||||||
| Low | 20.2 | 19.9 | 20.5 | 19.8 | 30.5 | |||
| Middle | 36.3 | 35.8 | 37.1 | 36.8 | 41.1 | |||
| High | 43.5 | 44.3 | 42.5 | 43.5 | 28.3 | |||
| Education level $, % | <0.001 | 0.0024 | ||||||
| <High school | 19.7 | 18.3 | 21.4 | 20.7 | 31.1 | |||
| High school | 25.6 | 24.0 | 27.6 | 27.7 | 27.0 | |||
| ≥college | 54.8 | 57.8 | 51.0 | 51.6 | 41.9 | |||
| Physical activity $, % | <0.001 | <0.001 | ||||||
| Inactivate | 36.9 | 32.6 | 42.5 | 41.3 | 58.0 | |||
| Moderate | 31.9 | 30.9 | 33.1 | 33.2 | 31.5 | |||
| Vigorous | 31.2 | 36.6 | 24.4 | 25.4 | 10.5 | |||
| Ever smoking, % | 42.7 | <0.001 | 38.8 | 47.6 | 47.1 | 54.0 | 0.0561 | |
| Dietary | Diet folate $, mcg | 402 (391, 412) | <0.001 | 413 (401, 426) | 384 (374, 394) | 384 (374, 395) | 352 (326, 377) | 0.0299 |
| Diet vitamin B12 $, mcg | 5.22 (4.93, 5.51) | 0.1149 | 5.09 (4.76, 5.43) | 5.37 (5.09, 5.64) | 5.42 (5.15, 5.69) | 4.47 (3.82, 5.12) | 0.0097 | |
| Taking vitamin B12 or folate supplement, % | 22.6 | 0.0318 | 23.6 | 21.4 | 21.4 | 21.0 | 0.8893 | |
| Physical examinations | BMI $, kg/m2 | 28.4 (28.1, 28.6) | <0.001 | 24.4 (24.2, 24.5) | 33.2 (32.8, 33.5) | 32.6 (32.3, 32.8) | 37.9 (36.3, 39.5) | <0.001 |
| Waist circumference, cm | 97.6 (96.9, 98.3) | <0.001 | 88.3 (87.9, 88.8) | 110.0 (109.2, 110.8) | 109.0 (109.0, 110.0) | 119.0 (116.0, 122.0) | <0.001 | |
| SBP $, mmHg | 124.2 (123.3, 125.0) | <0.001 | 124.0 (123.0, 125.0) | 129.0 (128.0, 130.0) | 130.0 (129.0, 131.0) | 129.0 (126.0, 132.0) | 0.7686 | |
| DBP $, mmHg | 71.6 (71.1, 72.0) | <0.001 | 69.8 (69.4, 70.3) | 73.3 (72.6, 74.0) | 73.1 (72.4, 73.8) | 69.2 (67.9, 70.6) | <0.001 | |
| Biochemical Laboratory Tests | Glucose $, mmol/L | 5.67 (5.59, 5.75) | <0.001 | 5.40 (5.35, 5.44) | 6.17 (6.06, 6.29) | 6.17 (6.05, 6.29) | 7.19 (6.67, 7.70) | <0.001 |
| Total cholesterol $, mmol/L | 5.24 (5.20, 5.28) | <0.001 | 5.14 (5.11, 5.18) | 5.45 (5.40, 5.51) | 5.49 (5.43, 5.55) | 5.01 (4.87, 5.14) | <0.001 | |
| TG$ #, mmol/L | 1.32 (1.29, 1.35) | <0.001 | 1.04 (1.02, 1.07) | 1.84 (1.79, 1.89) | 1.85 (1.80, 1.90) | 1.69 (1.55, 1.84) | 0.0322 | |
| HDL $, mmol/L | 1.34 (1.33, 1.36) | <0.001 | 1.47 (1.45, 1.48) | 1.20 (1.18, 1.22) | 1.20 (1.19, 1.22) | 1.21 (1.15, 1.26) | 0.8820 | |
| ALT, U/L | 24.9 (24.2, 25.5) | <0.001 | 21.3 (20.8, 21.7) | 28.8 (27.4, 30.1) | 27.5 (26.2, 28.7) | 34.8 (30.5, 39.1) | 0.0021 | |
| AST, U/L | 24.3 (24.0, 24.5) | <0.001 | 23.8 (23.6, 24.1) | 25.0 (24.7, 25.4) | 24.2 (23.8, 24.5) | 34.0 (30.2, 37.9) | <0.001 | |
| GGT, U/L | 26.0 (25.2, 26.9) | <0.001 | 19.7 (19.2, 20.3) | 34.6 (33.1, 36.2) | 33.9 (32.6, 35.1) | 43.2 (35.6, 50.9) | 0.0166 | |
| CRP $, mg/dL | 0.43 (0.41, 0.46) | <0.001 | 0.29 (0.27, 0.31) | 0.61 (0.56, 0.66) | 0.61 (0.56, 0.65) | 0.68 (0.55, 0.80) | 0.2187 | |
| eGFR, mL/min per 1.73 m2 | 92.5 (91.3, 93.7) | 0.6370 | 89.3 (88.3, 90.3) | 89.0 (88.0, 90.0) | 87.0 (86.0, 88.0) | 82.0 (79.3, 84.7) | <0.001 | |
| Ferritin $, μg/L | 123 (118, 128) | <0.001 | 115 (110, 120) | 148 (141, 155) | 147 (140, 155) | 180 (146, 213) | 0.0816 | |
| Vitamin B12 markers | MMA #, nmol/L | 139.5 (135.7, 143.4) | 0.4490 | 145.5 (141.2, 148.4) | 144.0 (139.8, 146.9) | 145.5 (141.2, 149.9) | 174.2 (164.0, 184.9) | <0.001 |
| HCY #, μmol/L | 8.30 (8.12, 8.48) | 0.1880 | 8.58 (8.41, 8,76) | 8.67 (8.50, 8.85) | 8.76 (8.58, 8.94) | 9.78 (9.21, 10.49) | 0.0011 | |
| Folate $#, serum (nmol/L) | 29.99 (29.15, 30.86) | <0.001 | 32.46 (31.19, 33.45) | 29.37 (28.50, 29.96) | 29.96 (29.08, 31.19) | 29.67 (27.66, 31.82) | 0.7216 | |
| Folate $#, RBC (nmol/L RBC) | 648.5 (633.3, 663.9) | <0.001 | 649.4 (632.1, 667.1) | 679.3 (663.8, 695.1) | 690.2 (673.8, 707.0) | 695.1 (659.8, 732.2) | 0.7940 | |
| Vitamin B12 $#, pmol/L | 347.5 (339.4, 355.8) | <0.001 | 364.3 (353.9, 375.0) | 330.6 (324.1, 337.0) | 333.0 (326.4, 340.0) | 306.7 (289.7, 325.1) | 0.0112 | |
| Red blood cell markers | RBC $ (million cells/μL) | 4.74 (4.71, 4.76) | <0.001 | 4.67 (4.65, 4.70) | 4.80 (4.77, 4.82) | 4.80 (4.77, 4.82) | 4.64 (4.58, 4.70) | <0.001 |
| RDW $ (%) | 12.67 (12.63, 12.70) | <0.001 | 12.61 (12.57, 12.65) | 12.80 (12.75, 12.85) | 12.79 (12.74, 12.85) | 13.19 (13.01, 13.37) | <0.001 | |
| MCV $ (fL) | 90.24 (90.00, 90.47) | <0.001 | 91.15 (90.92, 91.38) | 89.74 (89.47, 90.01) | 89.94 (89.67, 90.20) | 90.23 (89.53, 90.94) | 0.3930 | |
| MCHC $ (g/dL) | 33.84 (33.77, 33.91) | 0.0029 | 33.80 (33.73, 33.86) | 33.88 (33.80, 33.95) | 33.87 (33.79, 33.94) | 33.89 (33.81, 33.98) | 0.5588 | |
| Metabolic disorders | Obesity, % | 32.3 | <0.001 | 5.1 | 66.8 | 65.8 | 80.0 | <0.001 |
| Hypertension, % | 39.5 | <0.001 | 28.3 | 53.6 | 51.6 | 80.7 | <0.001 | |
| Diabetes, % | 11.2 | <0.001 | 4.4 | 19.7 | 16.6 | 60.2 | <0.001 | |
| Dyscholestrolemia, % | 68.5 | <0.001 | 58.8 | 80.9 | 81.6 | 71.5 | <0.001 | |
| Comorbidities | CVD, % | 10.7 | <0.001 | 7.9 | 14.3 | 12.4 | 39.4 | <0.001 |
| COPD, % | 4.4 | 0.0304 | 3.7 | 5.3 | 4.9 | 11.0 | <0.001 | |
| Cancer, % | 9.3 | 0.3014 | 9.0 | 9.7 | 9.0 | 18.6 | <0.001 | |
| Advanced Fibrosis | ||||
|---|---|---|---|---|
| (OR) | NAFLD | Total | NAFLD | |
| Model 1 | MMA | 1.21 (1.10, 1.34) ** | 2.86 (2.43, 3.38) ** | 3.08 (2.40, 3.95) ** |
| HCY | 1.95 (1.66, 2.29) ** | 7.86 (6.08, 10.16) ** | 6.81 (4.63, 10.01) ** | |
| RBC-folate | 1.56 (1.32, 1.84) ** | 2.32 (1.77, 3.04) ** | 2.17 (1.49, 3.16) ** | |
| Model 2 | MMA | 0.95 (0.85, 1.07) | 1.60 (1.34, 1.91) ** | 1.73 (1.38, 2.16) ** |
| HCY | 1.12 (0.93, 1.34) | 3.03 (1.99, 4.62) ** | 2.46 (1.55, 3.89) ** | |
| RBC-folate | 1.42 (1.20, 1.67) ** | 1.09 (0.83, 1.44) | 1.13 (0.78, 1.63) | |
| Model 3 | MMA | 1.13 (0.95, 1.34) | 1.32 (1.05, 1.66) * | 1.36 (1.05, 1.77) * |
| HCY | 0.84 (0.55, 1.28) | 2.29 (1.35, 3.89) * | 1.77 (1.07, 2.93) * | |
| RBC-folate | 1.58 (1.20, 2.07) * | 1.05 (0.84, 1.31) | 1.27 (0.91, 1.77) | |
| Model 4 | MMA | 1.10 (0.89, 1.36) | 1.41 (1.10, 1.80) * | 1.39 (1.04, 1.85) * |
| HCY | 0.75 (0.47, 1.18) | 2.76 (1.49, 5.11) * | 1.95 (1.09, 3.46) * | |
| RBC-folate | 2.24 (1.58, 3.18) ** | 1.20 (0.90, 1.60) | 1.34 (0.89, 2.01) | |
| Model 5 | RBC-folate | 2.46 (1.72, 3.53) ** | 1.18 (0.88, 1.57) | 1.33 (0.88, 1.99) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Huang, Q.; Yang, L.; Zhang, R.; Gao, L.; Han, X.; Ji, L.; Zou, X. The Association between Non-Alcoholic Fatty Liver Disease (NAFLD) and Advanced Fibrosis with Serological Vitamin B12 Markers: Results from the NHANES 1999–2004. Nutrients 2022, 14, 1224. https://doi.org/10.3390/nu14061224
Li L, Huang Q, Yang L, Zhang R, Gao L, Han X, Ji L, Zou X. The Association between Non-Alcoholic Fatty Liver Disease (NAFLD) and Advanced Fibrosis with Serological Vitamin B12 Markers: Results from the NHANES 1999–2004. Nutrients. 2022; 14(6):1224. https://doi.org/10.3390/nu14061224
Chicago/Turabian StyleLi, Li, Qi Huang, Linjian Yang, Rui Zhang, Leili Gao, Xueyao Han, Linong Ji, and Xiantong Zou. 2022. "The Association between Non-Alcoholic Fatty Liver Disease (NAFLD) and Advanced Fibrosis with Serological Vitamin B12 Markers: Results from the NHANES 1999–2004" Nutrients 14, no. 6: 1224. https://doi.org/10.3390/nu14061224