The Effect of Oral Probiotics (Streptococcus Salivarius k12) on the Salivary Level of Secretory Immunoglobulin A, Salivation Rate, and Oral Biofilm: A Pilot Randomized Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval
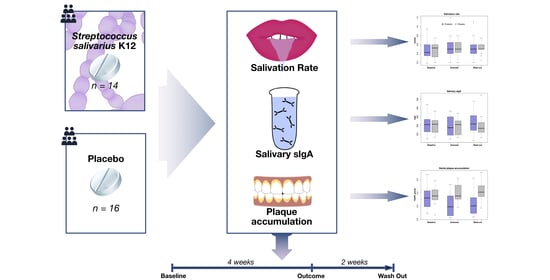
2.2. Study Design
2.3. Sampling Criteria
2.3.1. Inclusion Criteria
- Permanent dentition;
- Presence of more than 20 teeth;
- Absence of systemic and chronic diseases.
2.3.2. Exclusion Criteria
- More than 5 cavities requiring treatment;
- Refusal to sign informed consent;
- Taking supplements or lozenges containing probiotics or prebiotics 3 weeks before the study;
- Taking antibiotics (within 1 month before the study);
- Orthodontic and prosthetic treatment;
- Allergy to the components of the drugs used in the study;
- Use of other hygiene products, immunostimulants and antibacterials, probiotics, or prebiotics during the study;
- Refusal to take a given medication;
- Failure to attend check-ups.
2.4. Randomization
2.5. Interventions
2.6. Outcomes
2.7. Statistical Analysis
2.8. Data Management
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, F.; Liang, T.; Zhang, Z.; Liu, L.; Li, J.; Dong, W.; Zhang, H.; Bai, S.; Ma, L.; Kang, L. Effects of Altitude on Human Oral Microbes. AMB Express 2021, 11, 41. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, T.; Oge, S.; Nakata, S.; Ueno, Y.; Ukita, H.; Kousaka, R.; Miura, Y.; Yoshinari, N.; Yoshida, A. Gemella Haemolysans Inhibits the Growth of the Periodontal Pathogen Porphyromonas Gingivalis. Sci. Rep. 2021, 11, 11742. [Google Scholar] [CrossRef] [PubMed]
- Bowen, W.; Burne, R.; Wu, H.; Koo, H. Oral Biofilms: Pathogens, Matrix, and Polymicrobial Interactions in Microenvironments. Trends Microbiol. 2018, 26, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, M.; Coelho, A.; Lima, R.; Amaro, I.; Paula, A.; Marto, C.; Sousa, J.; Spagnuolo, G.; Marques Ferreira, M.; Carrilho, E. Efficacy and Patient’s Acceptance of Alternative Methods for Caries Removal—A Systematic Review. J. Clin. Med. 2020, 9, 3407. [Google Scholar] [CrossRef]
- Chokshi, A.; Mahesh, P.; Sharada, P.; Chokshi, K.; Anupriya, S.; Ashwini, B. A Correlative Study of the Levels of Salivary Streptococcus Mutans, Lactobacilli and Actinomyces with Dental Caries Experience in Subjects with Mixed and Permanent Dentition. J. Oral Maxillofac. Pathol. 2016, 20, 25. [Google Scholar] [CrossRef] [Green Version]
- Manmontri, C.; Nirunsittirat, A.; Piwat, S.; Wattanarat, O.; Pahumunto, N.; Makeudom, A.; Sastraruji, T.; Krisanaprakornkit, S.; Teanpaisan, R. Reduction of Streptococcus Mutans by Probiotic Milk: A Multicenter Randomized Controlled Trial. Clin. Oral Investig. 2020, 24, 2363–2374. [Google Scholar] [CrossRef]
- World Health Organization. Food and Agriculture Organization of the United Nations Probiotics in Food Health and Nutritional Properties and Guidelines for Evaluation. FAO Food Nutr. Pap. 2006, 85, 1–50. [Google Scholar]
- Chudzik, A.; Orzyłowska, A.; Rola, R.; Stanisz, G. Probiotics, Prebiotics and Postbiotics on Mitigation of Depression Symptoms: Modulation of the Brain–Gut–Microbiome Axis. Biomol. 2021, 11, 1000. [Google Scholar] [CrossRef]
- Vitellio, P.; Celano, G.; Bonfrate, L.; Gobbetti, M.; Portincasa, P.; de Angelis, M. Effects of Bifidobacterium Longum and Lactobacillus Rhamnosus on Gut Microbiota in Patients with Lactose Intolerance and Persisting Functional Gastrointestinal Symptoms: A Randomised, Double-Blind, Cross-over Study. Nutrients 2019, 11, 886. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, R.; Pilmann Laursen, R.; Bruun, S.; Larnkjær, A.; Mølgaard, C.; Michaelsen, K.; Høst, A. Probiotics in Late Infancy Reduce the Incidence of Eczema: A Randomized Controlled Trial. Pediatr. Allergy Immunol. 2019, 30, 335–340. [Google Scholar] [CrossRef]
- Campanella, V.; Syed, J.; Santacroce, L.; Saini, R.; Ballini, A.; Inchingolo, F. Oral Probiotics Influence Oral and Respiratory Tract Infections in Pediatric Population: A Randomized Double-Blinded Placebo-Controlled Pilot Study. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 8034–8041. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wang, B.; Li, Z.; Li, Y.; Liang, J. Alterations of Intestinal Flora and the Effects of Probiotics in Children with Recurrent Respiratory Tract Infection. World J. Pediatr. 2019, 15, 255–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, C.; Kleinman, J. Effect of Microbial Interactions on in Vitro Plaque Formation by Streptococcus Mutans. J. Dent. Res. 1974, 53, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Zare Javid, A.; Amerian, E.; Basir, L.; Ekrami, A.; Haghighizadeh, M.; Maghsoumi-Norouzabad, L. Effects of the Consumption of Probiotic Yogurt Containing Bifidobacterium Lactis Bb12 on the Levels of Streptococcus Mutans and Lactobacilli in Saliva of Students with Initial Stages of Dental Caries: A Double-Blind Randomized Controlled Trial. Caries Res. 2020, 54, 68–74. [Google Scholar] [CrossRef]
- Patil, R.; Dastoor, P.; Unde, M. Comparative Evaluation of Antimicrobial Effectiveness of Probiotic Milk and Fluoride Mouthrinse on Salivary Streptococcus Mutans Counts and Plaque Scores in Children—An in Vivo Experimental Study. J. Indian Soc. Pedod. Prev. Dent. 2019, 37, 378. [Google Scholar] [CrossRef]
- Doppalapudi, R.; Vundavalli, S.; Prabhat, M. Effect of Probiotic Bacteria on Oral Candida in Head- and Neck-Radiotherapy Patients: A Randomized Clinical Trial. J. Cancer Res. Ther. 2020, 16, 470–477. [Google Scholar] [CrossRef]
- Miyazima, T.; Ishikawa, K.; Mayer, M.; Saad, S.; Nakamae, A. Cheese Supplemented with Probiotics Reduced the Candida Levels in Denture Wearers-RCT. Oral Dis. 2017, 23, 919–925. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Rodrigues, C.; Stojanović-Radić, Z.; Dimitrijević, M.; Aleksić, A.; Neffe-Skocińska, K.; Zielińska, D.; Kołożyn-Krajewska, D.; Salehi, B.; Milton Prabu, S.; et al. Probiotics: Versatile Bioactive Components in Promoting Human Health. Medicina 2020, 56, 433. [Google Scholar] [CrossRef]
- Mu, Q.; Tavella, V.; Luo, X. Role of Lactobacillus Reuteri in Human Health and Diseases. Front. Microbiol. 2018, 9, 757. [Google Scholar] [CrossRef]
- Romani Vestman, N.; Chen, T.; Lif Holgerson, P.; Öhman, C.; Johansson, I. Oral Microbiota Shift after 12-Week Supplementation with Lactobacillus Reuteri DSM 17938 and PTA 5289; A Randomized Control Trial. PLoS ONE 2015, 10, e0125812. [Google Scholar] [CrossRef]
- Chen, X.; Daliri, E.; Kim, N.; Kim, J.; Yoo, D.; Oh, D. Microbial Etiology and Prevention of Dental Caries: Exploiting Natural Products to Inhibit Cariogenic Biofilms. Pathogens 2020, 9, 569. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.; López-López, A.; Nicolescu, T.; Perez-Vilaplana, S.; Boix-Amorós, A.; Dzidic, M.; Garcia, S.; Artacho, A.; Llena, C.; Mira, A. Topic Application of the Probiotic Streptococcus Dentisani Improves Clinical and Microbiological Parameters Associated With Oral Health. Front. Cell. Infect. Microbiol. 2020, 10, 465. [Google Scholar] [CrossRef] [PubMed]
- Vaisberg, M.; Paixão, V.; Almeida, E.; Santos, J.; Foster, R.; Rossi, M.; Pithon-Curi, T.; Gorjão, R.; Momesso, C.; Andrade, M.; et al. Daily Intake of Fermented Milk Containing Lactobacillus Casei Shirota (Lcs) Modulates Systemic and Upper Airways Immune/Inflammatory Responses in Marathon Runners. Nutrients 2019, 11, 1678. [Google Scholar] [CrossRef] [Green Version]
- Pahumunto, N.; Sophatha, B.; Piwat, S.; Teanpaisan, R. Increasing Salivary IgA and Reducing Streptococcus Mutans by Probiotic Lactobacillus Paracasei SD1: A Double-Blind, Randomized, Controlled Study. J. Dent. Sci. 2019, 14, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Malyshev, M.; Iordanishvili, A.; Prisyazhnyuk, O.; Bumai, A. The Effect of Probiotics on the Secretory Immunity of Saliva in Patients with Type 2 Diabetes. Stomatologiya 2019, 98, 26. [Google Scholar] [CrossRef] [PubMed]
- Herich, R. Is the Role of IgA in Local Immunity Completely Known? Food Agric. Immunol. 2017, 28, 223–237. [Google Scholar] [CrossRef] [Green Version]
- Soesilawati, P.; Notopuro, H.; Yuliati, Y.; Ariani, M.; Alwino Bayu Firdauzy, M. The Role of Salivary SIgA as Protection for Dental Caries Activity in Indonesian Children. Clin. Cosmet. Investig. Dent. 2019, 11, 291–295. [Google Scholar] [CrossRef] [Green Version]
- Bustamante, M.; Oomah, B.; Mosi-Roa, Y.; Rubilar, M.; Burgos-Díaz, C. Probiotics as an Adjunct Therapy for the Treatment of Halitosis, Dental Caries and Periodontitis. Probiotics Antimicrob. Proteins 2020, 12, 325–334. [Google Scholar] [CrossRef]
- Abranches, J.; Zeng, L.; Kajfasz, J.; Palmer, S.; Chakraborty, B.; Wen, Z.; Richards, V.; Brady, L.; Lemos, J. Biology of Oral Streptococci. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef]
- Mignolet, J.; Fontaine, L.; Kleerebezem, M.; Hols, P. Complete Genome Sequence of Streptococcus Salivarius HSISS4, a Human Commensal Bacterium Highly Prevalent in the Digestive Tract. Genome Announc. 2016, 4, e01637-15. [Google Scholar] [CrossRef] [Green Version]
- Poorni, S.; Srinivasan, M.; Nivedhitha, M. Probiotic Streptococcus Strains in Caries Prevention: A Systematic Review. J. Conserv. Dent. 2019, 22, 123. [Google Scholar] [CrossRef]
- Tanzer, J.; Kurasz, A.; Clive, J. Inhibition of Ecological Emergence of Mutans Streptococci Naturally Transmitted between Rats and Consequent Caries Inhibition by Streptococcus Salivarius TOVE-R Infection. Infect. Immun. 1985, 49, 76–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delorme, C.; Abraham, A.; Renault, P.; Guédon, E. Genomics of Streptococcus Salivarius, a Major Human Commensal. Infect. Genet. Evol. 2015, 33, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.; Chan, Y.; Lévesque, C. Complete Genome Sequence of Megaplasmid-Bearing Streptococcus Salivarius Strain LAB813, Isolated from the Dental Plaque of a Caries-Free Child. Microbiol. Resour. Announc. 2019, 8, e01092-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manning, J.; Dunne, E.; Wescombe, P.; Hale, J.; Mulholland, E.; Tagg, J.; Robins-Browne, R.; Satzke, C. Investigation of Streptococcus Salivarius-Mediated Inhibition of Pneumococcal Adherence to Pharyngeal Epithelial Cells. BMC Microbiol. 2016, 16, 225. [Google Scholar] [CrossRef] [Green Version]
- Yoo, H.; Jwa, S.; Kim, D.; Ji, Y. Inhibitory Effect of Streptococcus Salivarius K12 and M18 on Halitosis In Vitro. Clin. Exp. Dent. Res. 2020, 6, 207–214. [Google Scholar] [CrossRef] [Green Version]
- Mokhtar, M.; Rismayuddin, N.; Mat Yassim, A.; Ahmad, H.; Abdul Wahab, R.; Dashper, S.; Arzmi, M. Streptococcus Salivarius K12 Inhibits Candida Albicans Aggregation, Biofilm Formation and Dimorphism. Biofouling 2021, 37, 767–776. [Google Scholar] [CrossRef]
- Dodoo, C.; Stapleton, P.; Basit, A.; Gaisford, S. The Potential of Streptococcus Salivarius Oral Films in the Management of Dental Caries: An Inkjet Printing Approach. Int. J. Pharm. 2020, 591, 119962. [Google Scholar] [CrossRef]
- di Pierro, F.; Zanvit, A.; Nobili, P.; Risso, P.; Fornaini, C. Cariogram Outcome after 90 Days of Oral Treatment with Streptococcus Salivarius M18 in Children at High Risk for Dental Caries: Results of a Randomized, Controlled Study. Clin. Cosmet. Investig. Dent. 2015, 7, 107. [Google Scholar] [CrossRef] [Green Version]
- Zupancic, K.; Kriksic, V.; Kovacevic, I.; Kovacevic, D. Influence of Oral Probiotic Streptococcus Salivarius K12 on Ear and Oral Cavity Health in Humans: Systematic Review. Probiotics Antimicrob. Proteins 2017, 9, 102–110. [Google Scholar] [CrossRef]
- Turesky, S.; Gilmore, N.; Glickman, I. Reduced Plaque Formation by the Chloromethyl Analogue of Victamine C. J. Periodontol. 1970, 41, 41–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuki, O.; Furutani, C.; Mizota, Y.; Wakita, A.; Mimura, S.; Kihara, T.; Ohara, M.; Okada, Y.; Okada, M.; Nikawa, H. Effect of Bovine Milk Fermented with Lactobacillus Rhamnosus L8020 on Periodontal Disease in Individuals with Intellectual Disability: A Randomized Clinical Trial. J. Appl. Oral Sci. 2019, 27, e20180564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, P.; Baez, R. Oral Health Surveys Basic Methods, 5th ed.; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Ericson, D.; Hamberg, K.; Bratthall, G.; Sinkiewicz-Enggren, G.; Ljunggren, L. Salivary IgA Response to Probiotic Bacteria and Mutans Streptococci after the Use of Chewing Gum Containing Lactobacillus Reuteri. Pathog. Dis. 2013, 68, 82–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, A.; Pyne, D.; Saunders, P.; Fricker, P. Oral Administration of the Probiotic Lactobacillus Fermentum VRI-003 and Mucosal Immunity in Endurance Athletes. Br. J. Sports Med. 2010, 44, 222–226. [Google Scholar] [CrossRef] [Green Version]
- Invernici, M.; Furlaneto, F.; Salvador, S.; Ouwehand, A.; Salminen, S.; Mantziari, A.; Vinderola, G.; Ervolino, E.; Santana, S.; Silva, P.; et al. Bifidobacterium Animalis Subsp Lactis HN019 Presents Antimicrobial Potential against Periodontopathogens and Modulates the Immunological Response of Oral Mucosa in Periodontitis Patients. PLoS ONE 2020, 15, e0238425. [Google Scholar] [CrossRef]
- Braathen, G.; Ingildsen, V.; Twetman, S.; Ericson, D.; Jørgensen, M. Presence of Lactobacillus Reuteri in Saliva Coincide with Higher Salivary IgA in Young Adults after Intake of Probiotic Lozenges. Benef. Microbes 2017, 8, 17–22. [Google Scholar] [CrossRef] [Green Version]
- Surono, I.; Koestomo, F.; Novitasari, N.; Zakaria, F.; Yulianasari, K. Novel Probiotic Enterococcus Faecium IS-27526 Supplementation Increased Total Salivary SIgA Level and Bodyweight of Pre-School Children: A Pilot Study. Anaerobe 2011, 17, 496–500. [Google Scholar] [CrossRef]
- Kotani, Y.; Shinkai, S.; Okamatsu, H.; Toba, M.; Ogawa, K.; Yoshida, H.; Fukaya, T.; Fujiwara, Y.; Chaves, P.; Kakumoto, K.; et al. Oral Intake of Lactobacillus Pentosus Strain B240 Accelerates Salivary Immunoglobulin A Secretion in the Elderly: A Randomized, Placebo-Controlled, Double-Blind Trial. Immun. Ageing 2010, 7, 11. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.; Kuo, Y.; Chen, C.; Huang, Y.; Hsu, C.; Lin, J.; Liu, C.; Chen, J.; Hsia, K.; Ho, H. Viable and Heat-Killed Probiotic Strains Improve Oral Immunity by Elevating the IgA Concentration in the Oral Mucosa. Curr. Microbiol. 2021, 78, 3541–3549. [Google Scholar] [CrossRef]
- Harbige, L.; Pinto, E.; Allgrove, J.; Thomas, L. Immune Response of Healthy Adults to the Ingested Probiotic Lactobacillus Casei Shirota. Scand. J. Immunol. 2016, 84, 353–364. [Google Scholar] [CrossRef] [Green Version]
- Lefevre, M.; Racedo, S.; Ripert, G.; Housez, B.; Cazaubiel, M.; Maudet, C.; Jüsten, P.; Marteau, P.; Urdaci, M. Probiotic Strain Bacillus Subtilis CU1 Stimulates Immune System of Elderly during Common Infectious Disease Period: A Randomized, Double-Blind Placebo-Controlled Study. Immun. Ageing 2015, 12, 24. [Google Scholar] [CrossRef] [Green Version]
- Paineau, D.; Carcano, D.; Leyer, G.; Darquy, S.; Alyanakian, M.; Simoneau, G.; Bergmann, J.; Brassart, D.; Bornet, F.; Ouwehand, A. Effects of Seven Potential Probiotic Strains on Specific Immune Responses in Healthy Adults: A Double-Blind, Randomized, Controlled Trial. FEMS Immunol. Med. Microbiol. 2008, 53, 107–113. [Google Scholar] [CrossRef] [Green Version]
- Jørgensen, M.; Keller, M.; Kragelund, C.; Hamberg, K.; Ericson, D.; Nielsen, C.; Twetman, S. Lactobacillus Reuteri Supplements Do Not Affect Salivary IgA or Cytokine Levels in Healthy Subjects: A Randomized, Double-Blind, Placebo-Controlled, Cross-over Trial. Acta Odontol. Scand. 2016, 74, 399–404. [Google Scholar] [CrossRef]
- Valle, M.; Vieira, I.; Fino, L.; Gallina, D.; Esteves, A.; da Cunha, D.; Cabral, L.; Benatti, F.; Marostica Junior, M.; Batista, Â.; et al. Immune Status, Well-Being and Gut Microbiota in Military Supplemented with Synbiotic Ice Cream and Submitted to Field Training: A Randomised Clinical Trial. Br. J. Nutr. 2021, 126, 1–15. [Google Scholar] [CrossRef]
- Gill, S.; Teixeira, A.; Rosado, F.; Cox, M.; Costa, R. High-Dose Probiotic Supplementation Containing Lactobacillus Casei for 7 Days Does Not Enhance Salivary Antimicrobial Protein Responses to Exertional Heat Stress Compared With Placebo. Int. J. Sport Nutr. Exerc. Metab. 2016, 26, 150–160. [Google Scholar] [CrossRef]
- Childs, C.; Röytiö, H.; Alhoniemi, E.; Fekete, A.; Forssten, S.; Hudjec, N.; Lim, Y.; Steger, C.; Yaqoob, P.; Tuohy, K.; et al. Xylo-Oligosaccharides Alone or in Synbiotic Combination with Bifidobacterium Animalis Subsp. Lactis Induce Bifidogenesis and Modulate Markers of Immune Function in Healthy Adults: A Double-Blind, Placebo-Controlled, Randomised, Factorial Cross-over Study. Br. J. Nutr. 2014, 111, 1945–1956. [Google Scholar] [CrossRef] [Green Version]
- Ebrahimpour-Koujan, S.; Milajerdi, A.; Larijani, B.; Esmaillzadeh, A. Effects of Probiotics on Salivary Cytokines and Immunoglobulines: A Systematic Review and Meta-Analysis on Clinical Trials. Sci. Rep. 2020, 10, 11800. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Saruta, J.; Takahashi, T.; To, M.; Shimizu, T.; Hayashi, T.; Morozumi, T.; Kubota, N.; Kamata, Y.; Makino, S.; et al. Effect of Ingesting Yogurt Fermented with Lactobacillus Delbrueckii Ssp. Bulgaricus OLL1073R-1 on Influenza Virus-Bound Salivary IgA in Elderly Residents of Nursing Homes: A Randomized Controlled Trial. Acta Odontol. Scand. 2019, 77, 517–524. [Google Scholar] [CrossRef]
- Sanghvi, U.; Chhabra, T.; Sethuraman, R. Effect of Probiotics on the Amount and PH of Saliva in Edentulous Patients: A Prospective Study. J. Indian Prosthodont. Soc. 2018, 18, 277. [Google Scholar] [CrossRef]
- Ibrahim, N.; Ooi, F.; Chen, C.; Muhamad, A. Effects of Probiotics Supplementation and Circuit Training on Immune Responses among Sedentary Young Males. J. Sports Med. Phys. Fit. 2018, 58, 1102–1109. [Google Scholar] [CrossRef]
- Alp, S.; Baka, Z. Effects of Probiotics on Salivary Streptecoccus Mutans and Lactobacillus Levels in Orthodontic Patients. Am. J. Orthod. Dentofac. Orthop. 2018, 154, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Jäsberg, H.; Tervahartiala, T.; Sorsa, T.; Söderling, E.; Haukioja, A. Probiotic Intervention Influences the Salivary Levels of Matrix Metalloproteinase (MMP)-9 and Tissue Inhibitor of Metalloproteinases (TIMP)-1 in Healthy Adults. Arch. Oral Biol. 2018, 85, 58–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishihara, T.; Suzuki, N.; Yoneda, M.; Hirofuji, T. Effects of Lactobacillus Salivarius-Containing Tablets on Caries Risk Factors: A Randomized Open-Label Clinical Trial. BMC Oral Health 2014, 14, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamuro, K.; Kotani, Y.; Toba, M.; Kakumoto, K.; Kohda, N. Comparison of Salivary IgA Secretion Rate Collected by the Aspiration Method and Swab Method. Biosci. Microbiota Food Health 2013, 32, 107–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burton, J.; Drummond, B.; Chilcott, C.; Tagg, J.; Thomson, W.; Hale, J.; Wescombe, P. Influence of the Probiotic Streptococcus Salivarius Strain M18 on Indices of Dental Health in Children: A Randomized Double-Blind, Placebo-Controlled Trial. J. Med. Microbiol. 2013, 62, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Nadelman, P.; Magno, M.; Masterson, D.; da Cruz, A.; Maia, L. Are Dairy Products Containing Probiotics Beneficial for Oral Health? A Systematic Review and Meta-Analysis. Clin. Oral Investig. 2018, 22, 2763–2785. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, S.; Lauritano, D.; Candotto, V.; Silvestre, F.; Nardi, G. Oral Probiotics in the Management of Gingivitis in Diabetic Patients: A Double Blinded Randomized Controlled Study. J. Biol. Regul. Homeost. Agents 2017, 31, 197–202. [Google Scholar]
- Gruner, D.; Paris, S.; Schwendicke, F. Probiotics for Managing Caries and Periodontitis: Systematic Review and Meta-Analysis. J. Dent. 2016, 48, 16–25. [Google Scholar] [CrossRef]
- Montero, E.; Iniesta, M.; Rodrigo, M.; Marín, M.; Figuero, E.; Herrera, D.; Sanz, M. Clinical and Microbiological Effects of the Adjunctive Use of Probiotics in the Treatment of Gingivitis: A Randomized Controlled Clinical Trial. J. Clin. Periodontol. 2017, 44, 708–716. [Google Scholar] [CrossRef]
- Cogulu, D.; Sabah, E.; Kutukculer, N.; Ozkinay, F. Evaluation of the Relationship between Caries Indices and Salivary Secretory IgA, Salivary PH, Buffering Capacity and Flow Rate in Children with Down’s Syndrome. Arch. Oral Biol. 2006, 51, 23–28. [Google Scholar] [CrossRef]
- Wu, Z.; Gong, Y.; Wang, C.; Lin, J.; Zhao, J. Association between Salivary S-IgA Concentration and Dental Caries: A Systematic Review and Meta-Analysis. Biosci. Rep. 2020, 40, BSR20203208. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Goel, M.; Nagpal, R.; Kar, A.; Rapsang, E.; Matani, P. Evaluation of Total Salivary Secretory Immunoglobulin A and Mi/Fans-Specific SIgA among Children Having Dissimilar Caries Status. J. Contemp. Dent. Pract. 2018, 19, 651–655. [Google Scholar] [PubMed]
- Matos-Gomes, N.; Katsurayama, M.; Makimoto, F.; Santana, L.; Paredes-Garcia, E.; Becker, M.; Dos-Santos, M. Psychological Stress and Its Influence on Salivary Flow Rate, Total Protein Concentration and IgA, IgG and IgM Titers. Neuroimmunomodulation 2010, 17, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Ke, J.; Chung, C.; Chen, C.; Hwang, T.; Chou, M.; Wong, A.; Hu, C.; Lee, Y. Relationship between Unstimulated Salivary Flow Rate and Saliva Composition of Healthy Children in Taiwan. Chang. Gung Med. J. 2008, 31, 281–286. [Google Scholar]
- Brandtzaeg, P. Human Secretory Immunoglobulins. VII. Concentrations of parotid IgA and other secretory proteins in relation to the rate of flow and duration of secretory stimulus. Arch. Oral Biol. 1971, 16, 1295–1310. [Google Scholar] [CrossRef]
- Bratthall, D.; Gahnberg, L.; Krasse, B. Method for Detecting IgA Antibodies to Streptococcus Mutans Serotypes in Parotid Saliva. Arch. Oral Biol. 1978, 23, 843–849. [Google Scholar] [CrossRef]
- Rockenbach, M.; Marinho, S.; Veeck, E.; Lindemann, L.; Shinkai, R. Salivary Flow Rate, PH, and Concentrations of Calcium, Phosphate, and SIgA in Brazilian Pregnant and Non-Pregnant Women. Head Face Med. 2006, 2, 44. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, Y.; Mizoguchi, H.; Masamura, K.; Nagaya, T. No Relationship of Salivary Flow Rate or Secretory Immunoglobulin A to Dental Caries in Children. Environ. Health Prev. Med. 1997, 2, 122–125. [Google Scholar] [CrossRef] [Green Version]
- Gråhn, E.; Tenovuo, J.; Lehtonen, O.; Eerola, E.; Vilja, P. Antimicrobial Systems of Human Whole Saliva in Relation to Dental Caries, Cariogenic Bacteria, and Gingival Inflammation in Young Adults. Acta Odontol. Scand. 1988, 46, 67–74. [Google Scholar] [CrossRef]
- Ørstavik, D.; Brandtzaeg, P. Secretion of Parotid IgA in Relation to Gingival Inflammation and Dental Caries Experience in Man. Arch. Oral Biol. 1975, 20, 701–704. [Google Scholar] [CrossRef]
- Kugler, J.; Hess, M.; Haake, D. Secretion of Salivary Immunoglobulin a in Relation to Age, Saliva Flow, Mood States, Secretion of Albumin, Cortisol, and Catecholamines in Saliva. J. Clin. Immunol. 1992, 12, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Ericson, D.; Bratthall, D.; Björck, L.; Kronvall, G. Β2-Microglobulin in Saliva and Its Relation to Flow Rate in Different Glands in Man. Arch. Oral Biol. 1982, 27, 679–682. [Google Scholar] [CrossRef]
- Singh, R.; Damle, S.; Chawla, A. Salivary Mutans Streptococci and Lactobacilli Modulations in Young Children on Consumption of Probiotic Ice-Cream Containing Bifidobacterium Lactis Bb12 and Lactobacillus Acidophilus La5. Acta Odontol. Scand. 2011, 69, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Twetman, S.; Keller, M.; Lee, L.; Yucel-Lindberg, T.; Pedersen, A. Effect of Probiotic Lozenges Containing Lactobacillus Reuteri on Oral Wound Healing: A Pilot Study. Benef. Microbes 2018, 9, 691–696. [Google Scholar] [CrossRef] [PubMed]


| Screening Visit | Baseline Visit | 4 Weeks | 6 Weeks | |
|---|---|---|---|---|
| Eligibility assessment: | ||||
| Inclusion | X | |||
| Exclusion | X | X | X | X |
| DMFT | X | X | X | X |
| TQHPI | X | X | X | X |
| Oral hygiene instructions | X | |||
| PMA | X | X | X | |
| Unstimulated salivary flow rate | X | X | X | |
| Salivary sIgA | X | X | X |
| Group | Dietary Supplement Composition | Intervention |
|---|---|---|
| Group 1—probiotic (“Bactoblis”) | Streptococcus salivarius K12 (≥1 × 109 CFU in 1 tablet), fructose (sweetener), maltodextrin, silicon dioxide, magnesium stearate (vegetable), flavoring (strawberry) | Dissolve the lozenges in the mouth once a day for 4 weeks |
| Group 2—placebo | Fructose (sweetener), maltodextrin, silicon dioxide, magnesium stearate (vegetable), flavoring (strawberry) |
| Parameter | Total (n = 30) | Probiotics (n = 14) | Placebo (n = 16) | Statistical Significance |
|---|---|---|---|---|
| Sex, n (%) | ||||
| Female | 27 (90) | 13 (93) | 14 (87.5) | p-value = 1.0 a |
| Male | 3 (10) | 1 (7) | 2 (12.5) | |
| Age | ||||
| m (sd) | 21.2 (0.8) | 21.4 (0.9) | 20.9 (0.6) | p-value = 0.171 b |
| Median [Q1; Q3] | 21 [21; 22] | 21 [21; 22] | 21 [20.75; 21] | |
| min-max | 20–24 | 21–24 | 20–22 | |
| DMFT | ||||
| Median [Q1; Q3] | 9 [6.25; 12.5] | 9 [7.5; 10.75] | 10.5 [5.75; 14] | p-value = 0.4892 b |
| min-max | 0–20 | 0–20 | 2–17 | |
| Decay | ||||
| Median [Q1; Q3] | 2.5 [2; 4] | 3.5 [2; 4] | 2 [2; 3] | p-value = 0.3389 b |
| min-max | 0–5 | 0–5 | 0–5 |
| Parameter | Probiotics (n = 14) | Placebo (n = 16) | Statistical Analysis |
|---|---|---|---|
| sIgA, mg/L, m (sd) | |||
| Baseline | 226 (130) | 205 (92) | Arm: F = 0.385, p-value = 0.54 |
| Outcome | 200 (113) | 191 (97) | Time: F = 0.572, p-value = 0.568 |
| Washout | 227 (119) | 196 (114) | Arm*Time: F = 0.16, p-value = 0.853 a |
| Salivation, mL/min, m (sd) | |||
| Baseline | 0.47 (0.20) | 0.48 (0.18) | Arm: F = 0.002, p-value = 0.969 |
| Outcome | 0.55 (0.25) | 0.53 (0.17) | Time: F = 2.952, p-value = 0.060 |
| Washout | 0.53 (0.22) | 0.53 (0.13) | Arm*Time: F = 0.234, p-value = 0.792 a |
| TQHPI, median [Q1; Q3] | |||
| Baseline | 2.8 [2.5; 3.1] | 2.9 [2.7; 3.1] | p-value = 0.5744 b |
| Outcome | 2.5 [2.2; 2.9] | 2.9 [2.8; 3.2] | p-value = 0.01114 b |
| Washout | 2.5 [2.3; 2.8] | 3.0 [2.9; 3.3] | p-value = 0.009286 b |
| Within-group comparisons | p-value = 0.02437 c | p-value = 0.1642 c | |
| PMA > 0, units, median [Q1; Q3] | n = 3 | n = 4 | |
| Baseline | 4 [3; 6.5] | 2.5 [2; 3.2] | p-value = 0.4587 b |
| Outcome | 0 [0; 1.5] | 3.5 [2.2; 4.2] | p-value = 0.2664 b |
| Washout | 0 [0; 0] | 3.5 [2.2; 4.2] | p-value = 0.1187 b |
| Within-group comparisons | p-value = 0.06081 c | p-value = 0.5836 c |
| Probiotics (n = 14) | Placebo (n = 16) | Significance b | |
|---|---|---|---|
| Level of sIgA | |||
| Baseline, n (%) | |||
| Low | 3 (21.5) | 4 (25.0) | p-value = 0.883 |
| Normal | 8 (57.0) | 10 (62.5) | |
| High | 3 (21.5) | 2 (12.5) | |
| Outcome, n (%) | |||
| Low | 4 (28.5) | 5 (31) | p-value = 0.8959 |
| Normal | 6 (43.0) | 8 (50) | |
| High | 4 (28.5) | 3 (19) | |
| Washout, n (%) | |||
| Low | 3 (21.5) | 2 (12.5) | p-value = 0.4369 |
| Normal | 7 (50.0) | 12 (75.0) | |
| High | 4 (28.5) | 2 (12.5) | |
| PMA | |||
| Baseline, n (%) | |||
| PMA = 0 | 11 (79) | 12 (75) | p-value = 1.0 |
| PMA > 0 | 3 (21) | 4 (25) | |
| Outcome, n (%) | |||
| PMA = 0 | 13 (93) | 13 (81) | p-value = 0.6015 |
| PMA > 0 | 1 (7) | 3 (19) | |
| Washout, n (%) | |||
| PMA = 0 | 14 (100) | 13 (81) | p-value = 0.2276 |
| PMA > 0 | - | 3 (19) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babina, K.; Salikhova, D.; Polyakova, M.; Svitich, O.; Samoylikov, R.; Ahmad El-Abed, S.; Zaytsev, A.; Novozhilova, N. The Effect of Oral Probiotics (Streptococcus Salivarius k12) on the Salivary Level of Secretory Immunoglobulin A, Salivation Rate, and Oral Biofilm: A Pilot Randomized Clinical Trial. Nutrients 2022, 14, 1124. https://doi.org/10.3390/nu14051124
Babina K, Salikhova D, Polyakova M, Svitich O, Samoylikov R, Ahmad El-Abed S, Zaytsev A, Novozhilova N. The Effect of Oral Probiotics (Streptococcus Salivarius k12) on the Salivary Level of Secretory Immunoglobulin A, Salivation Rate, and Oral Biofilm: A Pilot Randomized Clinical Trial. Nutrients. 2022; 14(5):1124. https://doi.org/10.3390/nu14051124
Chicago/Turabian StyleBabina, Ksenia, Dilara Salikhova, Maria Polyakova, Oxana Svitich, Roman Samoylikov, Samya Ahmad El-Abed, Alexandr Zaytsev, and Nina Novozhilova. 2022. "The Effect of Oral Probiotics (Streptococcus Salivarius k12) on the Salivary Level of Secretory Immunoglobulin A, Salivation Rate, and Oral Biofilm: A Pilot Randomized Clinical Trial" Nutrients 14, no. 5: 1124. https://doi.org/10.3390/nu14051124