Supplementing Soy-Based Diet with Creatine in Rats: Implications for Cardiac Cell Signaling and Response to Doxorubicin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
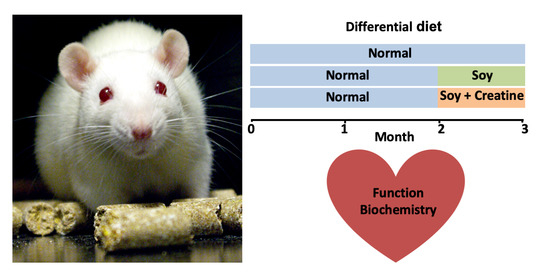
2.2. Animals
2.3. Rat Heart Perfusion
2.4. Metabolites, Oxidative Damage/Antioxidant Status, and DNA Integrity
2.5. Immunoblotting
2.6. Data Analysis
3. Results
3.1. Heart Function
3.2. Creatine and Adenylate Levels
3.3. Cell Signaling Pathways
3.4. Oxidative Damage, Antioxidant Status, and DNA Integrity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, L.W.; Demark-Wahnefried, W. Diet, exercise, and complementary therapies after primary treatment for cancer. Lancet Oncol. 2006, 7, 1017–1026. [Google Scholar] [CrossRef]
- Minotti, G.; Menna, P.; Salvatorelli, E.; Cairo, G.; Gianni, L. Anthracyclines: Molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol. Rev. 2004, 56, 185–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eschenhagen, T.; Force, T.; Ewer, M.S.; de Keulenaer, G.W.; Suter, T.M.; Anker, S.D.; Avkiran, M.; de Azambuja, E.; Balligand, J.L.; Brutsaert, D.L.; et al. Cardiovascular side effects of cancer therapies: A position statement from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2011, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gianni, L.; Herman, E.H.; Lipshultz, S.E.; Minotti, G.; Sarvazyan, N.; Sawyer, D.B. Anthracycline cardiotoxicity: From bench to bedside. J. Clin. Oncol. 2008, 26, 3777–3784. [Google Scholar] [CrossRef] [Green Version]
- Sterba, M.; Popelova, O.; Vavrova, A.; Jirkovsky, E.; Kovarikova, P.; Gersl, V.; Simunek, T. Oxidative stress, redox signaling, and metal chelation in anthracycline cardiotoxicity and pharmacological cardioprotection. Antioxid. Redox Signal. 2013, 18, 899–929. [Google Scholar] [CrossRef] [Green Version]
- Lipshultz, S.E.; Cochran, T.R.; Franco, V.I.; Miller, T.L. Treatment-related cardiotoxicity in survivors of childhood cancer. Nat. Rev. Clin. Oncol. 2013, 10, 697–710. [Google Scholar] [CrossRef]
- Lipshultz, S.E.; Karnik, R.; Sambatakos, P.; Franco, V.I.; Ross, S.W.; Miller, T.L. Anthracycline-related cardiotoxicity in childhood cancer survivors. Curr. Opin. Cardiol. 2014, 29, 103–112. [Google Scholar] [CrossRef]
- Jones, L.W.; Alfano, C.M. Exercise-oncology research: Past, present, and future. Acta Oncol. 2013, 52, 195–215. [Google Scholar] [CrossRef]
- Jones, L.W.; Dewhirst, M.W. Therapeutic properties of aerobic training after a cancer diagnosis: More than a one-trick pony? J. Natl. Cancer Inst. 2014, 106, dju042. [Google Scholar] [CrossRef] [Green Version]
- Scott, J.M.; Koelwyn, G.J.; Hornsby, W.E.; Khouri, M.; Peppercorn, J.; Douglas, P.S.; Jones, L.W. Exercise therapy as treatment for cardiovascular and oncologic disease after a diagnosis of early-stage cancer. Semin. Oncol. 2013, 40, 218–228. [Google Scholar] [CrossRef]
- Scott, J.M.; Lakoski, S.; Mackey, J.R.; Douglas, P.S.; Haykowsky, M.J.; Jones, L.W. The potential role of aerobic exercise to modulate cardiotoxicity of molecularly targeted cancer therapeutics. Oncologist 2013, 18, 221–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipshultz, S.E.; Adams, M.J.; Colan, S.D.; Constine, L.S.; Herman, E.H.; Hsu, D.T.; Hudson, M.M.; Kremer, L.C.; Landy, D.C.; Miller, T.L.; et al. Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: Pathophysiology, course, monitoring, management, prevention, and research directions: A scientific statement from the American Heart Association. Circulation 2013, 128, 1927–1995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kreider, R.B.; Stout, J.R. Creatine in Health and Disease. Nutrients 2021, 13, 447. [Google Scholar] [CrossRef] [PubMed]
- Wyss, M.; Kaddurah-Daouk, R. Creatine and creatinine metabolism. Physiol. Rev. 2000, 80, 1107–1213. [Google Scholar] [CrossRef] [PubMed]
- Hanna-El-Daher, L.; Braissant, O. Creatine synthesis and exchanges between brain cells: What can be learned from human creatine deficiencies and various experimental models? Amino Acids 2016, 48, 1877–1895. [Google Scholar] [CrossRef] [PubMed]
- Wallimann, T.; Tokarska-Schlattner, M.; Schlattner, U. The creatine kinase system and pleiotropic effects of creatine. Amino Acids 2011, 40, 1271–1296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlattner, U.; Tokarska-Schlattner, M.; Wallimann, T. Mitochondrial creatine kinase in human health and disease. Biochim. Biophys. Acta 2006, 1762, 164–180. [Google Scholar] [CrossRef]
- Lygate, C.A.; Bohl, S.; ten Hove, M.; Faller, K.M.; Ostrowski, P.J.; Zervou, S.; Medway, D.J.; Aksentijevic, D.; Sebag-Montefiore, L.; Wallis, J.; et al. Moderate elevation of intracellular creatine by targeting the creatine transporter protects mice from acute myocardial infarction. Cardiovasc. Res. 2012, 96, 466–475. [Google Scholar] [CrossRef] [Green Version]
- Balestrino, M. Role of Creatine in the Heart: Health and Disease. Nutrients 2021, 13, 1215. [Google Scholar] [CrossRef]
- Santos, R.V.; Batista, M.L., Jr.; Caperuto, E.C.; Costa Rosa, L.F. Chronic supplementation of creatine and vitamins C and E increases survival and improves biochemical parameters after Doxorubicin treatment in rats. Clin. Exp. Pharm. Physiol 2007, 34, 1294–1299. [Google Scholar] [CrossRef]
- Caretti, A.; Bianciardi, P.; Sala, G.; Terruzzi, C.; Lucchina, F.; Samaja, M. Supplementation of creatine and ribose prevents apoptosis in ischemic cardiomyocytes. Cell. Physiol. Biochem. 2010, 26, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Santacruz, L.; Darrabie, M.D.; Mantilla, J.G.; Mishra, R.; Feger, B.J.; Jacobs, D.O. Creatine supplementation reduces doxorubicin-induced cardiomyocellular injury. Cardiovasc. Toxicol. 2015, 15, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Fimognari, C.; Sestili, P.; Lenzi, M.; Bucchini, A.; Cantelli-Forti, G.; Hrelia, P. RNA as a new target for toxic and protective agents. Mutat. Res. 2008, 648, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Rohlfsen, C.; Leppo, M.K.; Chacko, V.P.; Wang, Y.; Steenbergen, C.; Weiss, R.G. Creatine kinase-overexpression improves myocardial energetics, contractile dysfunction and survival in murine doxorubicin cardiotoxicity. PLoS ONE 2013, 8, e74675. [Google Scholar] [CrossRef] [Green Version]
- Sestili, P.; Martinelli, C.; Colombo, E.; Barbieri, E.; Potenza, L.; Sartini, S.; Fimognari, C. Creatine as an antioxidant. Amino Acids 2011, 40, 1385–1396. [Google Scholar] [CrossRef]
- Tokarska-Schlattner, M.; Epand, R.F.; Meiler, F.; Zandomeneghi, G.; Neumann, D.; Widmer, H.R.; Meier, B.H.; Epand, R.M.; Saks, V.; Wallimann, T.; et al. Phosphocreatine interacts with phospholipids, affects membrane properties and exerts membrane-protective effects. PLoS ONE 2012, 7, e43178. [Google Scholar] [CrossRef] [Green Version]
- Messina, M.; Messina, V. The role of soy in vegetarian diets. Nutrients 2010, 2, 855–888. [Google Scholar] [CrossRef] [Green Version]
- Konhilas, J.P.; Leinwand, L.A. The effects of biological sex and diet on the development of heart failure. Circulation 2007, 116, 2747–2759. [Google Scholar] [CrossRef]
- Sacks, F.M.; Lichtenstein, A.; Van Horn, L.; Harris, W.; Kris-Etherton, P.; Winston, M.; for the American Heart Association Nutrition Committee. Soy protein, isoflavones, and cardiovascular health: An American Heart Association Science Advisory for professionals from the Nutrition Committee. Circulation 2006, 113, 1034–1044. [Google Scholar] [CrossRef] [Green Version]
- Erdman, J.W., Jr. AHA Science Advisory: Soy protein and cardiovascular disease: A statement for healthcare professionals from the Nutrition Committee of the AHA. Circulation 2000, 102, 2555–2559. [Google Scholar] [CrossRef] [Green Version]
- Tokarska-Schlattner, M.; Zaugg, M.; da Silva, R.; Lucchinetti, E.; Schaub, M.C.; Wallimann, T.; Schlattner, U. Acute toxicity of doxorubicin on isolated perfused heart: Response of kinases regulating energy supply. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H37–H47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tokarska-Schlattner, M.; Lucchinetti, E.; Zaugg, M.; Kay, L.; Gratia, S.; Guzun, R.; Saks, V.; Schlattner, U. Early effects of doxorubicin in perfused heart: Transcriptional profiling reveals inhibition of cellular stress response genes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R1075–R1088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gratia, S.; Kay, L.; Michelland, S.; Seve, M.; Schlattner, U.; Tokarska-Schlattner, M. Cardiac phosphoproteome reveals cell signaling events involved in doxorubicin cardiotoxicity. J. Proteom. 2012, 75, 4705–4716. [Google Scholar] [CrossRef] [PubMed]
- Gratia, S.; Kay, L.; Potenza, L.; Seffouh, A.; Novel-Chate, V.; Schnebelen, C.; Sestili, P.; Schlattner, U.; Tokarska-Schlattner, M. Inhibition of AMPK signalling by doxorubicin: At the crossroads of the cardiac responses to energetic, oxidative, and genotoxic stress. Cardiovasc. Res. 2012, 95, 290–299. [Google Scholar] [CrossRef] [Green Version]
- Hininger-Favier, I.; Benaraba, R.; Coves, S.; Anderson, R.A.; Roussel, A.M. Green tea extract decreases oxidative stress and improves insulin sensitivity in an animal model of insulin resistance, the fructose-fed rat. J. Am. Coll. Nutr. 2009, 28, 355–361. [Google Scholar] [CrossRef]
- Faure, P.; Lafond, J.L. Measurement of plasma sulfhydryl and carbonyl groups as a possible indicator of protein oxydation. In Analysis of Free Radicals in Biological Systems; Favier, A.E., Cadet, J., Kalnyanaraman, M., Fontecave, M., Pierre, J.L., Eds.; Birkäuser Basel: Boston, MA, USA; Berlin, Germany, 1995; pp. 237–248. [Google Scholar]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Richard, M.J.; Portal, B.; Meo, J.; Coudray, C.; Hadjian, A.; Favier, A. Malondialdehyde kit evaluated for determining plasma and lipoprotein fractions that react with thiobarbituric acid. Clin. Chem. 1992, 38, 704–709. [Google Scholar] [CrossRef]
- Tokarska-Schlattner, M.; Zaugg, M.; Zuppinger, C.; Wallimann, T.; Schlattner, U. New insights into doxorubicin-induced cardiotoxicity: The critical role of cellular energetics. J. Mol. Cell. Cardiol. 2006, 41, 389–405. [Google Scholar] [CrossRef]
- Tokarska-Schlattner, M.; Wallimann, T.; Schlattner, U. Multiple interference of anthracyclines with mitochondrial creatine kinases: Preferential damage of the cardiac isoenzyme and its implications for drug cardiotoxicity. Mol. Pharmacol. 2002, 61, 516–523. [Google Scholar] [CrossRef] [Green Version]
- Mousseau, M.; Faure, H.; Hininger, I.; Bayet-Robert, M.; Favier, A. Leukocyte 8-oxo-7,8-dihydro-2’-deoxyguanosine and comet assay in epirubicin-treated patients. Free Radic. Res. 2005, 39, 837–843. [Google Scholar] [CrossRef]
- Hardie, D.G. Sensing of energy and nutrients by AMP-activated protein kinase. Am. J. Clin. Nutr. 2011, 93, 891S–896S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawaguchi, T.; Takemura, G.; Kanamori, H.; Takeyama, T.; Watanabe, T.; Morishita, K.; Ogino, A.; Tsujimoto, A.; Goto, K.; Maruyama, R.; et al. Prior starvation mitigates acute doxorubicin cardiotoxicity through restoration of autophagy in affected cardiomyocytes. Cardiovasc. Res. 2012, 96, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Mitra, M.S.; Donthamsetty, S.; White, B.; Latendresse, J.R.; Mehendale, H.M. Mechanism of protection of moderately diet restricted rats against doxorubicin-induced acute cardiotoxicity. Toxicol. Appl. Pharm. 2007, 225, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Cederroth, C.R.; Vinciguerra, M.; Gjinovci, A.; Kuhne, F.; Klein, M.; Cederroth, M.; Caille, D.; Suter, M.; Neumann, D.; James, R.W.; et al. Dietary phytoestrogens activate AMP-activated protein kinase with improvement in lipid and glucose metabolism. Diabetes 2008, 57, 1176–1185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, C.E.; Yun, H.; Lee, E.B.; Min, B.I.; Bae, H.; Choe, W.; Kang, I.; Kim, S.S.; Ha, J. The antioxidant effects of genistein are associated with AMP-activated protein kinase activation and PTEN induction in prostate cancer cells. J. Med. Food 2010, 13, 815–820. [Google Scholar] [CrossRef]
- Hawley, S.A.; Ross, F.A.; Chevtzoff, C.; Green, K.A.; Evans, A.; Fogarty, S.; Towler, M.C.; Brown, L.J.; Ogunbayo, O.A.; Evans, A.M.; et al. Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab. 2010, 11, 554–565. [Google Scholar] [CrossRef] [Green Version]
- Hardie, D.G. AMP-activated protein kinase: An energy sensor that regulates all aspects of cell function. Genes Dev. 2011, 25, 1895–1908. [Google Scholar] [CrossRef] [Green Version]
- Eijnde, B.O.; Derave, W.; Wojtaszewski, J.F.; Richter, E.A.; Hespel, P. AMP kinase expression and activity in human skeletal muscle: Effects of immobilization, retraining, and creatine supplementation. J. Appl. Physiol. 2005, 98, 1228–1233. [Google Scholar] [CrossRef]
- Ceddia, R.B.; Sweeney, G. Creatine supplementation increases glucose oxidation and AMPK phosphorylation and reduces lactate production in L6 rat skeletal muscle cells. J. Physiol. 2004, 555, 409–421. [Google Scholar] [CrossRef]
- Petti, C.; Vegetti, C.; Molla, A.; Bersani, I.; Cleris, L.; Mustard, K.J.; Formelli, F.; Hardie, G.D.; Sensi, M.; Anichini, A. AMPK activators inhibit the proliferation of human melanomas bearing the activated MAPK pathway. Melanoma Res. 2012, 22, 341–350. [Google Scholar] [CrossRef]
- Hawley, S.A.; Ross, F.A.; Gowans, G.J.; Tibarewal, P.; Leslie, N.R.; Hardie, D.G. Phosphorylation by Akt within the ST loop of AMPK-alpha1 down-regulates its activation in tumour cells. Biochem. J. 2014, 459, 275–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esteve-Puig, R.; Canals, F.; Colome, N.; Merlino, G.; Recio, J.A. Uncoupling of the LKB1-AMPKalpha energy sensor pathway by growth factors and oncogenic BRAF. PLoS ONE 2009, 4, e4771. [Google Scholar] [CrossRef]
- Messina, M. A brief historical overview of the past two decades of soy and isoflavone research. J. Nutr. 2010, 140, 1350S–1354S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, H.; Bowen, R.; Cai, Q.; Barnes, S.; Wang, Y. Antioxidant and antipromotional effects of the soybean isoflavone genistein. Proc. Soc. Exp. Biol. Med. 1995, 208, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Polkowski, K.; Mazurek, A.P. Biological properties of genistein. A review of in vitro and in vivo data. Acta Pol. Pharm. 2000, 57, 135–155. [Google Scholar]
- Nakamura, Y.; Yogosawa, S.; Izutani, Y.; Watanabe, H.; Otsuji, E.; Sakai, T. A combination of indol-3-carbinol and genistein synergistically induces apoptosis in human colon cancer HT-29 cells by inhibiting Akt phosphorylation and progression of autophagy. Mol. Cancer 2009, 8, 100. [Google Scholar] [CrossRef] [Green Version]
- Gong, L.; Li, Y.; Nedeljkovic-Kurepa, A.; Sarkar, F.H. Inactivation of NF-kappaB by genistein is mediated via Akt signaling pathway in breast cancer cells. Oncogene 2003, 22, 4702–4709. [Google Scholar] [CrossRef] [Green Version]
- El Touny, L.H.; Banerjee, P.P. Akt GSK-3 pathway as a target in genistein-induced inhibition of TRAMP prostate cancer progression toward a poorly differentiated phenotype. Carcinogenesis 2007, 28, 1710–1717. [Google Scholar] [CrossRef] [Green Version]
- Deldicque, L.; Theisen, D.; Bertrand, L.; Hespel, P.; Hue, L.; Francaux, M. Creatine enhances differentiation of myogenic C2C12 cells by activating both p38 and Akt/PKB pathways. Am. J. Physiol. Cell Physiol. 2007, 293, C1263–C1271. [Google Scholar] [CrossRef] [Green Version]
- Hespel, P.; Derave, W. Ergogenic effects of creatine in sports and rehabilitation. Subcell. Biochem. 2007, 46, 245–259. [Google Scholar]
- Bonilla, D.A.; Moreno, Y.; Rawson, E.S.; Forero, D.A.; Stout, J.R.; Kerksick, C.M.; Roberts, M.D.; Kreider, R.B. A Convergent Functional Genomics Analysis to Identify Biological Regulators Mediating Effects of Creatine Supplementation. Nutrients 2021, 13, 2521. [Google Scholar] [CrossRef] [PubMed]
- Kreider, R.B.; Kalman, D.S.; Antonio, J.; Ziegenfuss, T.N.; Wildman, R.; Collins, R.; Candow, D.G.; Kleiner, S.M.; Almada, A.L.; Lopez, H.L. International Society of Sports Nutrition position stand: Safety and efficacy of creatine supplementation in exercise, sport, and medicine. J. Int. Soc. Sports Nutr. 2017, 14, 18. [Google Scholar] [CrossRef] [PubMed]
- Roschel, H.; Gualano, B.; Ostojic, S.M.; Rawson, E.S. Creatine Supplementation and Brain Health. Nutrients 2021, 13, 586. [Google Scholar] [CrossRef] [PubMed]
- Schlattner, U.; Mockli, N.; Speer, O.; Werner, S.; Wallimann, T. Creatine kinase and creatine transporter in normal, wounded, and diseased skin. J. Investig. Dermatol. 2002, 118, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Taniyama, Y.; Walsh, K. Elevated myocardial Akt signaling ameliorates doxorubicin-induced congestive heart failure and promotes heart growth. J. Mol. Cell. Cardiol. 2002, 34, 1241–1247. [Google Scholar] [CrossRef]
- Eijnde, B.O.; Lebacq, J.; Ramaekers, M.; Hespel, P. Effect of muscle creatine content manipulation on contractile properties in mouse muscles. Muscle Nerve 2004, 29, 428–435. [Google Scholar] [CrossRef]
- Sestili, P.; Barbieri, E.; Martinelli, C.; Battistelli, M.; Guescini, M.; Vallorani, L.; Casadei, L.; D’Emilio, A.; Falcieri, E.; Piccoli, G.; et al. Creatine supplementation prevents the inhibition of myogenic differentiation in oxidatively injured C2C12 murine myoblasts. Mol. Nutr. Food Res. 2009, 53, 1187–1204. [Google Scholar] [CrossRef]
- Boehm, E.; Chan, S.; Monfared, M.; Wallimann, T.; Clarke, K.; Neubauer, S. Creatine transporter activity and content in the rat heart supplemented by and depleted of creatine. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E399–E406. [Google Scholar] [CrossRef]
- Mahn, K.; Borras, C.; Knock, G.A.; Taylor, P.; Khan, I.Y.; Sugden, D.; Poston, L.; Ward, J.P.; Sharpe, R.M.; Vina, J.; et al. Dietary soy isoflavone induced increases in antioxidant and eNOS gene expression lead to improved endothelial function and reduced blood pressure in vivo. FASEB J. 2005, 19, 1755–1757. [Google Scholar] [CrossRef]
- Douglas, G.; Armitage, J.A.; Taylor, P.D.; Lawson, J.R.; Mann, G.E.; Poston, L. Cardiovascular consequences of life-long exposure to dietary isoflavones in the rat. J. Physiol. 2006, 571, 477–487. [Google Scholar] [CrossRef]
- Siow, R.C.; Mann, G.E. Dietary isoflavones and vascular protection: Activation of cellular antioxidant defenses by SERMs or hormesis? Mol. Asp. Med. 2010, 31, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Si, H.; Liu, D. Genistein, a soy phytoestrogen, upregulates the expression of human endothelial nitric oxide synthase and lowers blood pressure in spontaneously hypertensive rats. J. Nutr. 2008, 138, 297–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mozaffarian, D.; Appel, L.J.; Van Horn, L. Components of a cardioprotective diet: New insights. Circulation 2011, 123, 2870–2891. [Google Scholar] [CrossRef]
- Aksentijevic, D.; Zervou, S.; Faller, K.M.; McAndrew, D.J.; Schneider, J.E.; Neubauer, S.; Lygate, C.A. Myocardial creatine levels do not influence response to acute oxidative stress in isolated perfused heart. PLoS ONE 2014, 9, e109021. [Google Scholar] [CrossRef] [Green Version]
- Record, I.R.; Dreosti, I.E.; McInerney, J.K. The antioxidant activity of genistein. J. Nutr. Biochem. 1995, 6, 481–485. [Google Scholar] [CrossRef]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef] [Green Version]
- Borras, C.; Gambini, J.; Gomez-Cabrera, M.C.; Sastre, J.; Pallardo, F.V.; Mann, G.E.; Vina, J. Genistein, a soy isoflavone, up-regulates expression of antioxidant genes: Involvement of estrogen receptors, ERK1/2, and NFkappaB. FASEB J. 2006, 20, 2136–2138. [Google Scholar] [CrossRef]
- Zang, M.; Xu, S.; Maitland-Toolan, K.A.; Zuccollo, A.; Hou, X.; Jiang, B.; Wierzbicki, M.; Verbeuren, T.J.; Cohen, R.A. Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes 2006, 55, 2180–2191. [Google Scholar] [CrossRef] [Green Version]
- Davies, K.J. Oxidative stress, antioxidant defenses, and damage removal, repair, and replacement systems. IUBMB Life 2000, 50, 279–289. [Google Scholar] [CrossRef]
- Altieri, P.; Barisione, C.; Lazzarini, E.; Garuti, A.; Bezante, G.P.; Canepa, M.; Spallarossa, P.; Tocchetti, C.G.; Bollini, S.; Brunelli, C.; et al. Testosterone Antagonizes Doxorubicin-Induced Senescence of Cardiomyocytes. J. Am. Heart Assoc. 2016, 5, e002383. [Google Scholar] [CrossRef] [Green Version]
- Green, D.M.; Grigoriev, Y.A.; Nan, B.; Takashima, J.R.; Norkool, P.A.; D’Angio, G.J.; Breslow, N.E. Congestive heart failure after treatment for Wilms’ tumor: A report from the National Wilms’ Tumor Study group. J. Clin. Oncol. 2001, 19, 1926–1934. [Google Scholar] [CrossRef]
- Lipshultz, S.E.; Lipsitz, S.R.; Mone, S.M.; Goorin, A.M.; Sallan, S.E.; Sanders, S.P.; Orav, E.J.; Gelber, R.D.; Colan, S.D. Female sex and higher drug dose as risk factors for late cardiotoxic effects of doxorubicin therapy for childhood cancer. N. Engl. J. Med. 1995, 332, 1738–1743. [Google Scholar] [CrossRef]
- Moulin, M.; Piquereau, J.; Mateo, P.; Fortin, D.; Rucker-Martin, C.; Gressette, M.; Lefebvre, F.; Gresikova, M.; Solgadi, A.; Veksler, V.; et al. Sexual dimorphism of doxorubicin-mediated cardiotoxicity: Potential role of energy metabolism remodeling. Circ. Heart Fail. 2015, 8, 98–108. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Knapton, A.; Lipshultz, S.E.; Cochran, T.R.; Hiraragi, H.; Herman, E.H. Sex-related differences in mast cell activity and doxorubicin toxicity: A study in spontaneously hypertensive rats. Toxicol. Pathol. 2014, 42, 361–375. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kay, L.; Potenza, L.; Hininger-Favier, I.; Roth, H.; Attia, S.; Tellier, C.; Zuppinger, C.; Calcabrini, C.; Sestili, P.; Wallimann, T.; et al. Supplementing Soy-Based Diet with Creatine in Rats: Implications for Cardiac Cell Signaling and Response to Doxorubicin. Nutrients 2022, 14, 583. https://doi.org/10.3390/nu14030583
Kay L, Potenza L, Hininger-Favier I, Roth H, Attia S, Tellier C, Zuppinger C, Calcabrini C, Sestili P, Wallimann T, et al. Supplementing Soy-Based Diet with Creatine in Rats: Implications for Cardiac Cell Signaling and Response to Doxorubicin. Nutrients. 2022; 14(3):583. https://doi.org/10.3390/nu14030583
Chicago/Turabian StyleKay, Laurence, Lucia Potenza, Isabelle Hininger-Favier, Hubert Roth, Stéphane Attia, Cindy Tellier, Christian Zuppinger, Cinzia Calcabrini, Piero Sestili, Theo Wallimann, and et al. 2022. "Supplementing Soy-Based Diet with Creatine in Rats: Implications for Cardiac Cell Signaling and Response to Doxorubicin" Nutrients 14, no. 3: 583. https://doi.org/10.3390/nu14030583