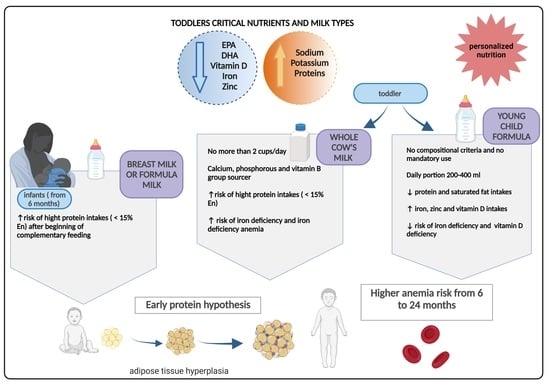
Which Milk during the Second Year of Life: A Personalized Choice for a Healthy Future?
Abstract
:1. Nutritional Requirements during the Second Year of Life
2. Type of Cow’s Milks and Growing-Up Milk
3. Nutritional Risks in Toddlers
3.1. Growth, Obesity, and Protein Leverage Hypothesis
3.2. Micronutrient Deficiencies
4. Which Milk Is the Best Choice during the Second Year of life? Evidence from Clinical Trials
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beluska-Turkan, K.; Korczak, R.; Hartell, B.; Moskal, K.; Maukonen, J.; Alexander, D.E.; Salem, N.; Harkness, L.; Ayad, W.; Szaro, J.; et al. Nutritional Gaps and Supplementation in the First 1000 Days. Nutrients 2019, 11, 2891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Food Safety Authority (EFSA). Dietary Reference Values for the EU. Available online: https://multimedia.efsa.europa.eu/drvs/index.htm (accessed on 10 August 2021).
- Riley, L.K.; Rupert, J.; Boucher, O. Nutrition in Toddlers. Am. Fam. Physician 2018, 98, 227–233. [Google Scholar] [PubMed]
- Fewtrell, M.; Bronsky, J.; Campoy, C.; Domellöf, M.; Embleton, N.; Fidler Mis, N.; Hojsak, I.; Hulst, J.M.; Indrio, F.; Lapillonne, A.; et al. Complementary Feeding: A Position Paper by the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Domellöf, M.; Braegger, C.; Campoy, C.; Colomb, V.; Decsi, T.; Fewtrell, M.; Hojsak, I.; Mihatsch, W.; Molgaard, C.; Shamir, R.; et al. Iron Requirements of Infants and Toddlers. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 119–129. [Google Scholar] [CrossRef]
- Hojsak, I.; Bronsky, J.; Campoy, C.; Domellöf, M.; Embleton, N.; Fidler Mis, N.; Hulst, J.; Indrio, F.; Lapillonne, A.; Mølgaard, C.; et al. Young Child Formula: A Position Paper by the ESPGHAN Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 177–185. [Google Scholar] [CrossRef]
- Koletzko, B.; Godfrey, K.M.; Poston, L.; Szajewska, H.; van Goudoever, J.B.; de Waard, M.; Brands, B.; Grivell, R.M.; Deussen, A.R.; Dodd, J.M.; et al. Nutrition During Pregnancy, Lactation and Early Childhood and Its Implications for Maternal and Long-Term Child Health: The Early Nutrition Project Recommendations. Ann. Nutr. Metab. 2019, 74, 93–106. [Google Scholar] [CrossRef]
- Hopkins, D.; Steer, C.D.; Northstone, K.; Emmett, P.M. Effects on Childhood Body Habitus of Feeding Large Volumes of Cow or Formula Milk Compared with Breastfeeding in the Latter Part of Infancy. Am. J. Clin. Nutr. 2015, 102, 1096–1103. [Google Scholar] [CrossRef] [Green Version]
- Marangoni, F.; Pellegrino, L.; Verduci, E.; Ghiselli, A.; Bernabei, R.; Calvani, R.; Cetin, I.; Giampietro, M.; Perticone, F.; Piretta, L.; et al. Cow’s Milk Consumption and Health: A Health Professional’s Guide. J. Am. Coll. Nutr. 2019, 38, 197–208. [Google Scholar] [CrossRef]
- Gnagnarella, P.; Salvini, S.; Parpinel, M. Food Composition Database for Epidemiological Studies in Italy. Available online: http://www.bda-ieo.it/ (accessed on 10 August 2021).
- Taylor, M.W.; MacGibbon, A.K.H. Milk Lipids|Fatty Acids. In Encyclopedia of Dairy Sciences, 2nd ed.; Fuquay, J.W., Ed.; Academic Press: San Diego, CA, USA, 2011; pp. 655–659. ISBN 978-0-12-374407-4. [Google Scholar]
- European Food Safety Authority (EFSA) Panel on Dietetic Products, Nutrition and Allergies. Scientific Opinion on Nutrient Requirements and Dietary Intakes of Infants and Young Children in the European Union. EFSA J. 2013, 11, 3408. [Google Scholar]
- Centro di Ricerca Alimenti e Nutrizione CREA. Linee Guida per una Sana Alimentazione 2018; Revisione; Centro di Ricerca Alimenti e Nutrizione CREA: Roma, Italy, 2019.
- Società Italiana di Pediatria (SIP) Piramide Alimentare Transculturale. Available online: https://sip.it/2017/10/30/piramide-alimentare-1/ (accessed on 8 July 2021).
- Vanderhout, S.M.; Aglipay, M.; Torabi, N.; Jüni, P.; da Costa, B.R.; Birken, C.S.; O’Connor, D.L.; Thorpe, K.E.; Maguire, J.L. Whole Milk Compared with Reduced-Fat Milk and Childhood Overweight: A Systematic Review and Meta-Analysis. Am. J. Clin. Nutr. 2020, 111, 266–279. [Google Scholar] [CrossRef]
- Verduci, E.; Bronsky, J.; Embleton, N.; Gerasimidis, K.; Indrio, F.; Köglmeier, J.; de Koning, B.; Lapillonne, A.; Moltu, S.J.; Norsa, L.; et al. Role of Dietary Factors, Food Habits, and Lifestyle in Childhood Obesity Development: A Position Paper from the European Society for Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2021, 72, 769–783. [Google Scholar] [CrossRef]
- Verduci, E.; D’Elios, S.; Cerrato, L.; Comberiati, P.; Calvani, M.; Palazzo, S.; Martelli, A.; Landi, M.; Trikamjee, T.; Peroni, D.G. Cow’s Milk Substitutes for Children: Nutritional Aspects of Milk from Different Mammalian Species, Special Formula and Plant-Based Beverages. Nutrients 2019, 11, 1739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verduci, E.; Di Profio, E.; Cerrato, L.; Nuzzi, G.; Riva, L.; Vizzari, G.; D’Auria, E.; Giannì, M.L.; Zuccotti, G.; Peroni, D.G. Use of Soy-Based Formulas and Cow’s Milk Allergy: Lights and Shadows. Front. Pediatr. 2020, 8, 591988. [Google Scholar] [CrossRef] [PubMed]
- Lovell, A.L.; Davies, P.S.W.; Hill, R.J.; Milne, T.; Matsuyama, M.; Jiang, Y.; Chen, R.X.; Grant, C.C.; Wall, C.R. A Comparison of the Effect of a Growing Up Milk-Lite (GUMLi) v. Cows’ Milk on Longitudinal Dietary Patterns and Nutrient Intakes in Children Aged 12-23 Months: The GUMLi Randomised Controlled Trial. Br. J. Nutr. 2019, 121, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Suthutvoravut, U.; Abiodun, P.O.; Chomtho, S.; Chongviriyaphan, N.; Cruchet, S.; Davies, P.S.W.; Fuchs, G.J.; Gopalan, S.; van Goudoever, J.B.; Nel, E.R.; et al. Composition of Follow-Up Formula for Young Children Aged 12–36 Months: Recommendations of an International Expert Group Coordinated by the Nutrition Association of Thailand and the Early Nutrition Academy. Ann. Nutr. Metab. 2015, 67, 119–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Food Safety Authority (EFSA) Scientific Opinion on the Essential Composition of Infant and Follow-on Formulae. EFSA J. 2014, 12, 3760. [CrossRef] [Green Version]
- Lippman, H.E.; Desjeux, J.-F.; Ding, Z.-Y.; Tontisirin, K.; Uauy, R.; Pedro, R.A.; Van Dael, P. Nutrient Recommendations for Growing-up Milk: A Report of an Expert Panel. Crit. Rev. Food Sci. Nutr. 2016, 56, 141–145. [Google Scholar] [CrossRef]
- Harris, J.L.; Pomeranz, J.L. Infant Formula and Toddler Milk Marketing: Opportunities to Address Harmful Practices and Improve Young Children’s Diets. Nutr. Rev. 2020, 78, 866–883. [Google Scholar] [CrossRef]
- Ogden, C.L.; Carroll, M.D.; Kit, B.K.; Flegal, K.M. Prevalence of Childhood and Adult Obesity in the United States, 2011–2012. JAMA 2014, 311, 806–814. [Google Scholar] [CrossRef] [Green Version]
- Consensus Nazionale Su Diagnosi, Trattamento e Prevenzione Dell’Obesità Del Bambino e Dell’adolescente-Siedp-Società Italiana Endocrinologia e Diabetologia Pediatrica. Available online: http://www.siedp.it/pagina/778/consensus+nazionale+su+diagnosi%2C+trattamento+e+prevenzione+dell%27obesita+del+bambino+e+dell%27adolescente (accessed on 8 July 2021).
- Roy, S.M.; Spivack, J.G.; Faith, M.S.; Chesi, A.; Mitchell, J.A.; Kelly, A.; Grant, S.F.A.; McCormack, S.E.; Zemel, B.S. Infant BMI or Weight-for-Length and Obesity Risk in Early Childhood. Pediatrics 2016, 137, e20153492. [Google Scholar] [CrossRef] [Green Version]
- Hardwick, J.; Sidnell, A. Infant Nutrition—Diet between 6 and 24 Months, Implications for Paediatric Growth, Overweight and Obesity. Nutr. Bull. 2014, 39, 354–363. [Google Scholar] [CrossRef] [Green Version]
- Weng, S.F.; Redsell, S.A.; Swift, J.A.; Yang, M.; Glazebrook, C.P. Systematic Review and Meta-Analyses of Risk Factors for Childhood Overweight Identifiable during Infancy. Arch. Dis. Child. 2012, 97, 1019–1026. [Google Scholar] [CrossRef] [Green Version]
- Arisaka, O.; Ichikawa, G.; Koyama, S.; Sairenchi, T. Childhood Obesity: Rapid Weight Gain in Early Childhood and Subsequent Cardiometabolic Risk. Clin. Pediatr. Endocrinol. 2020, 29, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Skouteris, H.; Nagle, C.; Fowler, M.; Kent, B.; Sahota, P.; Morris, H. Interventions Designed to Promote Exclusive Breastfeeding in High-Income Countries: A Systematic Review. Breastfeed. Med. Off. J. Acad. Breastfeed. Med. 2014, 9, 113–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theurich, M.A.; Davanzo, R.; Busck-Rasmussen, M.; Díaz-Gómez, N.M.; Brennan, C.; Kylberg, E.; Bærug, A.; McHugh, L.; Weikert, C.; Abraham, K.; et al. Breastfeeding Rates and Programs in Europe: A Survey of 11 National Breastfeeding Committees and Representatives. J. Pediatr. Gastroenterol. Nutr. 2019, 68, 400–407. [Google Scholar] [CrossRef]
- Michaelsen, K.F.; Greer, F.R. Protein Needs Early in Life and Long-Term Health. Am. J. Clin. Nutr. 2014, 99, 718S–722S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savarino, G.; Corsello, A.; Corsello, G. Macronutrient Balance and Micronutrient Amounts through Growth and Development. Ital. J. Pediatr. 2021, 47, 109. [Google Scholar] [CrossRef]
- Tang, M. Protein Intake during the First Two Years of Life and Its Association with Growth and Risk of Overweight. Int. J. Environ. Res. Public. Health 2018, 15, 1742. [Google Scholar] [CrossRef] [Green Version]
- Günther, A.L.B.; Remer, T.; Kroke, A.; Buyken, A.E. Early Protein Intake and Later Obesity Risk: Which Protein Sources at Which Time Points throughout Infancy and Childhood Are Important for Body Mass Index and Body Fat Percentage at 7 y of Age? Am. J. Clin. Nutr. 2007, 86, 1765–1772. [Google Scholar] [CrossRef]
- Scaglioni, S.; Agostoni, C.; Notaris, R.D.; Radaelli, G.; Radice, N.; Valenti, M.; Giovannini, M.; Riva, E. Early Macronutrient Intake and Overweight at Five Years of Age. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 2000, 24, 777–781. [Google Scholar] [CrossRef] [Green Version]
- Luque, V.; Closa-Monasterolo, R.; Escribano, J.; Ferré, N. Early Programming by Protein Intake: The Effect of Protein on Adiposity Development and the Growth and Functionality of Vital Organs. Nutr. Metab. Insights 2015, 8, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; von Kries, R.; Closa, R.; Monasterolo, R.C.; Escribano, J.; Subías, J.E.; Scaglioni, S.; Giovannini, M.; Beyer, J.; Demmelmair, H.; et al. Can Infant Feeding Choices Modulate Later Obesity Risk? Am. J. Clin. Nutr. 2009, 89, 1502S–1508S. [Google Scholar] [CrossRef] [Green Version]
- Koletzko, B.; von Kries, R.; Closa, R.; Escribano, J.; Scaglioni, S.; Giovannini, M.; Beyer, J.; Demmelmair, H.; Gruszfeld, D.; Dobrzanska, A.; et al. Lower Protein in Infant Formula Is Associated with Lower Weight up to Age 2 y: A Randomized Clinical Trial. Am. J. Clin. Nutr. 2009, 89, 1836–1845. [Google Scholar] [CrossRef] [Green Version]
- Lacroix, M.; Bos, C.; Léonil, J.; Airinei, G.; Luengo, C.; Daré, S.; Benamouzig, R.; Fouillet, H.; Fauquant, J.; Tomé, D.; et al. Compared with Casein or Total Milk Protein, Digestion of Milk Soluble Proteins Is Too Rapid to Sustain the Anabolic Postprandial Amino Acid Requirement. Am. J. Clin. Nutr. 2006, 84, 1070–1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Moya, T.; López-Nicolás, R.; Planes, D.; González-Bermúdez, C.A.; Ros-Berruezo, G.; Frontela-Saseta, C. In Vitro Modulation of Gut Microbiota by Whey Protein to Preserve Intestinal Health. Food Funct. 2017, 8, 3053–3063. [Google Scholar] [CrossRef]
- Aslam, H.; Marx, W.; Rocks, T.; Loughman, A.; Chandrasekaran, V.; Ruusunen, A.; Dawson, S.L.; West, M.; Mullarkey, E.; Pasco, J.A.; et al. The Effects of Dairy and Dairy Derivatives on the Gut Microbiota: A Systematic Literature Review. Gut Microbes 2020, 12, 1799533. [Google Scholar] [CrossRef] [PubMed]
- Ferré, N.; Luque, V.; Closa-Monasterolo, R.; Zaragoza-Jordana, M.; Gispert-Llauradó, M.; Grote, V.; Koletzko, B.; Escribano, J. Association of Protein Intake during the Second Year of Life with Weight Gain-Related Outcomes in Childhood: A Systematic Review. Nutrients 2021, 13, 583. [Google Scholar] [CrossRef]
- Agostoni, C.; Caroli, M. Role of Fats in the First Two Years of Life as Related to Later Development of NCDs. Nutr. Metab. Cardiovasc. Dis. NMCD 2012, 22, 775–780. [Google Scholar] [CrossRef]
- Rolland-Cachera, M.F.; Maillot, M.; Deheeger, M.; Souberbielle, J.C.; Péneau, S.; Hercberg, S. Association of Nutrition in Early Life with Body Fat and Serum Leptin at Adult Age. Int. J. Obes. 2013, 37, 1116–1122. [Google Scholar] [CrossRef] [Green Version]
- Uwaezuoke, S.N.; Eneh, C.I.; Ndu, I.K. Relationship Between Exclusive Breastfeeding and Lower Risk of Childhood Obesity: A Narrative Review of Published Evidence. Clin. Med. Insights Pediatr. 2017, 11, 1179556517690196. [Google Scholar] [CrossRef]
- Leung, A.K.; Marchand, V.; Sauve, R.S. The ‘Picky Eater’: The Toddler or Preschooler Who Does Not Eat. Paediatr. Child Health 2012, 17, 455–457. [Google Scholar] [CrossRef]
- Domellöf, M. Iron and Other Micronutrient Deficiencies in Low-Birthweight Infants. Nestle Nutr. Inst. Workshop Ser. 2013, 74, 197–206. [Google Scholar] [CrossRef] [Green Version]
- Ackland, M.L.; Michalczyk, A.A. Zinc and Infant Nutrition. Arch. Biochem. Biophys. 2016, 611, 51–57. [Google Scholar] [CrossRef]
- Corbo, M.D.; Lam, J. Zinc Deficiency and Its Management in the Pediatric Population: A Literature Review and Proposed Etiologic Classification. J. Am. Acad. Dermatol. 2013, 69, 616–624. [Google Scholar] [CrossRef]
- Brotanek, J.M.; Gosz, J.; Weitzman, M.; Flores, G. Iron Deficiency in Early Childhood in the United States: Risk Factors and Racial/Ethnic Disparities. Pediatrics 2007, 120, 568–575. [Google Scholar] [CrossRef] [Green Version]
- Baker, R.D.; Greer, F.R.; The Committee on Nutrition. Diagnosis and Prevention of Iron Deficiency and Iron-Deficiency Anemia in Infants and Young Children (0–3 Years of Age). Pediatrics 2010, 126, 1040–1050. [Google Scholar] [CrossRef] [Green Version]
- Joo, E.Y.; Kim, K.Y.; Kim, D.H.; Lee, J.-E.; Kim, S.K. Iron Deficiency Anemia in Infants and Toddlers. Blood Res. 2016, 51, 268–273. [Google Scholar] [CrossRef] [Green Version]
- Ziegler, E.E. Consumption of Cow’s Milk as a Cause of Iron Deficiency in Infants and Toddlers. Nutr. Rev. 2011, 69, S37–S42. [Google Scholar] [CrossRef] [PubMed]
- Keim, S.A.; Branum, A.M. Dietary Intake of Polyunsaturated Fatty Acids and Fish among US Children 12–60 Months of Age. Matern. Child Nutr. 2015, 11, 987–998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atlantis, E.; Cochrane, B. The Association of Dietary Intake and Supplementation of Specific Polyunsaturated Fatty Acids with Inflammation and Functional Capacity in Chronic Obstructive Pulmonary Disease: A Systematic Review. Int. J. Evid. Based Healthc. 2016, 14, 53–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauritzen, L.; Brambilla, P.; Mazzocchi, A.; Harsløf, L.B.S.; Ciappolino, V.; Agostoni, C. DHA Effects in Brain Development and Function. Nutrients 2016, 8, 6. [Google Scholar] [CrossRef] [Green Version]
- Antonucci, R.; Locci, C.; Clemente, M.G.; Chicconi, E.; Antonucci, L. Vitamin D Deficiency in Childhood: Old Lessons and Current Challenges. J. Pediatr. Endocrinol. Metab. JPEM 2018, 31, 247–260. [Google Scholar] [CrossRef]
- Walton, J.; Flynn, A. Nutritional Adequacy of Diets Containing Growing up Milks or Unfortified Cow’s Milk in Irish Children (Aged 12–24 Months). Food Nutr. Res. 2013, 57, 21836. [Google Scholar] [CrossRef] [Green Version]
- Wall, C.R.; Hill, R.J.; Lovell, A.L.; Matsuyama, M.; Milne, T.; Grant, C.C.; Jiang, Y.; Chen, R.X.; Wouldes, T.A.; Davies, P.S.W. A Multicenter, Double-Blind, Randomized, Placebo-Controlled Trial to Evaluate the Effect of Consuming Growing Up Milk “Lite” on Body Composition in Children Aged 12–23 Mo. Am. J. Clin. Nutr. 2019, 109, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, C.; Udam, T.R.; Lauritzen, L.; Mølgaard, C.; Juul, A.; Michaelsen, K.F. Animal Protein Intake, Serum Insulin-like Growth Factor I, and Growth in Healthy 2.5-y-Old Danish Children. Am. J. Clin. Nutr. 2004, 80, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Lovell, A.L.; Davies, P.S.W.; Hill, R.J.; Milne, T.; Matsuyama, M.; Jiang, Y.; Chen, R.X.; Wouldes, T.A.; Heath, A.-L.M.; Grant, C.C.; et al. Compared with Cow Milk, a Growing-Up Milk Increases Vitamin D and Iron Status in Healthy Children at 2 Years of Age: The Growing-Up Milk-Lite (GUMLi) Randomized Controlled Trial. J. Nutr. 2018, 148, 1570–1579. [Google Scholar] [CrossRef] [PubMed]
- Lovell, A.L.; Milne, T.; Jiang, Y.; Chen, R.X.; Grant, C.C.; Wall, C.R. Evaluation of the Effect of a Growing up Milk Lite vs. Cow’s Milk on Diet Quality and Dietary Intakes in Early Childhood: The Growing up Milk Lite (GUMLi) Randomised Controlled Trial. Nutrients 2019, 11, 203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lovell, A.L.; Davies, P.S.W.; Hill, R.J.; Milne, T.; Matsuyama, M.; Jiang, Y.; Chen, R.X.; Heath, A.-L.M.; Grant, C.C.; Wall, C.R. Validation and Calibration of the Eating Assessment in Toddlers FFQ (EAT FFQ) for Children, Used in the Growing Up Milk-Lite (GUMLi) Randomised Controlled Trial. Br. J. Nutr. 2021, 125, 183–193. [Google Scholar] [CrossRef]
- Sazawal, S.; Dhingra, U.; Dhingra, P.; Hiremath, G.; Sarkar, A.; Dutta, A.; Menon, V.P.; Black, R.E. Micronutrient Fortified Milk Improves Iron Status, Anemia and Growth among Children 1–4 Years: A Double Masked, Randomized, Controlled Trial. PLoS ONE 2010, 5, e12167. [Google Scholar] [CrossRef] [Green Version]
- Atkins, L.A.; McNaughton, S.A.; Spence, A.C.; Szymlek-Gay, E.A. Dietary Patterns of Australian Pre-Schoolers and Associations with Haem and Non-Haem Iron Intakes. Eur. J. Nutr. 2021, 60, 3059–3070. [Google Scholar] [CrossRef] [PubMed]
- Houghton, L.A.; Gray, A.R.; Szymlek-Gay, E.A.; Heath, A.-L.M.; Ferguson, E.L. Vitamin D-Fortified Milk Achieves the Targeted Serum 25-Hydroxyvitamin D Concentration without Affecting That of Parathyroid Hormone in New Zealand Toddlers. J. Nutr. 2011, 141, 1840–1846. [Google Scholar] [CrossRef] [Green Version]
- Lovell, A.L.; Milne, T.; Matsuyama, M.; Hill, R.J.; Davies, P.S.W.; Grant, C.C.; Wall, C.R. Protein Intake, IGF-1 Concentrations, and Growth in the Second Year of Life in Children Receiving Growing Up Milk-Lite (GUMLi) or Cow’s Milk (CM) Intervention. Front. Nutr. 2021, 8, 666228. [Google Scholar] [CrossRef]

| Proteins | |
|---|---|
| Pro kg | 1.14–0.90 g/kg a day |
| Fats | 35–40% %En |
| Saturated fatty acids | As low as possible |
| Linoleic Acid (LA) | 4% |
| Alpha-Linolenic Acid (ALA) | 0.5% |
| EPA + DHA | 250 mg + 100 mg (during the second year of life) |
| Trans fatty acids | As low as possible |
| Carbohydrates | 45–60% %En |
| Sugars | <10% |
| Fiber | 10 g |
| Minerals | |
| Sodium (g) | 1.1 |
| Potassium (g) | 0.8 |
| Chloride (g) | 1.7 |
| Calcium (mg) | 450 |
| Phosphorous (mg) | 250 |
| Magnesium (mg) | 170–230 |
| Iron (mg) | 7 |
| Zinc (mg) | 4.3 |
| Copper (mg) | 0.7–1 |
| Manganese (mg) | 0.5 |
| Fluoride (mg) | 0.6 |
| Iodine (μg) | 90 |
| Selenium (μg) | 15 |
| Vitamins | |
| Vitamin A (μg) | 250 |
| Vitamin D (μg) | 15 |
| Vitamin E (mg) | 6–9 |
| Vitamin K (μg) | 12 |
| Vitamin B1 or thiamine (mg) | 0.1 |
| Vitamin B2 or riboflavin (mg) | 0.6 |
| Vitamin B3 or niacin (mg) | 1.6 |
| Vitamin B5 or pantothenic acid (mg) | 4 |
| Vitamin B6 or pyridoxine (mg) | 0.6 |
| Vitamin B7 or biotin (μg) | 20 |
| Vitamin B9 or folic acid (μg) | 120 |
| Vitamin B12 or cobalamin (μg) | 1.5 |
| Vitamin C (mg) | 20 |
| Whole Cow’s Milk | Partially Skimmed Milk | Skimmed Milk | |
|---|---|---|---|
| Energy, kcal | 64 | 46 | 36 |
| Water, g | 87 | 88.5 | 90.5 |
| Carbohydrate, g | 4.9 | 5.0 | 5.3 |
| Protein, g | 3.3 | 3.5 | 3.6 |
| Fat, g | 3.6 | 1.5 | 0.2 |
| Polyunsaturated Fatty acids (PUFA), g | 0.12 | 0.08 | 0.01 |
| Linoleic acid, g | 0.07 | 0.05 | 0.01 |
| Linolenic acid, g | 0.05 | 0.03 | traces |
| Eicosapentaenoic acid (EPA), g | 0.00 | 0.00 | 0.00 |
| Docosahexaenoic acid (DHA), g | 0.00 | 0.00 | 0.00 |
| Calcium, mg | 119 | 120 | 125 |
| Sodium, mg | 50 | 46 | 52 |
| Phosphorus, mg | 93 | 94 | 97 |
| Iron, mg | 0.1 | 0.1 | 0.1 |
| Zinc, mg | 0.38 | 0.37 | 0.59 |
| Potassium, mg | 150 | 170 | 150 |
| Magnesium, mg | 12 | 11 | 11 |
| Selenium, µg | 1.6 | 1.6 | 1 |
| Vitamin D, µg | 0.03 | 0.01 | traces |
| Vitamin A, µg (RE-eq) | 43 | 21 | traces |
| Vitamin E, mg | 0.07 | 0.04 | traces |
| Vitamin C, mg | 1 | 1 | 1 |
| Whole Cow’s Milk (Mean) | Young Child Formula, (Median) | EFSA Recommendation for Follow-On Formula (Min-Max or Min) | |
|---|---|---|---|
| Energy (kcal/100 g) | 69 | 67 | 60–70 |
| Proteins (g/100 kcal) | 4.8 | 2.6 | 1.6–2.5 |
| Casein | 1.7 | ||
| Whey protein | 0.7 | ||
| Fats (g/100 kcal) | 6.1 | 4.3 | 4.4–6 |
| Saturated fat (g/100 kcal) | 1.4 | ||
| Monounsaturated (g/100 kcal) | 1.9 | ||
| Polyunsaturated (g/100 kcal) | 0.9 | ||
| Linoleic acid (g/100 kcal) | 0.07 | 0.8 | 0.5–1.2 |
| Arachidonic acid (g/100 kcal) | 0 | ||
| Alpha-linolenic acid n−3 (mg/100 kcal) | 103 | 50–100 | |
| Eicosapentaenoic acid (EPA) (mg/100 kcal) | 19 | ||
| Docosahexaenoic acid (DHA) (mg/100 kcal) | 6.4 | 20–50 | |
| Trans fatty acids | 6.4 | <3% of total fatty acids | |
| Carbohydrates (g/100 kcal) | 6.8 | 12.6 | 9–14 |
| Total sugars (g/100 kcal) | 9.9 | <20% of total CHO | |
| Lactose (g/100 kcal) | 9 | >4.5 | |
| Sucrose (g/100 kcal) | 2.1 | ||
| Glucose (g/100 kcal) | 0.5 | 0 | |
| Maltose (g/100 kcal) | 0.2 | ||
| Maltodextrin (g/100 kcal) | 4.1 | ||
| Fiber | 0.8 | ||
| Minerals | |||
| Sodium (mg) | 64.3 | 40.4 | 25 |
| Potassium (mg) | 215.1 | 126.8 | 80 |
| Chloride (mg) | 146.5 | 75 | 60 |
| Calcium (mg) | 176.7 | 126.9 | 50 |
| Phosphorous (mg) | 138.3 | 77.6 | 25 |
| Magnesium (mg) | 16.8 | 10.4 | 5 |
| Iron (mg) | <0.1 | 1.8 | 0.6 |
| Zinc (mg) | 0.6 | 1.1 | 0.5 |
| Copper (mg) | 0 | 0.1 | 0.06 |
| Manganese (mg) | 0 | 0 | 1 |
| Fluoride (mg) | 0 | not necessary | |
| Iodine (μg) | 23 | 20 | 15 |
| Selenium (μg) | 1.9 | 2.4 | 3 |
| Chromium (μg) | 1.4 | not necessary | |
| Molybdenum (μg) | 4.2 | 0.4 | |
| Vitamins | |||
| Vitamin A (μg) | 57.5 | 101.6 | 70 |
| Vitamin D (μg) | 0.1 | 2.1 | 2 |
| Vitamin E (mg) | 0.1 | 1.6 | 0.6 |
| Vitamin K (μg) | 0 | 7.5 | 1 |
| Vitamin B1 or thiamine (μg) | 0 | 0.1 | 0.04 |
| Vitamin B2 or riboflavin (μg) | 0.3 | 0.2 | 0.06 |
| Vitamin B3 or niacin (mg) | 1.0 | 0.9 | 0.4 |
| Vitamin B5 or pantothenic acid (mg) | 0 | 0.7 | 0.4 |
| Vitamin B6 or pyridoxine (μg) | 0 | 0.1 | 0.02 |
| Vitamin B7 or biotin (μg) | 4.3 | 3.1 | 1 |
| Vitamin B9 or folic acid (μg) | 9.1 | 22.4 | 15 |
| Vitamin B12 or cobalamin (μg) | 0.7 | 0.3 | 0.1 |
| Vitamin C (mg) | 1.9 | 15.4 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verduci, E.; Di Profio, E.; Corsello, A.; Scatigno, L.; Fiore, G.; Bosetti, A.; Zuccotti, G.V. Which Milk during the Second Year of Life: A Personalized Choice for a Healthy Future? Nutrients 2021, 13, 3412. https://doi.org/10.3390/nu13103412
Verduci E, Di Profio E, Corsello A, Scatigno L, Fiore G, Bosetti A, Zuccotti GV. Which Milk during the Second Year of Life: A Personalized Choice for a Healthy Future? Nutrients. 2021; 13(10):3412. https://doi.org/10.3390/nu13103412
Chicago/Turabian StyleVerduci, Elvira, Elisabetta Di Profio, Antonio Corsello, Lorenzo Scatigno, Giulia Fiore, Alessandra Bosetti, and Gian Vincenzo Zuccotti. 2021. "Which Milk during the Second Year of Life: A Personalized Choice for a Healthy Future?" Nutrients 13, no. 10: 3412. https://doi.org/10.3390/nu13103412