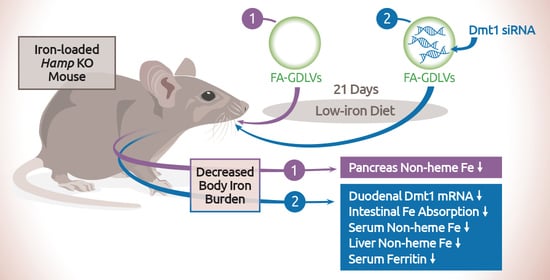
Oral Administration of Ginger-Derived Lipid Nanoparticles and Dmt1 siRNA Potentiates the Effect of Dietary Iron Restriction and Mitigates Pre-Existing Iron Overload in Hamp KO Mice
Abstract
:1. Introduction
“…it is likely that the combination of phlebotomy and diet modifications is the best treatment for hemochromatosis. Changes in nutrition can perhaps reduce the number of required phlebotomies. That this preventive method for iron overload gets virtually no attention is quite remarkable because–although (almost) no research is done–it is expected that bloodletting also has an impact on other health aspects, such as the vitamin status of patients”.
“Despite the limited quantitative evidence and the lack of randomized, prospective trials, dietary interventions that modify iron intake and bioavailability may affect iron accumulation in HH patients. Although this measure may be welcome in patients willing to contribute to their disease management, limited data exist on the clinical and quality-of-life benefit.” [6].
2. Materials and Methods
2.1. Preparation of Folic Acid-Conjugated Ginger Nanoparticle-derived Lipid Vectors (FA-GDLVs)
2.2. Animal Studies
2.3. Hematologic and Iron Parameters
2.4. Iron Absorption Study
2.5. MPO Assay
2.6. Western Blotting
2.7. Quantitative Real-Time PCR
2.8. Statistical Analyses
3. Results
3.1. FA-GDLVs/siRNA Exposure Had No Detrimental Effects on Experimental Mice
3.2. FA-GDLV-Mediated siRNA Delivery Downregulated Duodenal Dmt1 mRNA Expression
3.3. Duodenal DMT1 and FPN1 Protein Levels Were Unaffected by FA-GDLV-siRNA Delivery
3.4. GDLV/siRNA Exposure Decreased Liver and Pancreas Non-heme Iron Content
3.5. Dmt1 Knock Down Reduced Parenchymal Iron Loading and Serum Non-heme Iron Levels
3.6. FA-GDLVs/Dmt1 siRNA Administration Blunted Intestinal Iron (59Fe) Absorption and Altered Tissue Iron Distribution
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Institutional Animal Care and Use (IACUC) Statement
References
- Gulec, S.; Anderson, G.J.; Collins, J.F. Mechanistic and regulatory aspects of intestinal iron absorption. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 307, G397–G409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nemeth, E.; Tuttle, M.S.; Powelson, J.; Vaughn, M.B.; Donovan, A.; Ward, D.M.; Ganz, T.; Kaplan, J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 2004, 306, 2090–2093. [Google Scholar] [CrossRef] [Green Version]
- Brissot, P.; Pietrangelo, A.; Adams, P.C.; De Graaff, B.; McLaren, C.E.; Loreal, O. Haemochromatosis. Nat. Rev. Dis. Prim. 2018, 4, 18016. [Google Scholar] [CrossRef] [PubMed]
- Brissot, P.; Troadec, M.B.; Loreal, O. Intestinal absorption of iron in HFE-1 hemochromatosis: Local or systemic process? J. Hepatol. 2004, 40, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Kowdley, K.V.; Brown, K.E.; Ahn, J.; Sundaram, V. ACG Clinical guideline: Hereditary hemochromatosis. Am. J. Gastroenterol. 2019, 114, 1202–1218. [Google Scholar] [CrossRef]
- Moretti, D.; Van Doorn, G.M.; Swinkels, D.W.; Melse-Boonstra, A. Relevance of dietary iron intake and bioavailability in the management of HFE hemochromatosis: A systematic review. Am. J. Clin. Nutr. 2013, 98, 468–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffiths, W.J.; Sly, W.S.; Cox, T.M. Intestinal iron uptake determined by divalent metal transporter is enhanced in HFE-deficient mice with hemochromatosis. Gastroenterology 2001, 120, 1420–1429. [Google Scholar] [CrossRef] [PubMed]
- Fleming, R.E.; Migas, M.C.; Zhou, X.; Jiang, J.; Britton, R.S.; Brunt, E.M.; Tomatsu, S.; Waheed, A.; Bacon, B.R.; Sly, W.S. Mechanism of increased iron absorption in murine model of hereditary hemochromatosis: Increased duodenal expression of the iron transporter DMT1. Proc. Natl. Acad. Sci. USA 1999, 96, 3143–3148. [Google Scholar] [CrossRef] [Green Version]
- Stuart, K.A.; Anderson, G.J.; Frazer, D.M.; Powell, L.W.; McCullen, M.; Fletcher, L.M.; Crawford, D.H. Duodenal expression of iron transport molecules in untreated haemochromatosis subjects. Gut 2003, 52, 953–959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hubert, N.; Hentze, M.W. Previously uncharacterized isoforms of divalent metal transporter (DMT)-1: Implications for regulation and cellular function. Proc. Natl. Acad. Sci. USA 2002, 99, 12345–12350. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhang, M.; Flores, S.R.L.; Woloshun, R.R.; Yang, C.; Yin, L.; Xiang, P.; Xu, X.; Garrick, M.D.; Vidyasagar, S.; et al. Oral gavage of ginger nanoparticle-derived lipid vectors carrying Dmt1 siRNA blunts iron loading in murine hereditary hemochromatosis. Mol. Ther. 2019, 27, 493–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Wang, X.; Han, M.K.; Collins, J.F.; Merlin, D. Oral administration of ginger-derived nanolipids loaded with siRNA as a novel approach for efficient siRNA drug delivery to treat ulcerative colitis. Nanomedicine 2017, 12, 1927–1943. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.; Yang, C.; Collins, J.F.; Merlin, D. Preparation and characterization of ginger lipid-derived nanoparticles for colon-targeted siRNA delivery. Bio-Protocol 2020, 10, e3685. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.; Yang, C.; Viennois, E.; Zhang, M.; Merlin, D. Isolation, purification, and characterization of ginger-derived nanoparticles (GDNPs) from ginger, rhizome of zingiber officinale. Bio-Protocol 2019, 9, e3390. [Google Scholar] [CrossRef] [PubMed]
- Shayeghi, M.; Latunde-Dada, G.O.; Oakhill, J.S.; Laftah, A.H.; Takeuchi, K.; Halliday, N.; Khan, Y.; Warley, A.; McCann, F.E.; Hider, R.C.; et al. Identification of an intestinal heme transporter. Cell 2005, 122, 789–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, A.; Jansen, M.; Sakaris, A.; Min, S.H.; Chattopadhyay, S.; Tsai, E.; Sandoval, C.; Zhao, R.; Akabas, M.H.; Goldman, I.D. Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption. Cell 2006, 127, 917–928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Collins, J.F.; Merlin, D. Do ginger-derived nanoparticles represent an attractive treatment strategy for inflammatory bowel diseases? Nanomedicine 2016, 11, 3035–3037. [Google Scholar] [CrossRef] [Green Version]
- Doguer, C.; Ha, J.H.; Gulec, S.; Vulpe, C.D.; Anderson, G.J.; Collins, J.F. Intestinal hephaestin potentiates iron absorption in weanling, adult, and pregnant mice under physiological conditions. Blood Adv. 2017, 1, 1335–1346. [Google Scholar] [CrossRef] [Green Version]
- Ha, J.H.; Doguer, C.; Collins, J.F. Consumption of a high-iron diet disrupts homeostatic regulation of intestinal copper absorption in adolescent mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 313, G535–G360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gulec, S.; Collins, J.F. Investigation of iron metabolism in mice expressing a mutant Menke’s copper transporting ATPase (Atp7a) protein with diminished activity (Brindled; Mo (Br) (/y)). PLoS ONE 2013, 8, e66010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wheby, M.S.; Jones, L.G.; Crosby, W.H. Studies on iron absorption. Intestinal regulatory mechanisms. J. Clin. Investig. 1964, 43, 1433–1442. [Google Scholar] [CrossRef]
- Jiang, L.; Ranganathan, P.; Lu, Y.; Kim, C.; Collins, J.F. Exploration of the copper-related compensatory response in the Belgrade rat model of genetic iron deficiency. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G877–G886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gulec, S.; Collins, J.F. Silencing the Menkes copper-transporting ATPase (Atp7a) gene in rat intestinal epithelial (IEC-6) cells increases iron flux via transcriptional induction of ferroportin 1 (Fpn1). J. Nutr. 2014, 144, 12–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, L.; Collins, J.F. Transcription factors Sp1 and Hif2alpha mediate induction of the copper-transporting ATPase (Atp7a) gene in intestinal epithelial cells during hypoxia. J. Biol. Chem. 2013, 288, 23943–23952. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Viennois, E.; Prasad, M.; Zhang, Y.; Wang, L.; Zhang, Z.; Han, M.K.; Xiao, B.; Xu, C.; Srinivasan, S.; et al. Edible ginger-derived nanoparticles: A novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials 2016, 101, 321–340. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Xiao, B.; Wang, H.; Han, M.K.; Zhang, Z.; Viennois, E.; Xu, C.; Merlin, D. Edible ginger-derived nano-lipids loaded with doxorubicin as a novel drug-delivery approach for colon cancer therapy. Mol. Ther. 2016, 24, 1783–1796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kautz, L.; Jung, G.; Valore, E.V.; Rivella, S.; Nemeth, E.; Ganz, T. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat. Genet. 2014, 46, 678–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semenza, G.L. Hypoxia-inducible factors in physiology and medicine. Cell 2012, 148, 399–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carthew, R.W.; Sontheimer, E.J. Origins and mechanisms of miRNAs and siRNAs. Cell 2009, 136, 642–655. [Google Scholar] [CrossRef] [Green Version]
- Shawki, A.; Anthony, S.R.; Nose, Y.; Engevik, M.A.; Niespodzany, E.J.; Barrientos, T.; Ohrvik, H.; Worrell, R.T.; Thiele, D.J.; Mackenzie, B. Intestinal DMT1 is critical for iron absorption in the mouse but is not required for the absorption of copper or manganese. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G635–G647. [Google Scholar] [CrossRef] [Green Version]
- Canonne-Hergaux, F.; Gruenheid, S.; Ponka, P.; Gros, P. Cellular and subcellular localization of the Nramp2 iron transporter in the intestinal brush border and regulation by dietary iron. Blood 1999, 93, 4406–4417. [Google Scholar] [CrossRef] [PubMed]
- Gruenheid, S.; Canonne-Hergaux, F.; Gauthier, S.; Hackam, D.J.; Grinstein, S.; Gros, P. The iron transport protein NRAMP2 is an integral membrane glycoprotein that colocalizes with transferrin in recycling endosomes. J. Exp. Med. 1999, 189, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Knutson, M.D.; Oukka, M.; Koss, L.M.; Aydemir, F.; Wessling-Resnick, M. Iron release from macrophages after erythrophagocytosis is up-regulated by ferroportin 1 overexpression and down-regulated by hepcidin. Proc. Natl. Acad. Sci. USA 2005, 102, 1324–1328. [Google Scholar] [CrossRef] [Green Version]
- Lesbordes-Brion, J.C.; Viatte, L.; Bennoun, M.; Lou, D.Q.; Ramey, G.; Houbron, C.; Hamard, G.; Kahn, A.; Vaulont, S. Targeted disruption of the hepcidin 1 gene results in severe hemochromatosis. Blood 2006, 108, 1402–1405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, Q.Q.; Xu, X.Y.; Cao, S.Y.; Gan, R.Y.; Corke, H.; Beta, T.; Li, H.B. Bioactive compounds and bioactivities of ginger (Zingiber officinale Roscoe). Foods 2019, 8, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Knovich, M.A.; Coffman, L.G.; Torti, F.M.; Torti, S.V. Serum ferritin: Past, present and future. Biochim. Biophys. Acta 2010, 1800, 760–769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masaratana, P.; Laftah, A.H.; Latunde-Dada, G.O.; Vaulont, S.; Simpson, R.J.; McKie, A.T. Iron absorption in hepcidin1 knockout mice. Br. J. Nutr. 2011, 105, 1583–1591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brissot, P.; Ropert, M.; Le Lan, C.; Loreal, O. Non-transferrin bound iron: A key role in iron overload and iron toxicity. Biochim. Biophys. Acta 2012, 1820, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Cadieux, J.A.; Zhang, Z.; Mattice, M.; Brownlie-Cutts, A.; Fu, J.; Ratkay, L.G.; Kwan, R.; Thompson, J.; Sanghara, J.; Zhong, J.; et al. Synthesis and biological evaluation of substituted pyrazoles as blockers of divalent metal transporter 1 (DMT1). Bioorg. Med. Chem. Lett. 2012, 22, 90–95. [Google Scholar] [CrossRef]
- Wetli, H.A.; Buckett, P.D.; Wessling-Resnick, M. Small-molecule screening identifies the selanazal drug ebselen as a potent inhibitor of DMT1-mediated iron uptake. Chem. Biol. 2006, 13, 965–972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Kodumuru, V.; Sviridov, S.; Liu, S.; Chafeev, M.; Chowdhury, S.; Chakka, N.; Sun, J.; Gauthier, S.J.; Mattice, M.; et al. Discovery of benzylisothioureas as potent divalent metal transporter 1 (DMT1) inhibitors. Bioorg. Med. Chem. Lett. 2012, 22, 5108–5113. [Google Scholar] [CrossRef] [PubMed]
- Montalbetti, N.; Simonin, A.; Simonin, C.; Awale, M.; Reymond, J.L.; Hediger, M.A. Discovery and characterization of a novel non-competitive inhibitor of the divalent metal transporter DMT1/SLC11A2. Biochem. Pharmacol. 2015, 96, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Turcu, A.L.; Versini, A.; Khene, N.; Gaillet, C.; Caneque, T.; Muller, S.; Rodriguez, R. DMT1 inhibitors kill cancer stem cells by blocking lysosomal iron translocation. Chemistry 2020, 26, 7369–7373. [Google Scholar] [CrossRef] [PubMed]
- Manatschal, C.; Pujol-Gimenez, J.; Poirier, M.; Reymond, J.L.; Hediger, M.A.; Dutzler, R. Mechanistic basis of the inhibition of SLC11/NRAMP-mediated metal ion transport by bis-isothiourea substituted compounds. Elife 2019, 8, e51913. [Google Scholar] [CrossRef] [PubMed]
- Pulli, B.; Ali, M.; Forghani, R.; Schob, S.; Hsieh, K.L.; Wojtkiewicz, G.; Linnoila, J.J.; Chen, J.W. Measuring myeloperoxidase activity in biological samples. PLoS ONE 2013, 8, e67976. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| Dmt1 | GTGATCCTGACCCGGTCTATCG | TGAGGATGGGTATGAGAGCAAAGG |
| Epo | ATGAAGACTTGCAGCGTGGA | AGGCCCAGAGGAATCAGTAG |
| Erfe | ACTCACCAAGCAGCCAAGAA | TTCTCCAGCCCCATCACAGT |
| TNF-α | CACAAGATGCTGGGACAGTGA | TCCTTGATGGTGGTGCATGA |
| IL-6 | CTGCAAGAGACTTCCATCCAGTT | AGGGAAGGCCGTGGTTGT |
| CypA | CTTACGACAAGCAGCCCTTCATG | AGCTGTTTTTAACTCACTGCTGTTGTA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zhang, M.; Woloshun, R.R.; Yu, Y.; Lee, J.K.; Flores, S.R.L.; Merlin, D.; Collins, J.F. Oral Administration of Ginger-Derived Lipid Nanoparticles and Dmt1 siRNA Potentiates the Effect of Dietary Iron Restriction and Mitigates Pre-Existing Iron Overload in Hamp KO Mice. Nutrients 2021, 13, 1686. https://doi.org/10.3390/nu13051686
Wang X, Zhang M, Woloshun RR, Yu Y, Lee JK, Flores SRL, Merlin D, Collins JF. Oral Administration of Ginger-Derived Lipid Nanoparticles and Dmt1 siRNA Potentiates the Effect of Dietary Iron Restriction and Mitigates Pre-Existing Iron Overload in Hamp KO Mice. Nutrients. 2021; 13(5):1686. https://doi.org/10.3390/nu13051686
Chicago/Turabian StyleWang, Xiaoyu, Mingzhen Zhang, Regina R. Woloshun, Yang Yu, Jennifer K. Lee, Shireen R. L. Flores, Didier Merlin, and James F. Collins. 2021. "Oral Administration of Ginger-Derived Lipid Nanoparticles and Dmt1 siRNA Potentiates the Effect of Dietary Iron Restriction and Mitigates Pre-Existing Iron Overload in Hamp KO Mice" Nutrients 13, no. 5: 1686. https://doi.org/10.3390/nu13051686