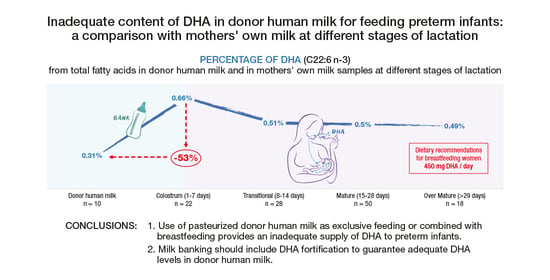
Inadequate Content of Docosahexaenoic Acid (DHA) of Donor Human Milk for Feeding Preterm Infants: A Comparison with Mother’s Own Milk at Different Stages of Lactation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants and Samples
2.3. Biochemical Analysis
2.4. Statistical Analysis
3. Results
3.1. Lipid Profile of the Main Families of Fatty Acids
3.2. Lipid Profile of SFAs
3.3. Lipid Profile of MUFAs
3.4. Lipid Profile of n-6 PUFAs
3.5. Lipid Profile of n-3 PUFAs
3.6. Lipid Quality Indexs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Le, H.D.; Meisel, J.A.; de Meijer, V.E.; Gura, K.M.; Puder, M. The essentiality of arachidonic acid and docosahexaenoic acid. Prostaglandins Leukot. Essent. Fat. Acids 2009, 81, 165–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harayama, T.; Shimizu, T. Roles of polyunsaturated fatty acids, from mediators to membranes. J. Lipid Res. 2020, 61, 1150–1160. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.R.; Kinnun, J.J.; Leng, X.; Williams, J.A.; Wassall, S.R. How polyunsaturated fatty acids modify molecular organization in membranes: Insight from NMR studies of model systems. Biochim. Biophys. Acta BBA Biomembr. 2015, 1848, 211–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Omega-3 Fatty Acids and Inflammatory Processes. Nutrients 2010, 2, 355–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rotstein, N.P.; Politi, L.E.; German, O.L.; Girotti, R. Protective Effect of Docosahexaenoic Acid on Oxidative Stress-Induced Apoptosis of Retina Photoreceptors. Investig. Opthalmol. Vis. Sci. 2003, 44, 2252–2259. [Google Scholar] [CrossRef]
- Lauritzen, L.; Brambilla, P.; Mazzocchi, A.; Harsløf, L.B.S.; Ciappolino, V.; Agostoni, C. DHA Effects in Brain Development and Function. Nutrients 2016, 8, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gil-Campos, M.; Dalmau Serra, J.; Comité de Nutrición de la Asociación Española de Pediatría. Importance of docosahexaenoic acid (DHA) functions and Recommendations for its ingestion in infants. An. Pediatr. Barc. 2010, 73, 142.e1–142.e8. (In Spanish) [Google Scholar] [CrossRef]
- Haggarty, P. Fatty Acid Supply to the Human Fetus. Annu. Rev. Nutr. 2010, 30, 237–255. [Google Scholar] [CrossRef]
- Baack, M.L.; Puumala, S.E.; Messier, S.E.; Pritchett, D.K.; Harris, W.S. What is the relationship between gestational age and docosahexaenoic acid (DHA) and arachidonic acid (ARA) levels? Prostaglandins Leukot. Essent. Fat. Acids 2015, 100, 5–11. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Lavoie, P.M.; Lacaze-Masmonteil, T.; Rhainds, M.; Marc, I. Omega-3 Long-Chain Polyunsaturated Fatty Acids for Extremely Preterm Infants: A Systematic Review. Pediatrics 2014, 134, 120–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuipers, R.S.; Luxwolda, M.F.; Offringa, P.J.; Boersma, E.R.; Dijck-Brouwer, D.J.; Muskiet, F.A. Fetal intrauterine whole body linoleic, arachidonic and docosahexaenoic acid contents and accretion rates. Prostaglandins Leukot. Essent. Fat. Acids 2012, 86, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.L.; Rouse, C.A. Docosahexaenoic acid and the preterm infant. Matern. Health Neonatol. Perinatol. 2017, 3, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueno, H.M.; Higurashi, S.; Shimomura, Y.; Wakui, R.; Matsuura, H.; Shiota, M.; Kubouchi, H.; Yamamura, J.-I.; Toba, Y.; Kobayashi, T. Association of DHA Concentration in Human Breast Milk with Maternal Diet and Use of Supplements: A Cross-Sectional Analysis of Data from the Japanese Human Milk Study Cohort. Curr. Dev. Nutr. 2020, 4, nzaa105. [Google Scholar] [CrossRef] [PubMed]
- Sherry, C.; Oliver, J.; Marriage, B. Docosahexaenoic acid supplementation in lactating women increases breast milk and plasma docosahexaenoic acid concentrations and alters infant omega 6:3 fatty acid ratio. Prostaglandins Leukot. Essent. Fat. Acids 2015, 95, 63–69. [Google Scholar] [CrossRef] [Green Version]
- Juber, B.A.; Jackson, K.H.; Johnson, K.B.; Harris, W.S.; Baack, M.L. Breast milk DHA levels may increase after informing women: A community-based cohort study from South Dakota USA. Int. Breastfeed. J. 2016, 12, 7. [Google Scholar] [CrossRef] [Green Version]
- Forsyth, S.; Gautier, S.; Salem, N., Jr. Global estimates of dietary intake of docosahexaenoic acid andarachidonic acid in developing and developed countries. Ann. Nutr. Metab. 2016, 68, 258–267. [Google Scholar] [CrossRef] [Green Version]
- GOED. Global Organization for EPA and DHA-35. Available online: http://www.goedomega3.com (accessed on 22 February 2021).
- Underwood, M.A. Human Milk for the Premature Infant. Pediatr. Clin. N. Am. 2013, 60, 189–207. [Google Scholar] [CrossRef] [Green Version]
- Luna, M.S.; Martin, S.C.; Gómez-De-Orgaz, C.S. Human milk bank and personalized nutrition in the NICU: A narrative review. Eur. J. Nucl. Med. Mol. Imaging 2021, 180, 1327–1333. [Google Scholar] [CrossRef]
- Bauer, J.; Gerss, J. Longitudinal analysis of macronutrients and minerals in human milk produced by mothers of preterm infants. Clin. Nutr. 2011, 30, 215–220. [Google Scholar] [CrossRef]
- Paul, V.K.; Singh, M.; Srivastava, L.M.; Arora, N.K.; Deorari, A.K. Macronutrient and energy content of breast milk of mothers delivering prematurely. Indian J. Pediatr. 1997, 64, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Sahin, S.; Ozdemir, T.; Katipoglu, N.; Akcan, A.B.; Turkmen, M.K. Comparison of Changes in Breast Milk Macronutrient Content During the First Month in Preterm and Term Infants. Breastfeed. Med. 2020, 15, 56–62. [Google Scholar] [CrossRef]
- Iranpour, R.; Kelishadi, R.; Babaie, S.; Khosravi-Darani, K.; Farajian, S. Comparison of long chain polyunsaturated fatty acid content in human milk in preterm and term deliveries and its correlation with mothers’ diet. J. Res. Med. Sci. 2013, 18, 1–5. [Google Scholar]
- Thakkar, S.K.; De Castro, C.A.; Beauport, L.; Tolsa, J.-F.; Fumeaux, C.J.F.; Affolter, M.; Giuffrida, F. Temporal Progression of Fatty Acids in Preterm and Term Human Milk of Mothers from Switzerland. Nutrients 2019, 11, 112. [Google Scholar] [CrossRef] [Green Version]
- Floris, L.; Stahl, B.; Abrahamse-Berkeveld, M.; Teller, I. Human milk fatty acid profile across lactational stages after term and preterm delivery: A pooled data analysis. Prostaglandins Leukot. Essent. Fat. Acids 2020, 156, 102023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ESPGHAN Committee on Nutrition; Arslanoglu, S.; Corpeleijn, W.; Moro, G.; Braegger, C.; Campoy, C.; Colomb, V.; Decsi, T.; Domellöf, M.; Fewtrell, M.; et al. Donor human milk for preterm infants: Current evidence and research directions. J. Pediatr. Gastroenter. Nutr. 2013, 57, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Lepage, G.; Roy, C.C. Direct transesterification of all classes of lipids in a one-step reaction. J. Lipid Res. 1986, 27, 114–120. [Google Scholar] [CrossRef]
- Baack, M.L.; Norris, A.W.; Yao, J.; Colaizy, T. Long-chain polyunsaturated fatty acid levels in US donor human milk: Meeting the needs of premature infants? J. Perinatol. 2012, 32, 598–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sauerwald, U.C.; Fink, M.M.; Demmelmair, H.; Schoenaich, P.V.; Rauh-Pfeiffer, A.A.; Koletzko, B. Effect of Different Levels of Docosahexaenoic Acid Supply on Fatty Acid Status and Linoleic and α-Linolenic Acid Conversion in Preterm Infants. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, D.R.; Wheaton, D.K.; James, K.J.; Tuazon, M.; Diersen-Schade, D.A.; Harris, C.L.; Stolz, S.; Berseth, C.L. Do-cosahexaenoic acid in red blood cells of term infants receiving two levels of long-chain polyunsaturated fatty acids. J. Pediatr. Gastroenter. Nutr. 2006, 42, 287–292. [Google Scholar] [CrossRef]
- Jensen, C.L.; Maude, M.; Anderson, R.E.; Heird, W.C. Effect of docosahexaenoic acid supplementation of lactating women on the fatty acid composition of breast milk lipids and maternal and infant plasma phospholipids. Am. J. Clin. Nutr. 2000, 71, 292s–299s. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calder, P.C. Docosahexaenoic Acid. Ann. Nutr. Metab. 2016, 69, 8–21. [Google Scholar] [CrossRef]
- Kuratko, C.N.; Barrett, E.C.; Nelson, E.B.; Salem, J.N. The Relationship of Docosahexaenoic Acid (DHA) with Learning and Behavior in Healthy Children: A Review. Nutrients 2013, 5, 2777–2810. [Google Scholar] [CrossRef] [Green Version]
- Fares, S.; Sethom, M.; Feki, M.; Cheour, M.; Sanhaji, H.; Kacem, S.; Kaabachi, N. Fatty acids profile in preterm Colostrum of Tunisian women. Association with selected maternal characteristics. Prostaglandins Leukot. Essent. Fat. Acids 2016, 112, 32–36. [Google Scholar] [CrossRef]
- Kovács, A.; Funke, S.; Marosvölgyi, T.; Burus, I.; Decsi, T. Fatty Acids in Early Human Milk after Preterm and Full-Term Delivery. J. Pediatr. Gastroenterol. Nutr. 2005, 41, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Smithers, L.; Markrides, M.; Gibson, R. Human milk fatty acids from lactating mothers of preterm infants: A study revealing wide intra- and inter-individual variation. Prostaglandins Leukot. Essent. Fat. Acids 2010, 83, 9–13. [Google Scholar] [CrossRef]
- Chen, J.; Liu, H. Nutritional Indices for Assessing Fatty Acids: A Mini-Review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef]
- Miller, J.; Tonkin, E.; Damarell, R.A.; McPhee, A.J.; Suganuma, M.; Suganuma, H.; Middleton, P.F.; Makrides, M.; Collins, C.T. A Systematic Review and Meta-Analysis of Human Milk Feeding and Morbidity in Very Low Birth Weight Infants. Nutrients 2018, 10, 707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, C.R.; DaSilva, D.A.; Cluette-Brown, J.E.; DiMonda, C.; Hamill, A.; Bhutta, A.Q.; Coronel, E.; Wilschanski, M.; Stephens, A.J.; Driscoll, D.F.; et al. Decreased Postnatal Docosahexaenoic and Arachidonic Acid Blood Levels in Premature Infants are Associated with Neonatal Morbidities. J. Pediatr. 2011, 159, 743–749.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, C.T.; Makrides, M.; McPhee, A.J.; Sullivan, T.R.; Davis, P.G.; Thio, M.; Simmer, K.; Rajadurai, V.S.; Travadi, J.; Berry, M.J.; et al. Docosahexaenoic Acid and Bronchopulmonary Dysplasia in Preterm Infants. N. Engl. J. Med. 2017, 376, 1245–1255. [Google Scholar] [CrossRef]
- De Oliveira, S.C.; Bellanger, A.; Ménard, O.; Pladys, P.; Le Gouar, Y.; Dirson, E.; Kroell, F.; Dupont, D.; Deglaire, A.; Bourlieu-Lacanal, C. Impact of human milk pasteurization on gastric digestion in preterm infants: A randomized controlled trial. Am. J. Clin. Nutr. 2017, 105, 379–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nessel, I.; De Rooy, L.; Khashu, M.; Murphy, J.L.; Dyall, S.C. Long-Chain Polyunsaturated Fatty Acids and Lipid Peroxidation Products in Donor Human Milk in the United Kingdom: Results from the LIMIT 2-Centre Cross-Sectional Study. J. Parenter. Enter. Nutr. 2020, 44, 1501–1509. [Google Scholar] [CrossRef] [PubMed]



| Stages of Lact Ation (Number of Mothers) | Mean (Standard Deviation) |
|---|---|
| Colostrum (1–7 days) (n = 22) | |
| Maternal age, years | 31.4 (5.9) |
| Weeks of gestation | 28.5 (2.2) |
| Birth weight of neonates, g | 1063 (382.3) |
| Transitional milk (8–14 days) (n = 28) | |
| Maternal age, years | 31.1 (6.9) |
| Weeks of gestation | 28.9 (2.2) |
| Birth weight of neonates, g | 1103 (329.2) |
| Mature milk (15–28 days) (n = 50) | |
| Maternal age, years | 33.1 (6.0) |
| Weeks of gestation | 29.1 (2.1) |
| Birth weight of neonates, g | 1118.6 (277.8) |
| Mature milk (≥29 days) (n = 18) | |
| Maternal age, years | 34.5 (7.0) |
| Weeks of gestation | 28.7 (2.3) |
| Birth weight of neonates, g | 1123.0 (231.3) |
| Fatty Acids | Donor Human Milk (n = 10) | Stages of Lactation | |||
|---|---|---|---|---|---|
| Colostrum (1–7 Days) (n = 22) | Transitional (8–14 Days) (n = 28) | Mature (15–28 Days) (n = 50) | Mature (≥29 Days) (n = 18) | ||
| Fatty acids families | |||||
| SFAs | 60.57 (4.62) | 48.06 (8.18) | 54.27 (7.24) | 53.26 (8.65) | 53.01 (8.04) |
| MUFAs | 18.79 (3.02) | 28.95 (8.47) | 24.47 (5.90) | 25.16 (8.80) | 26.71 (8.03) |
| PUFAs | 20.64 (3.11) | 23.26 (3.21) | 22.24 (2.59) | 21.81 (3.22) | 22.59 (5.17) |
| n-6 PUFAs | 18.94 (3.19) | 21.52 (3.0) | 20.46 (2.57) | 20.04 (3.22) | 21.19 (3.86) |
| n-3 PUFAS | 1.70 (0.44) | 1.95 (0.80) | 1.85 (0.67) | 1.78 (0.42) | 1.91 (0.69) |
| SFAs | |||||
| Lauric acid (C12:0) | 4.91 (0.93) | 3.82 (1.19) | 6.80 (1.48) | 6.54 (1.52) | 6.18 (1.05) |
| Myristic acid (C14:0) | 5.62 (1.24) | 5.62 (1.26) | 7.86 (2.13) | 7.23 (2.05) | 6.71 (1.75) |
| Palmitic acid (C16:0) | 19.32 (1.20) | 19.69 (1.96) | 18.69 (1.74) | 18.40 (2.33) | 19.08 (2.97) |
| Stearic acid (C18:0) | 30.11 (3.35) | 18.16 (7.84) | 20.08 (7.12) | 20.70 (8.23) | 22.16 (6.50) |
| MUFAs | |||||
| Oleic acid (C18:1 n9) | 13.60 (3.27) | 23.32 (8.11) | 19.55 (6.53) | 19.99 (7.94) | 19.65 (8.29) |
| n-6 PUFAs | |||||
| LA (C18:2 n6) | 16.74 (3.27) | 17.17 (2.63) | 17.54 (2.36) | 17.07 (2.70) | 18.17 (3.95) |
| AA (C20:4 n6) | 0.67 (0.09) | 1.06 (0.36) | 0.92 (0.21) | 0.74 (0.18) | 0.73 (0.12) |
| n-3 PUFAs | |||||
| ALA (C18:3 n3) | 1.09 (0.23) | 0.76 (0.31) | 0.93 (0.46) | 0.90 (0.34) | 0.89 (0.27) |
| DHA (C22:6 n3) | 0.31 (0.16) | 0.66 (0.14) | 0.51 (0.19) | 0.50 (0.26) | 0.49 (0.32) |
| DPA (C22:5 n3) | 0.13 (0.04) | 0.24 (0.12) | 0.15 (0.05) | 0.14 (0.06) | 0.15 (0.08) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castillo, F.; Castillo-Ferrer, F.-J.; Cordobilla, B.; Domingo, J.C. Inadequate Content of Docosahexaenoic Acid (DHA) of Donor Human Milk for Feeding Preterm Infants: A Comparison with Mother’s Own Milk at Different Stages of Lactation. Nutrients 2021, 13, 1300. https://doi.org/10.3390/nu13041300
Castillo F, Castillo-Ferrer F-J, Cordobilla B, Domingo JC. Inadequate Content of Docosahexaenoic Acid (DHA) of Donor Human Milk for Feeding Preterm Infants: A Comparison with Mother’s Own Milk at Different Stages of Lactation. Nutrients. 2021; 13(4):1300. https://doi.org/10.3390/nu13041300
Chicago/Turabian StyleCastillo, Félix, Félix-Joel Castillo-Ferrer, Begoña Cordobilla, and Joan Carles Domingo. 2021. "Inadequate Content of Docosahexaenoic Acid (DHA) of Donor Human Milk for Feeding Preterm Infants: A Comparison with Mother’s Own Milk at Different Stages of Lactation" Nutrients 13, no. 4: 1300. https://doi.org/10.3390/nu13041300