The Gut–Brain Axis and Its Role in Controlling Eating Behavior in Intestinal Inflammation
Abstract
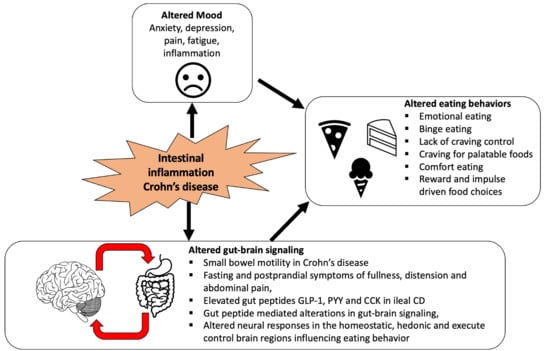
:1. Introduction
2. Methods
3. Eating Behavior in IBD
4. Physiology of Appetite Regulation
4.1. CCK
4.2. GLP-1
4.3. PYY
4.4. Ghrelin
5. Gut–Brain Axis
6. Intestinal Inflammation and Modulation of EEC Peptides
6.1. Inflammatory Response and Body Weight
6.2. Examples of Intestinal Inflammation in Mice and Humans
6.3. EEC Peptides and CD
6.4. Modulation of Gut–Brain Signaling in CD
7. The Effect of Intestinal and Systemic Inflammation on the CNS
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Poulia, K.-A.; Klek, S.; Doundoulakis, I.; Bouras, E.; Karayiannis, D.; Baschali, A.; Passakiotou, M.; Chourdakis, M. The two most popular malnutrition screening tools in the light of the new ESPEN consensus definition of the diagnostic criteria for malnutrition. Clin. Nutr. 2017, 36, 1130–1135. [Google Scholar] [CrossRef] [PubMed]
- Sonnenberg, A.; Collins, J.F. Vicious circles in inflammatory bowel disease. Inflamm. Bowel Dis. 2006, 12, 944–949. [Google Scholar] [CrossRef]
- Wędrychowicz, A.; Zając, A.; Tomasik, P. Advances in nutritional therapy in inflammatory bowel diseases: Review. World J. Gastroenterol. 2016, 22, 1045–1066. [Google Scholar] [CrossRef]
- De Graaf, C.; Blom, W.A.M.; Smeets, P.A.M.; Stafleu, A.; Hendriks, H.F.J. Biomarkers of satiation and satiety. Am. J. Clin. Nutr. 2004, 79, 946–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruen, C.M.; O’Halloran, F.; Cashman, K.D.; Giblin, L. The effects of food components on hormonal signalling in gastrointestinal enteroendocrine cells. Food Funct. 2012, 3, 1131–1143. [Google Scholar] [CrossRef] [PubMed]
- Satherley, R.-M.; Howard, R.; Higgs, S. The prevalence and predictors of disordered eating in women with coeliac disease. Appetite 2016, 107, 260–267. [Google Scholar] [CrossRef]
- Satherley, R.; Howard, R.; Higgs, S. Disordered eating practices in gastrointestinal disorders. Appetite 2015, 84, 240–250. [Google Scholar] [CrossRef]
- Limdi, J.K.; Aggarwal, D.; McLaughlin, J.T. Dietary Practices and Beliefs in Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2016, 22, 164–170. [Google Scholar] [CrossRef] [Green Version]
- Casanova, M.J.; Chaparro, M.; Molina, B.; Merino, O.; Batanero, R.; Dueñas-Sadornil, C.; Robledo, P.; Garcia-Albert, A.M.; Gómez-Sánchez, M.B.; Calvet, X.; et al. Prevalence of Malnutrition and Nutritional Characteristics of Patients with Inflammatory Bowel Disease. J. Crohn’s Colitis 2017, 11, 1430–1439. [Google Scholar] [CrossRef]
- De Vries, J.H.; Dijkhuizen, M.; Tap, P.; Witteman, B.J. Patient’s Dietary Beliefs and Behaviours in Inflammatory Bowel Disease. Dig. Dis. 2019, 37, 131–139. [Google Scholar] [CrossRef]
- Wardle, R.A.; Thapaliya, G.; Nowak, A.; Radford, S.; Dalton, M.; Finlayson, G.; Moran, G.W. An Examination of Appetite and Disordered Eating in Active Crohn’s Disease. J. Crohn’s Colitis 2018, 12, 819–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filippi, J.; Al-Jaouni, R.; Wiroth, J.B.; Hébuterne, X.; Schneider, S.M. Nutritional deficiencies in patients with Crohn’s disease in remission. Inflamm. Bowel Dis. 2006, 12, 185–191. [Google Scholar] [CrossRef]
- Aghdassi, E.; Wendland, B.E.; Stapleton, M.; Raman, M.; Allard, J.P. Adequacy of Nutritional Intake in a Canadian Population of Patients with Crohn’s Disease. J. Am. Diet. Assoc. 2007, 107, 1575–1580. [Google Scholar] [CrossRef]
- Wardle, R.A.; Wardle, A.J.; Charadava, C.; Ghosh, S.; Moran, G.W. Literature review: Impacts of socioeconomic status on the risk of inflammatory bowel disease and its outcomes. Eur. J. Gastroenterol. Hepatol. 2017, 29, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Hindryckx, P.; Laukens, D.; D’Amico, F.; Danese, S. Unmet Needs in IBD: The Case of Fatigue. Clin. Rev. Allergy Immunol. 2018, 55, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Pizzi, L.T.; Weston, C.M.; Goldfarb, N.I.; Moretti, D.; Cobb, N.; Howell, J.B.; Infantolino, A.; Dimarino, A.J.; Cohen, S. Impact of chronic conditions on quality of life in patients with inflammatory bowel disease. Inflamm. Bowel Dis. 2006, 12, 47–52. [Google Scholar] [CrossRef]
- Vavricka, S.R.; Schoepfer, A.; Scharl, M.; Lakatos, P.L.; Navarini, A.A.; Rogler, G. Extraintestinal Manifestations of Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2015, 21, 1982–1992. [Google Scholar] [CrossRef] [Green Version]
- Van Oudenhove, L.; McKie, S.; Lassman, D.; Uddin, B.; Paine, P.; Coen, S.; Gregory, L.; Tack, J.; Aziz, Q. Fatty acid–induced gut-brain signaling attenuates neural and behavioral effects of sad emotion in humans. J. Clin. Investig. 2011, 121, 3094–3099. [Google Scholar] [CrossRef] [Green Version]
- Cavicchia, P.P.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Ma, Y.; Ockene, I.S.; Hébert, J.R. A new dietary inflammatory index predicts interval changes in serum high-sensitivity C-reactive protein. J. Nutr. 2009, 139, 2365–2372. [Google Scholar] [CrossRef]
- Lamers, C.R.; De Roos, N.M.; Witteman BJ, M. The association between inflammatory potential of diet and disease activity: Results from a cross-sectional study in patients with inflammatory bowel disease. BMC Gastroenterol. 2020, 20, 316. [Google Scholar] [CrossRef]
- Dalley, J.W.; Everitt, B.J.; Robbins, T.W. Impulsivity, Compulsivity, and Top-Down Cognitive Control. Neuron 2011, 69, 680–694. [Google Scholar] [CrossRef] [Green Version]
- Evenden, J.L. Varieties of impulsivity. Psychopharmacology 1999, 146, 348–361. [Google Scholar] [CrossRef] [PubMed]
- Kagan, J. Reflection-impulsivity: The generality and dynamics of conceptual tempo. J. Abnorm. Psychol. 1966, 71, 17–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La Barbera, D.; Bonanno, B.; Rumeo, M.V.; Alabastro, V.; Frenda, M.; Massihnia, E.; Morgante, M.C.; Sideli, L.; Craxì, A.; Cappello, M.; et al. Alexithymia and personality traits of patients with inflammatory bowel disease. Sci. Rep. 2017, 7, srep41786. [Google Scholar] [CrossRef] [Green Version]
- Sybil, B.; GEysenck HJ, E. Impulsiveness and venturesomeness: Their position in a dimensional system of personality. Psychol. Rep. 1978, 43, 1247–1255. [Google Scholar]
- Hyphantis, T.; Antoniou, K.; Tomenson, B.; Tsianos, E.; Mavreas, V.; Creed, F. Is the personality characteristic ‘impulsive sensation seeking’ correlated to differences in current smoking between ulcerative colitis and Crohn’s disease patients? Gen. Hosp. Psychiatry 2010, 32, 57–65. [Google Scholar] [CrossRef]
- Reuter, M.; Netter, P. The influence of personality on nicotine craving: A hierarchical multivariate statistical prediction model. Neuropsychobiology 2001, 44, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Berridge, K.C.; Robinson, T.E.; Aldridge, J.W. Dissecting components of reward: ‘liking’, ‘wanting’, and learning. Curr. Opin. Pharmacol. 2010, 3, 65–73. [Google Scholar] [CrossRef] [Green Version]
- Sternini, C.; Anselmia, L.; Rozengurt, E. Enteroendocrine cells: A site of ‘taste’ in gastrointestinal chemosensing. Curr. Opin. Endocrinol. Diabetes Obes. 2008, 15, 73–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Posovszky, C.; Wabitsch, M. Regulation of appetite, satiation, and body weight by enteroendocrine cells. Part 1: Characteristics of enteroendocrine cells and their capability of weight regulation. Horm. Res. Paediatr. 2015, 83, 1–10. [Google Scholar] [CrossRef]
- Date, Y.; Murakami, N.; Toshinai, K.; Matsukura, S.; Niijima, A.; Matsuo, H.; Kangawa, K.; Nakazato, M. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology 2002, 123, 1120–1128. [Google Scholar] [CrossRef]
- Sukkar, S.G.; Vaccaro, A.; Ravera, G.B.; Borrini, C.; Gradaschi, R.; Sacchi-Nemours, A.M.; Cordera, R.; Andraghetti, G. Appetite control and gastrointestinal hormonal behavior (CCK, GLP-1, PYY 1-36) following low doses of a whey protein-rich nutraceutic. Med. J. Nutr. Metab. 2013, 6, 259–266. [Google Scholar] [CrossRef] [Green Version]
- Moran, G.W.; Leslie, F.C.; Levison, S.E.; Worthington, J.; McLaughlin, J.T. Enteroendocrine cells: Neglected players in gastrointestinal disorders? Therap. Adv. Gastroenterol. 2008, 1, 51–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furness, J.B.; Kunze, W.A.A.; Clerc, N., II. The intestine as a sensory organ: Neural, endocrine, and immune responses. Am. J. Physiol. Liver Physiol. 1999, 277, G922–G928. [Google Scholar] [CrossRef] [PubMed]
- Berthoud, H.-R.; Neuhuber, W.L. Functional and chemical anatomy of the afferent vagal system. Auton. Neurosci. 2000, 85, 1–17. [Google Scholar] [CrossRef]
- Berthoud, H.-R. Vagal and hormonal gut-brain communication: From satiation to satisfaction. Neurogastroenterol. Motil. 2008, 20, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.J. The role of gastrointestinal vagal afferents in the control of food intake: Current prospects. Nutrition 2000, 16, 866–873. [Google Scholar] [CrossRef]
- Perry, B.; Wang, Y. Appetite regulation and weight control: The role of gut hormones. Nutr. Diabetes 2012, 2, e26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cummings, D.E.; Overduin, J. Gastrointestinal regulation of food intake. J. Clin. Investig. 2007, 117, 13–23. [Google Scholar] [CrossRef]
- McLaughlin, J.T.; Lomax, R.B.; Hall, L.; Dockray, G.J.; Thompson, D.G.; Warhurst, G. Fatty acids stimulate cholecystokinin secretion via an acyl chain length-specific, Ca2+-dependent mechanism in the enteroendocrine cell line STC-1. J. Physiol. 1998, 513, 11–18. [Google Scholar] [CrossRef]
- Liou, A.P.; Lu, X.; Sei, Y.; Zhao, X.; Pechhold, S.; Carrero, R.J.; Raybould, H.E.; Wank, S. The G-protein-coupled receptor GPR40 directly mediates Long-chain fatty acid-induced secretion of cholecystokinin. Gastroenterology 2011, 140, 903–912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valassi, E.; Scacchi, M.; Cavagnini, F. Neuroendocrine control of food intake. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 158–168. [Google Scholar] [CrossRef]
- Huda MS, B.; Wilding JP, H.; Pinkney, J.H. Gut peptides and the regulation of appetite. Obes. Rev. 2006, 7, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Moran, T.; Sheng, B. Hyperphagia and Obesity of OLETF Rats Lacking CCK1 Receptors: Developmental Aspects. Dev. Psychobiol. 2007, 832–840. [Google Scholar] [CrossRef]
- Leslie, F.C.; Thompson, D.G.; McLaughlin, J.T.; Varro, A.; Dockray, G.J.; Mandal, B.K. Plasma cholecystokinin concentrations are elevated in acute upper gastrointestinal infections. QJM Mon. J. Assoc. Physicians 2003, 96, 870–871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crawley, J.N.; Corwin, R.L. Biological actions of cholecystokinin. Peptides 1994, 15, 731–755. [Google Scholar] [CrossRef]
- Matson, C.A.; Reid, D.F.; Cannon, T.A.; Ritter, R.C. Cholecystokinin and leptin act synergistically to reduce body weight. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 278, R882–R890. [Google Scholar] [CrossRef] [Green Version]
- Herrmann, C.; Göke, R.; Richter, G.; Fehmann, H.-C.; Arnold, R.; Göke, B. Glucagon-Like Peptide-1 and Glucose-Dependent Insulin-Releasing Polypeptide Plasma Levels in Response to Nutrients. Digestion 1995, 56, 117–126. [Google Scholar] [CrossRef]
- Holmes, G.M.; Browning, K.N.; Tong, M.; Qualls-Creekmore, E.; Travagli, R.A. Vagally mediated effects of glucagon-like peptide 1: In vitro and in vivo gastric actions. J. Physiol. 2009, 587, 4749–4759. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.E.; Brubaker, P.L. Glucagon-like peptide 1 secretion by the L-cell: The view from within. Diabetes 2006, 55, S70–S77. [Google Scholar] [CrossRef] [Green Version]
- Chaudhri, O.B.; Wynne, K.; Bloom, S.R. Can gut hormones control appetite and prevent obesity? Diabetes Care 2008, 31 (Suppl. S2), S284–S289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sisley, S.; Gutierrez-Aguilar, R.; Scott, M.; D’Alessio, D.A.; Sandoval, D.A.; Seeley, R.J. Neuronal GLP1R mediates liraglutide’s anorectic but not glucose-lowering effect. J. Clin. Investig. 2014, 124, 2456–2463. [Google Scholar] [CrossRef] [Green Version]
- Baggio, L.L.; Drucker, D.J. Glucagon-like peptide-1 receptors in the brain: Controlling food intake and body weight. J. Clin. Investig. 2014, 124, 4223–4226. [Google Scholar] [CrossRef] [Green Version]
- Secher, A.; Jelsing, J.; Baquero, A.F.; Hecksher-Sørensen, J.; Cowley, M.A.; Dalbøge, L.S.; Hansen, G.; Grove, K.L.; Pyke, C.; Raun, K.; et al. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J. Clin. Investig. 2014, 124, 4473–4488. [Google Scholar] [CrossRef] [Green Version]
- Farr, O.M.; Sofopoulos, M.; Tsoukas, M.A.; Dincer, F.; Thakkar, B.; Sahin-Efe, A.; Filippaios, A.; Bowers, J.; Srnka, A.; Gavrieli, A.; et al. GLP-1 receptors exist in the parietal cortex, hypothalamus and medulla of human brains and the GLP-1 analogue liraglutide alters brain activity related to highly desirable food cues in individuals with diabetes: A crossover, randomised, placebo-controlled trial. Diabetologia 2016, 59, 954–965. [Google Scholar] [CrossRef] [Green Version]
- Kulve, J.S.T.; Veltman, D.J.; Van Bloemendaal, L.; Groot, P.F.C.; Ruhé, H.G.; Barkhof, F.; Diamant, M.; Ijzerman, R.G. Endogenous GLP1 and GLP1 analogue alter CNS responses to palatable food consumption. J. Endocrinol. 2016, 229, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Filippatos, T.D.; Panagiotopoulou, T.V.; Elisaf, M.S. Adverse Effects of GLP-1 Receptor Agonists. Rev. Diabet. Stud. 2015, 202–230. [Google Scholar] [CrossRef] [Green Version]
- Batterham, R.L.; Cowley, M.A.; Small, C.J.; Herzog, H.; Cohen, M.A.; Dakin, C.L.; Wren, A.M.; Brynes, A.E.; Low, M.J.; Ghatei, M.A.; et al. Does gut hormone PYY3-36 decrease food intake in rodents? (reply). Nature 2004, 430, 3–4. [Google Scholar] [CrossRef]
- Anini, Y.; Fu-Cheng, X.; Cuber, J.C.; Kervran, A.; Chariot, J. Comparison of the postprandial release of peptide YY and proglucagon- derived peptides in the rat. Pflug. Arch. Eur. J. Physiol. 1999, 438, 299–306. [Google Scholar] [CrossRef]
- Lin, H.C.; Chey, W.Y.; Zhao, X. Release of distal gut peptide YY (PYY) by fat in proximal gut depends on CCK. Peptides 2000, 21, 1561–1563. [Google Scholar] [CrossRef]
- Kim, B.-J.; Carlson, O.D.; Jang, H.-J.; Elahi, D.; Berry, C.; Egan, J.M. Peptide YY is secreted after oral glucose administration in a gender-specific manner. J. Clin. Endocrinol. Metab. 2005, 90, 6665–6671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lumb, K.J.; DeCarr, L.B.; Milardo, L.F.; Mays, M.R.; Buckholz, T.M.; Fisk, S.E.; Pellegrino, C.M.; Ortiz, A.A.; Mahle, C.D. Novel selective neuropeptide Y2 receptor PEGylated peptide agonists reduce food intake and body weight in mice. J. Med. Chem. 2007, 50, 2264–2268. [Google Scholar] [CrossRef]
- Abbott, C.R.; Small, C.J.; Kennedy, A.R.; Neary, N.M.; Sajedi, A.; Ghatei, M.A.; Bloom, S.R. Blockade of the neuropeptide Y Y2 receptor with the specific antagonist BIIE0246 attenuates the effect of endogenous and exogenous peptide YY (3-36) on food intake. Brain Res. 2005, 1043, 139–144. [Google Scholar] [CrossRef]
- Vincent, R.P.; le Roux, C.W. The satiety hormone peptide YY as a regulator of appetite. J. Clin. Pathol. 2008, 61, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Koda, S.; Date, Y.; Murakami, N.; Shimbara, T.; Hanada, T.; Toshinai, K.; Niijima, A.; Furuya, M.; Inomata, N.; Osuye, K.; et al. The role of the vagal nerve in peripheral PYY 3-36-induced feeding reduction in rats. Endocrinology 2005, 146, 2369–2375. [Google Scholar] [CrossRef] [Green Version]
- Savage, A.P.; Adrian, T.E.; Carolan, G.; Chatterjee, V.K.; Bloom, S.R. Effects of peptide YY (PYY) on mouth to caecum intestinal transit time and on the rate of gastric emptying in healthy volunteers. Gut 1987, 28, 166–170. [Google Scholar] [CrossRef]
- le Roux, C.W.; Borg, C.M.; Murphy, K.G.; Vincent, R.P.; Ghatei, M.A.; Bloom, S.R. Supraphysiological doses of intravenous PYY3-36 cause nausea, but no additional reduction in food intake. Ann. Clin. Biochem. 2008, 45, 93–95. [Google Scholar] [CrossRef] [Green Version]
- Tschop, M.; Smiley, D.L.; Heiman, M.L. Ghrelin induces adiposity in rodents. Nature 2000, 407, 908–913. [Google Scholar] [CrossRef]
- Chen, H.Y.; Trumbauer, M.E.; Chen, A.S.; Weingarth, D.T.; Adams, J.R.; Frazier, E.G.; Shen, Z.; Marsh, D.J.; Feighner, S.D.; Guan, X.-M.; et al. Orexigenic action of peripheral ghrelin is mediated by neuropeptide Y and agouti-related protein. Endocrinology 2004, 145, 2607–2612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Tilve, D.; Heppner, K.; Kirchner, H.; Lockie, S.H.; Woods, S.C.; Smiley, D.L.; Tschöp, M.; Pfluger, P. Ghrelin-induced adiposity is independent of orexigenic effects. FASEB J. 2011, 25, 2814–2822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamazaki, M.; Nakamura, K.; Kobayashi, H.; Matsubara, M.; Hayashi, Y.; Kangawa, K.; Sakai, T. Regulational effect of ghrelin on growth hormone secretion from perifused rat anterior pituitary cells. J. Neuroendocrinol. 2002, 14, 156–162. [Google Scholar] [CrossRef]
- Chuang, J.-C.; Sakata, I.; Kohno, D.; Perello, M.; Osborne-Lawrence, S.; Repa, J.J.; Zigman, J.M. Ghrelin directly stimulates glucagon secretion from pancreatic α-cells. Mol. Endocrinol. 2011, 25, 1600–1611. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Prigeon, R.L.; Davis, H.W.; Bidlingmaier, M.; Kahn, S.E.; Cummings, D.E.; Tschop, M.H.; D’Alessio, D. Ghrelin suppresses glucose-stimulated insulin secretion and deteriorates glucose tolerance in healthy humans. Diabetes 2010, 59, 2145–2151. [Google Scholar] [CrossRef] [Green Version]
- Alfonso, A. Stress and obesity: The ghrelin connection. J. Neuroendocrinol. 2019, 31, e12693. [Google Scholar]
- Nikitopoulou, I.; Kampisiouli, E.; Jahaj, E.; Vassiliou, A.; Dimopoulou, I.; Mastora, Z.; Tsakiris, S.; Perreas, K.; Tzanela, M.; Routsi, C.; et al. Ghrelin alterations during experimental and human sepsis. Cytokine 2020, 127, 154937. [Google Scholar] [CrossRef] [PubMed]
- Baatar, D.; Patel, K.; Taub, D.D. The effects of ghrelin on inflammation and the immune system. Mol. Cell. Endocrinol. 2011, 340, 44–58. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.W.; Porte, D., Jr. Diabetes, Obesity, and the Brain. Science 2005, 307, 375–379. [Google Scholar] [CrossRef]
- Farr, O.M.; Li, C.-S.R.; Mantzoros, C.S. Central nervous system regulation of eating: Insights from human brain imaging. Metabolism 2016, 65, 699–713. [Google Scholar] [CrossRef] [Green Version]
- DiLeone, R.J.; Taylor, J.R.; Picciotto, M.R. The drive to eat: Comparisons and distinctions between mechanisms of food reward and drug addiction. Nat. Neurosci. 2012, 15, 1330–1335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelley, A.E.; Baldo, B.A.; Pratt, W.E.; Will, M.J. Corticostriatal-hypothalamic circuitry and food motivation: Integration of energy, action and reward. Physiol. Behav. 2005, 86, 773–795. [Google Scholar] [CrossRef]
- Morton, G.J.; Meek, T.H.; Schwartz, M.W. Neurobiology of food intake in health and disease. Nat. Rev. Neurosci. 2014, 15, 367–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziauddeen, H.; Alonso-alonso, M.; Hill, J.O.; Kelley, M.; Khan, N.A. Obesity and the Neurocognitive Basis of Food Reward and the Control of Intake. Adv. Nutr. 2015, 474–486. [Google Scholar] [CrossRef] [Green Version]
- Zanchi, D.; Depoorter, A.; Egloff, L.; Haller, S.; Mählmann, L.; Lang, U.E.; Drewe, J.; Beglinger, C.; Schmidt, A.; Borgwardt, S. The impact of gut hormones on the neural circuit of appetite and satiety: A systematic review. Neurosci. Biobehav. Rev. 2017, 80, 457–475. [Google Scholar] [CrossRef]
- McLaughlin, J.T.; McKie, S. Human brain responses to gastrointestinal nutrients and gut hormones. Curr. Opin. Pharmacol. 2016, 31, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gao, J.-H.; Liu, H.-L.; Fox, P.T. The temporal response of the brain after eating revealed by functional MRI. Nature 2000, 405, 1058–1062. [Google Scholar] [CrossRef] [PubMed]
- Smeets PA, M.; De Graaf, C.; Stafleu, A.; Van Osch MJ, P.; Van Der Grond, J. Functional MRI of human hypothalamic responses following glucose ingestion. Neuroimage 2005, 24, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Smeets, P.A.M.; de Graaf, C.; Stafleu, A.; van Osch, M.J.P.; van der Grond, J. Functional magnetic resonance imaging of human hypothalamic responses to sweet taste and calories. Am. J. Clin. Nutr. 2005, 82, 1011–1016. [Google Scholar] [CrossRef]
- Smeets, P.A.M.; Vidarsdottir, S.; De Graaf, C.; Stafleu, A.; Van Osch, M.J.P.; Viergever, M.A.; Pijl, H.; Van Der Grond, J. Oral glucose intake inhibits hypothalamic neuronal activity more effectively than glucose infusion. Am. J. Physiol. Endocrinol. Metab. 2007, 293, 754–758. [Google Scholar] [CrossRef] [Green Version]
- Little, T.J.; McKie, S.; Jones, R.B.; D’Amato, M.; Smith, C.P.; Kiss, O.; Thompson, D.G.; McLaughlin, J.T. Mapping glucose-mediated gut-to-brain signalling pathways in humans. Neuroimage 2014, 96, 1–11. [Google Scholar] [CrossRef]
- Jastreboff, A.M.; Sinha, R.; Arora, J.; Giannini, C.; Kubat, J.; Malik, S.; Van Name, M.A.; Santoro, N.; Savoye, M.; Duran, E.J.; et al. Altered brain response to drinking glucose and fructose in obese adolescents. Diabetes 2016, 65, 1929–1939. [Google Scholar] [CrossRef] [Green Version]
- Van Opstal, A.M.; van den Berg-Huysmans, A.A.; Hoeksma, M.; Blonk, C.; Pijl, H.; Rombouts, S.A.R.B.; van der Grond, J. The effect of consumption temperature on the homeostatic and hedonic responses to glucose ingestion in the hypothalamus and the reward system. Am. J. Clin. Nutr. 2018, 107, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Van Opstal, A.; Kaal, I.; Berg-Huysmans, A.V.D.; Hoeksma, M.; Blonk, C.; Pijl, H.; Rombouts, S.; Van Der Grond, J. Dietary sugars and non-caloric sweeteners elicit different homeostatic and hedonic responses in the brain. Nutrition 2019, 60, 80–86. [Google Scholar] [CrossRef]
- Purnell, J.Q.; Klopfenstein, B.A.; Stevens, A.A.; Havel, P.J.; Adams, S.H.; Dunn, T.N.; Krisky, C.; Rooney, W.D. Brain functional magnetic resonance imaging response to glucose and fructose infusions in humans. Diabetes Obes. Metab. 2011, 13, 229–234. [Google Scholar] [CrossRef] [Green Version]
- Stopyra, M.A.; Friederich, H.-C.; Sailer, S.; Pauen, S.; Bendszus, M.; Herzog, W.; Simon, J.J. The effect of intestinal glucose load on neural regulation of food craving. Nutr. Neurosci. 2019, 24, 109–118. [Google Scholar] [CrossRef]
- Lassman, D.J.; McKie, S.; Gregory, L.J.; Lal, S.; D’Amato, M.; Steele, I.; Varro, A.; Dockray, G.J.; Williams, S.C.; Thompson, D.G. Defining the Role of Cholecystokinin in the Lipid-Induced Human Brain Activation Matrix. Gastroenterology 2010, 138, 1514–1524. [Google Scholar] [CrossRef]
- Jones, R.B.; McKie, S.; Astbury, N.; Little, T.J.; Tivey, S.; Lassman, D.J.; McLaughlin, J.; Luckman, S.; Williams, S.R.; Dockray, G.J.; et al. Functional neuroimaging demonstrates that ghrelin inhibits the central nervous system response to ingested lipid. Gut 2012, 61, 1543–1551. [Google Scholar] [CrossRef]
- Eldeghaidy, S.; Marciani, L.; Hort, J.; Hollowood, T.; Singh, G.; Bush, D.; Foster, T.; Taylor, A.J.; Busch, J.; Spiller, R.C.; et al. Prior Consumption of a Fat Meal in Healthy Adults Modulates the Brain’s Response to Fat. J. Nutr. 2016, 146, 2187–2198. [Google Scholar] [CrossRef] [Green Version]
- Frank-Podlech, S.; Heinze, J.M.; Machann, J.; Scheffler, K.; Camps, G.; Fritsche, A.; Rosenberger, M.; Hinrichs, J.; Veit, R.; Preissl, H. Functional Connectivity within the Gustatory Network Is Altered by Fat Content and Oral Fat—A Pilot Study. Front. Neurosci. 2019, 13, 725. [Google Scholar] [CrossRef]
- Cifford, S.B.; Chou, T.C.; Elmquist, J.K. The Need to Feed: Homeostatic and Hedonic Control of Eating. Neuron 2002, 36, 199–211. [Google Scholar]
- Rossi, M.A.; Stuber, G.D. Overlapping Brain Circuits for Homeostatic and Hedonic Feeding. Cell Metab. 2018, 27, 42–56. [Google Scholar] [CrossRef] [PubMed]
- de Araujo, I.E.; Schatzker, M.; Small, D.M. Rethinking Food Reward. Annu. Rev. Psychol. 2020, 71, 139–164. [Google Scholar] [CrossRef]
- Yeomans, M.R.; Leitch, M.; Gould, N.J.; Mobini, S. Differential hedonic, sensory and behavioral changes associated with flavor-nutrient and flavor-flavor learning. Physiol. Behav. 2008, 93, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Brunstrom, J.M.; Mitchell, G.L. Flavor-nutrient learning in restrained and unrestrained eaters. Physiol. Behav. 2007, 90, 133–141. [Google Scholar] [CrossRef]
- De Araujo, I.E.; Lin, T.; Veldhuizen, M.G.; Small, D.M. Metabolic regulation of brain response to food cues. Curr. Biol. 2013, 23, 878–883. [Google Scholar] [CrossRef] [Green Version]
- Mahida, Y.R.; Wu, K.; Jewell, D.P. Enhanced production of interleukin 1-beta by mononuclear cells isolated from mucosa with active ulcerative colitis of Crohn’s disease. Gut 1989, 30, 835–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahida, Y.R.; Kurlac, L.; Gallagher, A.; Hawkey, C.J. High circulating concentrations of interleukin-6 in active Crohn ’ s disease but not ulcerative colitis. Gut 1991, 32, 1531–1534. [Google Scholar] [CrossRef] [Green Version]
- Macdonald, T.T.; Hutchings, P.; Choy, M.; Murch, S.; Cooke, A. Tumour necrosis factor-alpha and interferon-gamma production measured at the single cell level in normal and inflamed human intestine. Clin. Exp. Immunol. 1990, 81, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Chrousos, G. The Hypothalmic-pituitary-adrenal axis and immune-mediated inflammation. N. Engl. J. Med. 1995, 332, 1351–1362. [Google Scholar] [CrossRef] [PubMed]
- Plata-Salaman, C.R.; Oomura, Y.; Kai, Y. Tumor necrosis factor and interleukin-1 beta: Suppression of food intake by direct action in the central nervous system. Brain Res 1988, 448, 106–114. [Google Scholar] [CrossRef]
- Akiho, H. Cytokine-induced alterations of gastrointestinal motility in gastrointestinal disorders. World J. Gastrointest. Pathophysiol. 2011, 2, 72. [Google Scholar] [CrossRef]
- Plata-Salamán, C.R. Cytokines and feeding. Int. J. Obes. 2001, 25, S48–S52. [Google Scholar] [CrossRef] [PubMed]
- Mcdermott, J.R.; Leslie, F.C.; Thompson, D.G.; Grencis, R.K.; Mclaughlin, J.T. Immune control of food intake: Enteroendocrine cells are regulated by CD4+ T lymphocytes during small intestinal inflammtion. Neurogastroenterology 2006, 55, 492–497. [Google Scholar] [CrossRef] [Green Version]
- Besterman, H.S.; Cook, G.C.; Sarson, D.L.; Christofides, N.D.; Bryant, M.G.; Gregor, M.; Bloom, S.R. Gut hormones in tropical malabsorption. Br. Med. J. 1979, 2, 1252–1255. [Google Scholar] [CrossRef] [Green Version]
- Solteszb, X.W.; Andersson, J.A.R. Cholecystokinin increases small intestinal motility and reduces enteric bacterial overgrowth and translocation in rats with surgically induced acute liver failure. Digestion 1996, 57, 67–72. [Google Scholar]
- Freier, S.; Eran, M.; Faber, J. Effect of cholecystokinin and of its antagonist, of atropine, and of food on the release of immunoglobulin A and immunoglobulin G specific antibodies in the rat intestine. Gastroenterology 1987, 93, 1242–1246. [Google Scholar] [CrossRef]
- Moran, G.W.; Pennock, J.; Mclaughlin, J.T. Enteroendocrine cells in terminal ileal Crohn’s disease. J. Crohn’s Colitis 2012, 6, 871–880. [Google Scholar] [CrossRef] [Green Version]
- Moran, G.W.; Leslie, F.C.; McLaughlin, J.T. Crohn’s disease affecting the small bowel is associated with reduced appetite and elevated levels of circulating gut peptides. Clin. Nutr. 2013, 32, 404–411. [Google Scholar] [CrossRef]
- Keller, J.; Beglinger, C.; Holst, J.J.; Andresen, V.; Layer, P. Mechanisms of gastric emptying disturbances in chronic and acute inflammation of the distal gastrointestinal tract. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297, G861–G868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keller, J.; Binnewies, U.; Rösch, M.; Holst, J.J.; Beglinger, C.; Andresen, V.; Layer, P. Gastric emptying and disease activity in inflammatory bowel disease. Eur. J. Clin. Investig. 2015, 45, 1234–1242. [Google Scholar] [CrossRef]
- Khalaf, A.; Hoad, C.L.; Menys, A.; Nowak, A.; Radford, S.; Taylor, S.A.; Latief, K.; Lingaya, M.; Falcone, Y.; Singh, G.; et al. Gastrointestinal peptides and small-bowel hypomotility are possible causes for fasting and postprandial symptoms in active Crohn’s disease. Am. J. Clin. Nutr. 2019, 111, 131–140. [Google Scholar] [CrossRef]
- Jacobsen, S.H.; Olesen, S.C.; Dirksen, C.; Jørgensen, N.B.; Bojsen-Møller, K.N.; Kielgast, U.; Worm, D.; Almdal, T.; Naver, L.S.; Hvolris, L.E.; et al. Changes in gastrointestinal hormone responses, insulin sensitivity, and beta-cell function within 2 weeks after gastric bypass in non-diabetic subjects. Obes. Surg. 2012, 22, 1084–1096. [Google Scholar] [CrossRef]
- Dirksen, C.; Jørgensen, N.B.; Bojsen-Møller, K.N.; Kielgast, U.; Jacobsen, S.H.; Clausen, T.R.; Worm, D.; Hartmann, B.; Rehfeld, J.F.; Damgaard, M.; et al. Gut hormones, early dumping and resting energy expenditure in patients with good and poor weight loss response after Roux-en-Y gastric bypass. Int. J. Obes. 2013, 37, 1452–1459. [Google Scholar] [CrossRef] [Green Version]
- Scholtz, S.; Miras, A.D.; Chhina, N.; Prechtl, C.G.; Sleeth, M.L.; Daud, N.M.; Ismail, N.A.; Durighel, G.; Ahmed, A.R.; Olbers, T.; et al. Obese patients after gastric bypass surgery have lower brain-hedonic responses to food than after gastric banding. Gut 2014, 63, 891–902. [Google Scholar] [CrossRef] [Green Version]
- Kulve, J.S.T.; Veltman, D.J.; Gerdes, V.E.; Van Bloemendaal, L.; Barkhof, F.; Deacon, C.F.; Holst, J.J.; Drent, M.L.; Diamant, M.; Ijzerman, R.G. Elevated postoperative endogenous GLP-1 levels mediate effects of roux-en-Y gastric bypass on neural responsivity to food cues. Diabetes Care 2017, 40, 1522–1529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zoon, H.F.; De Bruijn, S.E.; Smeets, P.A.; De Graaf, C.; Janssen, I.M.; Schijns, W.; Aarts, E.O.; Jager, G.; Boesveldt, S. Altered neural responsivity to food cues in relation to food preferences, but not appetite-related hormone concentrations after RYGB-surgery. Behav. Brain Res. 2018, 353, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Goldstone, A.P.; Miras, A.D.; Scholtz, S.; Jackson, S.; Neff, K.J.; Pénicaud, L.; Geoghegan, J.; Chhina, N.; Durighel, G.; Bell, J.D.; et al. Link between increased satiety gut hormones and reduced food reward following gastric bypass surgery for obesity. J. Clin. Endocrinol. Metab. 2016, 101, 599–609. [Google Scholar] [CrossRef] [PubMed]
- De Silva, A.; Salem, V.; Long, C.J.; Makwana, A.; Newbould, R.D.; Rabiner, E.A.; Ghatei, M.A.; Bloom, S.R.; Matthews, P.M.; Beaver, J.D.; et al. The Gut Hormones PYY3-36 and GLP-17-36 amide Reduce Food Intake and Modulate Brain Activity in Appetite Centers in Humans Akila. Cell Metab. 2011, 14, 700–706. [Google Scholar] [CrossRef] [Green Version]
- Hubbard, C.S.; Becerra, L.; Heinz, N.; Ludwick, A.; Rasooly, T.; Wu, R.; Johnson, A.; Schechter, N.L.; Borsook, D.; Nurko, S. Abdominal pain, the adolescent and altered brain structure and function. PLoS ONE 2016, 11, e0156545. [Google Scholar] [CrossRef]
- Jones, M.P.; Dilley, J.B.; Drossman, D.; Crowell, M.D. Brain-gut connections in functional GI disorders: Anatomic and physiologic relationships. Neurogastroenterol. Motil. 2006, 18, 91–103. [Google Scholar] [CrossRef]
- Dantzer, R.; O’Connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat. Rev. Neurosci. 2008, 9, 46–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomann, A.K.; Thomann, P.A.; Wolf, R.C.; Hirjak, D.; Schmahl, C.; Ebert, M.P.; Szabo, K.; Reindl, W.; Griebe, M. Altered markers of brain development in Crohn’s disease with extraintestinal manifestations—A pilot study. PLoS ONE 2016, 11, e0163202. [Google Scholar] [CrossRef]
- Ratnakumaran, R.; Warren, L.; Gracie, D.J.; Sagar, R.C.; Hamlin, P.J.; Ford, A.C.; O’Connor, A. Fatigue in Inflammatory Bowel Disease Reflects Mood and Symptom-Reporting Behavior Rather Than Biochemical Activity or Anemia. Clin. Gastroenterol. Hepatol. 2018, 16, 1165–1167. [Google Scholar] [CrossRef] [Green Version]
- Van Erp, S.; Ercan, E.; Breedveld, P.; Brakenhoff, L.; Ghariq, E.; Schmid, S.; Van Osch, M.; Van Buchem, M.; Emmer, B.; Van Der Grond, J.; et al. Cerebral magnetic resonance imaging in quiescent Crohn’s disease patients with fatigue. World J. Gastroenterol. 2017, 23, 1018–1029. [Google Scholar] [CrossRef]
- Bao, C.; Liu, P.; Shi, Y.; Wu, L.; Jin, X.; Zeng, X.; Zhang, J.; Wang, D.; Liu, H.; Wu, H. Differences in brain gray matter volume in patients with Crohn’s disease with and without abdominal pain. Oncotarget 2017, 8, 93624–93632. [Google Scholar] [CrossRef] [Green Version]
- Bao, C.H.; Liu, P.; Liu, H.R.; Wu, L.Y.; Shi, Y.; Chen, W.F.; Qin, W.; Lu, Y.; Zhang, J.Y.; Jin, X.M.; et al. Alterations in brain gray matter structures in patients with Crohn’s disease and their correlation with psychological distress. J. Crohn’s Colitis 2015, 9, 532–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodhand, J.R.; Greig, F.I.; Koodun, Y.; McDermott, A.; Wahed, M.; Langmead, L.; Rampton, D.S. Do antidepressants influence the disease course in inflammatory bowel disease? A retrospective case-matched observational study. Inflamm. Bowel Dis. 2012, 18, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Casella, G.; Tontini, G.E.; Bassotti, G.; Pastorelli, L.; Villanacci, V.; Spina, L.; Baldini, V.; Vecchi, M. Neurological disorders and inflammatory bowel diseases. World J. Gastroenterol. 2014, 20, 8764–8782. [Google Scholar] [PubMed]
- Mrakotsky, C.; Anand, R.; Watson, C.; Vu, C.; Matos, A.; Friel, S.; Rivkin, M.; Snapper, S. New Evidence for Structural Brain Differences in Pediatric Crohn’s Disease: Impact of Underlying Disease Factors. Inflamm. Bowel Dis. 2016, 22, S6–S7. [Google Scholar] [CrossRef] [Green Version]
- Yeung, A.W.K. Structural and functional changes in the brain of patients with Crohn’s disease: An activation likelihood estimation meta-analysis. Brain Imaging Behav. 2020, 1–12. [Google Scholar] [CrossRef]

| Author, Country | Study Sample | Methods | Main Findings |
|---|---|---|---|
| Keller et al. 2009, GERMANY [118] | Active CD patients (n = 13) (4 ileal, 4 colonic, and 5 ileal-colonic), in active UC patients (n = 10), diverticulitis patients (n = 7)and HCs (n = 13) | Postprandial gut hormone levels (assessed by ELISA) and gastric emptying (assessed by 13C-octanoic acid breath test), in response to a standardized breakfast meal | A 3-fold increase in postprandial plasma CCK levels was found in active CD patients compared with HCs and was associated with delayed gastric emptying. Postprandial CCK plasma concentrations were significantly higher in CD patients with exclusively ileal CD compared with the patients with colonic and ileal-colonic CD. No difference in postprandial PYY and GLP-1 was found between CD and HCs. |
| Moran et al. 2012, UK [116] | Terminal ileal tissue from patients with (i) active ileal CD (n = 38), (ii) inactive ileal CD (n = 5), and colonic tissue from patients with (iii) active colonic CD (n = 12) (iv) inactive colonic CD (n = 4) and (v) HCs (n = 60) | Terminal ileal tissue from small or large bowel CD and HCs was analyzed for enteroendocrine marker expression by immunohistochemistry and quantitative polymerase chain reaction. Inflammation was graded by endoscopic, clinical, histological, and biochemical scoring | In ileal CD, GLP-1 and chromogranin A cells were increased 2.5-fold (p = 0.049) and 2-fold (p = 0.031), respectively. PYY cells were unchanged. Ileal EEC expression was unaffected in the presence of colonic CD. Phox2b was co-localized to EEC and showed a 1.5-fold increase in ileal disease. Significant mRNA increases were noted for chromogranin A (3.3-fold; p = 0.009), glucagon-like peptide 1 (3.1-fold; p = 0.007), and ubiquitination protein 4a (2.2-fold; p = 0.02). Neurogenin 3, an enteroendocrine transcription factor showed a 2-fold upregulation (p = 0.048). |
| Moran et al. 2013, UK [117] | Active ileal CD patients (n = 12), inactive ileal CD patients (n = 6), active colonic CD patients (n = 5), and HCs (n = 13) | Gut peptide responses to a mixed nutrient test meal were measured by ELISA. Symptoms were assessed by visual analogue score. A patient subset was re-studied in remission. | Ileal and colonic CD subjects displayed reduced appetite (p < 0.0001) before and after eating a mixed nutrient test meal compared with HCs. Total PYY was increased 2.2-fold (p = 0.04) and correlated with nausea (p = 0.036) and bloating (p = 0.037) scores only in small bowel CD compared with HCs. GLP-1 and GIP were not elevated. In remission, postprandial PYY and ghrelin reverted to control levels. |
| Keller et al. 2015, GERMANY [119] | Active CD patients (n = 14), active UC patients (n = 14), and HCs (n = 24) | Postprandial gastric emptying (measured by 13C-octanoic acid breath test), and gut hormone levels (measured by ELISA) in response to a test meal | Fasting CCK levels and maximal postprandial concentrations were similar between IBD patients and controls, however, patients with UC had significantly lower postprandial CCK levels than the controls and CD patients. No association between CCK plasma levels and gastric emptying was found. Fasting GLP-1, PYY, and postprandial PYY were similar between IBD and HCs. Postprandial GLP-1 responses were increased in IBD (including CD-control and UC-control) and were associated with delayed gastric emptying. The reassessment of IBD patients in remission showed accelerated gastric emptying and normal postprandial increase in GLP-1, indicating that the increased release of GLP-1 from the inflamed gut mucosa in IBD may result in delayed gastric emptying. |
| Wardle et al. 2018, UK [11] | Active CD patients (n = 30); HC (n = 31) | Disordered eating was assessed using validated questionnaires: Binge Eating Scale (BES); Power of Food Scale (PFS); Control of Eating Questionnaire (CoEQ); Dutch Eating Behavior Questionnaire (DEBQ); and Three Factor Eating Questionnaire (TFEQ). Food intake was assessed by 24-h dietary recall | Protein intake was lower in the CD cohort (p = 0.03) compared with HCs. Hospital Anxiety and Depression score was higher (p = 0.01) and CoEQ-Positive Mood (p = 0.001) lower in CD compared with HCs. CD patients were characterized by higher BES (p = 0.01) and lower CoEQ Craving Control (p = 0.027), with greater craving for Sweet (p = 0.043) and Savory (p = 0.021) foods relative to HCs. PFS food Present (food available but not physically present) (p = 0.005), DEBQ Emotional (p =< 0.001), and External Eating (the eating behavior triggered by external environmental stimuli, particularly, the presence of food, smell and/or taste, or even the time of day) (p = 0.022) were significantly higher than among HCs. |
| Khalaf et al. 2020, UK [120] | Active CD patients (n = 15) and 20 HCs (n = 20) | Small bowel motility (measured by MRI) and gut hormone levels (measured by ELISA) in response to a test meal | A decrease in fasting small bowel motility was observed in ileal CD patients compared with HCs. Fasting concentrations of GLP-1 and PYY were significantly greater in CD participants, compared with HCs (p ≤ 0.0001). The meal challenge induced a significant postprandial increase in aversive symptom scores (fullness, distention, bloating, abdominal pain, and sickness) in CD participants compared with HCs (p ≤ 0.05) |
| Yeung et al. 2020, Hong Kong [139] | Sixteen original studies comprised a total of 865 participants, where CD patients in remission (n = 486) and HCs (n = 379) were meta-analyzed | Original studies published until 2019 were identified from Scopus, Web of Science, and PubMed databases and included into the analysis if they reported relevant results from task-related or resting state functional magnetic resonance imaging (fMRI or rsfMRI) or voxel-based morphometry (VBM), in the form of standardized brain coordinates based on whole-brain analysis. The brain coordinates and sample size of significant results were extracted from eligible studies to be meta-analyzed with the activation likelihood estimation method using the GingerALE software | Compared to HCs, patients with CD had reduced resting state brain connectivity in the paracentral lobule (motor function) and cingulate gyrus (emotion and impulse control) as well as reduced grey matter volume in the medial frontal gyrus (executive function). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moran, G.W.; Thapaliya, G. The Gut–Brain Axis and Its Role in Controlling Eating Behavior in Intestinal Inflammation. Nutrients 2021, 13, 981. https://doi.org/10.3390/nu13030981
Moran GW, Thapaliya G. The Gut–Brain Axis and Its Role in Controlling Eating Behavior in Intestinal Inflammation. Nutrients. 2021; 13(3):981. https://doi.org/10.3390/nu13030981
Chicago/Turabian StyleMoran, Gordon William, and Gita Thapaliya. 2021. "The Gut–Brain Axis and Its Role in Controlling Eating Behavior in Intestinal Inflammation" Nutrients 13, no. 3: 981. https://doi.org/10.3390/nu13030981