Let Food Be Thy Medicine—Its Role in Crohn’s Disease
Abstract
:1. Introduction
2. Methods
3. Overview of Different Dietary Strategies
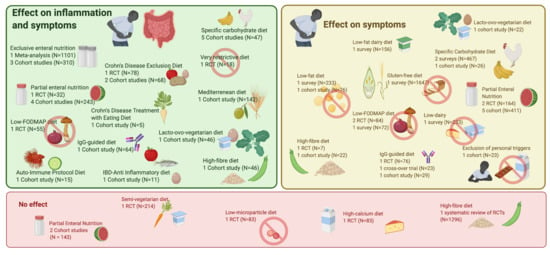
3.1. EEN (Exclusive Enteral Nutrition) and PEN (Partial Enteral Nutrition)
3.2. SCD (Specific Carbohydrate Diet)
3.3. Immunoglobulin G (IgG)-Guided Exclusion Diet
3.4. CDED (Crohn Disease Exclusion Diet)
3.5. CD-TREAT (Treatment-with-Eating Diet)
3.6. IBD-AID (Inflammatory Bowel Disease-Anti-Inflammatory Diet)
3.7. FIT (Food Influence on the Intestinal Microbiota Diet)
3.8. The Autoimmune Protocol Diet (AIP)/Paleolithic Diet
3.9. Mediterranean Diet
3.10. (Semi-)Vegetarian Diet
3.11. High-Fibre Diet
3.12. Fat Content—Dairy Products
3.13. Gluten-Free Diet
3.14. Low-FODMAP (Fermentable Oligo-, Di-, Monosaccharides and Polyols) Diet
3.15. Other Diets
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- De Souza, H.S.P.; Fiocchi, C. Immunopathogenesis of IBD: Current state of the art. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012, 142, 46–54.e42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberti, R.; Iannone, L.F.; Palleria, C.; De Sarro, C.; Spagnuolo, R.; Barbieri, M.A.; Vero, A.; Manti, A.; Pisana, V.; Fries, W.; et al. Safety profiles of biologic agents for inflammatory bowel diseases: A prospective pharmacovigilance study in Southern Italy. Curr. Med. Res. Opin. 2020, 36, 1457–1463. [Google Scholar] [CrossRef] [PubMed]
- Lamb, C.A.; Kennedy, N.A.; Raine, T.; Hendy, P.A.; Smith, P.J.; Limdi, J.K.; Hayee, B.; Lomer, M.C.E.; Parkes, G.C.; Selinger, C.; et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019, 68, s1–s106. [Google Scholar] [CrossRef] [Green Version]
- Cosnes, J.; Gowerrousseau, C.; Seksik, P.; Cortot, A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 2011, 140, 1785–1794.e4. [Google Scholar] [CrossRef]
- Ko, Y.; Butcher, R.; Leong, R.W. Epidemiological studies of migration and environmental risk factors in the inflammatory bowel diseases. World J. Gastroenterol. 2014, 20, 1238–1247. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef] [Green Version]
- Glassner, K.L.; Abraham, B.P.; Quigley, E.M.M. The microbiome and inflammatory bowel disease. J. Allergy Clin. Immunol. 2020, 145, 16–27. [Google Scholar] [CrossRef] [Green Version]
- Dziechciarz, P.; Horvath, A.; Shamir, R.; Szajewska, H. Meta-analysis: Enteral nutrition in active Crohn’s disease in children. Aliment. Pharmacol. Ther. 2007, 26, 795–806. [Google Scholar] [CrossRef]
- Levine, A.; Wine, E.; Assa, A.; Sigall Boneh, R.; Shaoul, R.; Kori, M.; Cohen, S.; Peleg, S.; Shamaly, H.; On, A.; et al. Crohn’s Disease Exclusion Diet Plus Partial Enteral Nutrition Induces Sustained Remission in a Randomized Controlled Trial. Gastroenterology 2019, 157, 440–450.e8. [Google Scholar] [CrossRef] [Green Version]
- Logan, M.; Clark, C.M.; Ijaz, U.Z.; Gervais, L.; Duncan, H.; Garrick, V.; Curtis, L.; Buchanan, E.; Cardigan, T.; Armstrong, L.; et al. The reduction of faecal calprotectin during exclusive enteral nutrition is lost rapidly after food re-introduction. Aliment. Pharmacol. Ther. 2019, 50, 664–674. [Google Scholar] [CrossRef]
- Grover, Z.; Burgess, C.; Muir, R.; Reilly, C.; Lewindon, P.J. Early mucosal healing with exclusive enteral nutrition is associated with improved outcomes in newly diagnosed children with Luminal Crohn’s disease. J. Crohn’s Colitis 2016, 10, 1159–1164. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Yu, J.; Lou, J.; Fang, Y.; Chen, J. Clinical Study Exclusive Enteral Nutrition versus Infliximab in Inducing Therapy of Pediatric Crohn’ s Disease. Gastroenterol. Res. Pract. 2017, 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonald PJ, F.V. What can Crohn’s patients eat? Eur. J. Clin. Nutr. 1988, 42, 703–708. [Google Scholar] [PubMed]
- Kakodkar, S.; Mutlu, E.A. Diet as a Therapeutic Option for Adult Inflammatory Bowel Disease. Gastroenterol. Clin. N. Am. 2017, 46, 745–767. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.; Ross, V.; Mahadevan, U. Popular exclusionary diets for inflammatory bowel disease: The search for a dietary culprit. Inflamm. Bowel Dis. 2014, 20, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Triggs, C.M.; Munday, K.; Hu, R.; Fraser, A.G.; Gearry, R.B.; Barclay, M.L.; Ferguson, L.R. Dietary factors in chronic inflammation: Food tolerances and intolerances of a New Zealand Caucasian Crohn’s disease population. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2010, 690, 123–138. [Google Scholar] [CrossRef]
- Cohen, A.B.; Lee, D.; Long, M.D.; Kappelman, M.D.; Martin, C.F.; Sandler, R.S.; James, D. Dietary Patterns and Self-Reported Associations of Diet with Symptoms of Inflammatory Bowel Disease. Dig. Dis. Sci. 2013, 58, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- Pittet, V.; Vaucher, C.; Maillard, M.H.; Girardin, M.; De Saussure, P.; Burnand, B.; Rogler, G.; Michetti, P. Information Needs and Concerns of Patients with Inflammatory Bowel Disease: What Can We Learn from Participants in a Bilingual Clinical Cohort? PLoS ONE 2016, 11, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vagianos, K.; Bector, S.; McConnell, J.; Bernstein, C.N. Nutrition assessment of patients with inflammatory bowel disease. J. Parenter. Enter. Nutr. 2007, 31, 311–319. [Google Scholar] [CrossRef]
- Filippi, J.; Al-Jaouni, R.; Wiroth, J.B.; Hébuterne, X.; Schneider, S.M. Nutritional deficiencies in patients with Crohn’s disease in remission. Inflamm. Bowel Dis. 2006, 12, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Dunleavy, K.A.; Ungaro, R.C.; Manning, L.; Gold, S.; Novak, J.; Colombel, J.F. Vitamin C deficiency in inflammatory bowel disease: The forgotten micronutrient. J. Crohn’s Colitis 2020, 14, S209. [Google Scholar] [CrossRef]
- Eadala, P.; Matthews, S.B.; Waud, J.P.; Green, J.T.; Campbell, A.K. Association of lactose sensitivity with inflammatory bowel disease—Demonstrated by analysis of genetic polymorphism, breath gases and symptoms. Aliment. Pharmacol. Ther. 2011, 34, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Durchschein, F.; Petritsch, W.; Hammer, H.F. Diet therapy for inflammatory bowel diseases: The established and the new. World J. Gastroenterol. 2016, 22, 2179–2194. [Google Scholar] [CrossRef] [PubMed]
- Wedlake, L.; Slack, N.; Andreyev, H.J.N.; Whelan, K. Fiber in the treatment and maintenance of inflammatory bowel disease: A systematic review of randomized controlled trials. Inflamm. Bowel Dis. 2014, 20, 576–586. [Google Scholar] [CrossRef] [PubMed]
- Narula, N.; Dhillon, A.; Zhang, D.; Sherlock, M.E.; Tondeur, M.; Zachos, M. Enteral nutritional therapy for induction of remission in Crohn’s disease. Cochrane Database Syst. Rev. 2018, 2018. [Google Scholar] [CrossRef]
- Takayuki, Y.; Maki, N.; Satoru, U.; Tatsushi, K.; Matsumoto, K. Impact of Elemental Diet on Mucosal Inflammation in Patients with Active Crohn’s Disease: Cytokine Production and Endoscopic and Histological Findings. Inflamm. Bowel Dis. 2005, 11, 580–588. [Google Scholar]
- Yang, Q.; Gao, X.; Chen, H.; Li, M.; Wu, X.; Zhi, M.; Lan, P.; Hu, P. Efficacy of exclusive enteral nutrition in complicated Crohn’s disease. Scand. J. Gastroenterol. 2017, 52, 995–1001. [Google Scholar] [CrossRef]
- Verma, S.; Holdsworth, C.D.; Giaffer, M.H. Does adjuvant nutritional support diminish steroid dependency in Crohn disease? Scand. J. Gastroenterol. 2001, 36, 383–388. [Google Scholar] [CrossRef]
- Esaki, M.; Matsumoto, T.; Nakamura, S.; Yada, S.; Fujisawa, K.; Jo, Y.; Iida, M. Factors affecting recurrence in patients with Crohn’s disease under nutritional therapy. Dis. Colon Rectum 2006, 49, 68–74. [Google Scholar] [CrossRef]
- Kamata, N.; Oshitani, N.; Watanabe, K.; Watanabe, K.; Hosomi, S.; Noguchi, A.; Yukawa, T.; Yamagami, H.; Shiba, M.; Tanigawa, T.; et al. Efficacy of Concomitant Elemental Diet Therapy in Scheduled Infliximab Therapy in Patients with Crohn’s Disease to Prevent Loss of Response. Dig. Dis. Sci. 2015, 60, 1382–1388. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, Y.; Cho, J.M.; Oh, S.H.; Kim, K.M. Therapeutic efficacy of oral enteral nutrition in pediatric crohn’s disease: A single center non-comparative retrospective study. Yonsei Med. J. 2016, 57, 1185–1191. [Google Scholar] [CrossRef] [Green Version]
- Schulman, J.M.; Pritzker, L.; Shaoul, R. Maintenance of Remission with Partial Enteral Nutrition Therapy in Pediatric Crohn’s Disease: A Retrospective Study. Can. J. Gastroenterol. Hepatol. 2017, 2017. [Google Scholar] [CrossRef]
- Ohara, N.; Mizushima, T.; Iijima, H.; Takahashi, H.; Hiyama, S.; Haraguchi, N.; Inoue, T.; Nishimura, J.; Shinzaki, S.; Hata, T.; et al. Adherence to an elemental diet for preventing postoperative recurrence of Crohn’s disease. Surg. Today 2017, 47, 1519–1525. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Takahama, K.; Kimura, T.; Mizuno, T.; Nagasaka, M.; Iwata, K.; Nakano, H.; Muramatsu, M.; Takazoe, M. Effect of concurrent elemental diet on infliximab treatment for Crohn’s disease. J. Gastroenterol. Hepatol. 2006, 21, 1143–1149. [Google Scholar] [CrossRef]
- Takagi, S.; Utsunomiya, K.; Kuriyama, S.; Yokoyama, H.; Takahashi, S.; Iwabuchi, M.; Takahashi, H.; Takahashi, S.; Kinouchi, Y.; Hiwatashi, N.; et al. Effectiveness of an “half elemental diet” as maintenance therapy for Crohn’s disease: A randomized-controlled trial. Aliment. Pharmacol. Ther. 2006, 24, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Nakahigashi, M.; Umegae, S.; Kitagawa, T.; Matsumoto, K. Impact of long-term enteral nutrition on clinical and endoscopic recurrence after resection for Crohn’s disease: A prospective, non-randomized, parallel, controlled study. Aliment. Pharmacol. Ther. 2007, 25, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Hartman, C.; Berkowitz, D.; Weiss, B.; Shaoul, R.; Levine, A.; Adiv, O.E.; Shapira, R.; Fradkin, A.; Wilschanski, M.; Tamir, A.; et al. Nutritional supplementation with polymeric diet enriched with transforming growth factor-beta 2 for children with Crohn’s disease. Isr. Med. Assoc. J. 2008, 10, 503–507. [Google Scholar] [PubMed]
- Yamamoto, T.; Nakahigashi, M.; Umegae, S.; Matsumoto, K. Prospective clinical trial: Enteral nutrition during maintenance infliximab in Crohn’s disease. J. Gastroenterol. 2010, 45, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Hanai, H.; Iida, T.; Takeuchi, K.; Arai, H.; Arai, O.; Abe, J.; Tanaka, T.; Maruyama, Y.; Ikeya, K.; Sugimoto, K.; et al. Nutritional therapy versus 6-mercaptopurine as maintenance therapy in patients with Crohn’s disease. Dig. Liver Dis. 2012, 44, 649–654. [Google Scholar] [CrossRef]
- Yamamoto, T.; Shiraki, M.; Nakahigashi, M.; Umegae, S.; Matsumoto, K. Enteral nutrition to suppress postoperative Crohn’s disease recurrence: A five-year prospective cohort study. Int. J. Colorectal Dis. 2013, 28, 335–340. [Google Scholar] [CrossRef]
- Kang, Y.; Kim, S.; Kim, S.Y.; Koh, H. Effect of short-term partial enteral nutrition on the treatment of younger patients with severe Crohn’s disease. Gut Liver 2015, 9, 87–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, T.; Nakahigashi, M.; Saniabadi, A.R.; Iwata, T.; Maruyama, Y.; Umegae, S.; Matsumoto, K. Impacts of long-term enteral nutrition on clinical and endoscopic disease activities and mucosal cytokines during remission in patients with Crohn’s disease: A prospective study. Inflamm. Bowel Dis. 2007, 13, 1493–1501. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Guo, Z.; Cao, L.; Xie, T.; Shen, W.; Li, Y.; Gong, J.; Zhu, W. Isolated colonic Crohn’s disease is associated with a reduced response to exclusive enteral nutrition compared to ileal or ileocolonic disease. Clin. Nutr. 2019, 38, 1629–1635. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.A.; Gold, B.D.; Oliva, S.; Lewis, J.; Stallworth, A.; Koch, B.; Eshee, L.; Mason, D. Clinical and mucosal improvement with specific carbohydrate diet in pediatric crohn disease. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Suskind, D.L.; Wahbeh, G.; Gregory, N.; Vendettuoli, H.; Christie, D. Nutritional therapy in pediatric crohn disease: The specific carbohydrate diet. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 87–91. [Google Scholar] [CrossRef] [Green Version]
- Kakodkar, S.; Farooqui, A.J.; Mikolaitis, S.L.; Mutlu, E.A. The Specific Carbohydrate Diet for Inflammatory Bowel Disease: A Case Series. J. Acad. Nutr. Diet. 2015, 115, 1226–1232. [Google Scholar] [CrossRef] [Green Version]
- Suskind, D.L.; Wahbeh, G.; Cohen, S.A.; Damman, C.J.; Klein, J.; Braly, K.; Shaffer, M.; Lee, D. Patients Perceive Clinical Benefit with the Specific Carbohydrate Diet for Inflammatory Bowel Disease. Dig. Dis. Sci. 2016, 61, 3255–3260. [Google Scholar] [CrossRef]
- Burgis, J.C.; Nguyen, K.; Park, K.T.; Cox, K. Response to strict and liberalized specific carbohydrate diet in pediatric Crohn’s disease. World J. Gastroenterol. 2016, 22, 2111–2117. [Google Scholar] [CrossRef] [PubMed]
- Obih, C.; Wahbeh, G.; Lee, D.; Braly, K.; Giefer, M.; Shaffer, M.L.; Nielson, H.; Suskind, D.L. Specific carbohydrate diet for pediatric inflammatory bowel disease in clinical practice within an academic IBD center. Nutrition 2016, 32, 418–425. [Google Scholar] [CrossRef]
- Wahbeh, G.T.; Ward, B.T.; Lee, D.Y.; Giefer, M.J.; Suskind, D.L. Lack of Mucosal Healing from Modified Specific Carbohydrate Diet in Pediatric Patients with Crohn Disease. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 289–292. [Google Scholar] [CrossRef]
- Suskind, D.L.; Cohen, S.A.; Brittnacher, M.J.; Wahbeh, G.; Lee, D.; Shaffer, M.L.; Braly, K.; Hayden, H.S.; Klein, J.; Gold, B.; et al. Clinical and Fecal Microbial Changes with Diet Therapy in Active Inflammatory Bowel Disease. J. Clin. Gastroenterol. 2018, 52, 155–163. [Google Scholar] [CrossRef]
- Bentz, S.; Hausmann, M.; Piberger, H.; Kellermeier, S.; Paul, S.; Held, L.; Falk, W.; Obermeier, F.; Fried, M.; Schölmerich, J.; et al. Clinical relevance of IgG antibodies against food antigens in Crohn’s disease: A double-blind cross-over diet intervention study. Digestion 2010, 81, 252–264. [Google Scholar] [CrossRef] [Green Version]
- Rajendran, N.; Kumar, D. Food-specific IgG4-guided exclusion diets improve symptoms in Crohn’s disease: A pilot study. Color. Dis. 2011, 13, 1009–1013. [Google Scholar] [CrossRef]
- Gunasekeera, V.; Mendall, M.A.; Chan, D.; Kumar, D. Treatment of Crohn’s Disease with an IgG4-Guided Exclusion Diet: A Randomized Controlled Trial. Dig. Dis. Sci. 2016, 61, 1148–1157. [Google Scholar] [CrossRef]
- Wang, G.; Ren, J.; Li, G.; Hu, Q.; Gu, G.; Ren, H.; Hong, Z.; Li, J. The utility of food antigen test in the diagnosis of Crohn’s disease and remission maintenance after exclusive enteral nutrition. Clin. Res. Hepatol. Gastroenterol. 2018, 42, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Sigall-Boneh, R.; Pfeffer-Gik, T.; Segal, I.; Zangen, T.; Boaz, M.; Levine, A. Partial enteral nutrition with a Crohn’s disease exclusion diet is effective for induction of remission in children and young adults with Crohn’s disease. Inflamm. Bowel Dis. 2014, 20, 1353–1360. [Google Scholar] [CrossRef]
- Sigall Boneh, R.; Shabat, C.S.; Yanai, H.; Chermesh, I.; Avraham, S.B.; Boaz, M.; Levine, A. Dietary therapy with the Crohn’s disease exclusion diet is a successful strategy for induction of Remission in children and adults failing biological therapy. J. Crohn’s Colitis 2017, 11, 1205–1212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svolos, V.; Hansen, R.; Nichols, B.; Quince, C.; Ijaz, U.Z.; Papadopoulou, R.T.; Edwards, C.A.; Watson, D.; Alghamdi, A.; Brejnrod, A.; et al. Treatment of Active Crohn’s Disease With an Ordinary Food-based Diet That Replicates Exclusive Enteral Nutrition. Gastroenterology 2019, 156, 1354–1367.e6. [Google Scholar] [CrossRef] [Green Version]
- Olendzki, B.C.; Silverstein, T.D.; Persuitte, G.M.; Ma, Y.; Baldwin, K.R.; Cave, D. An anti-inflammatory diet as treatment for inflammatory bowel disease: A ca...: Discovery Service for Endeavour College of Natural Health Library. Nutr. J. 2014, 13, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabino, J.; Lewis, J.D.; Colombel, J.F. Treating Inflammatory Bowel Disease With Diet: A Taste Test. Gastroenterology 2019, 157, 295–297. [Google Scholar] [CrossRef] [Green Version]
- Sabino, J.; Vieira-Silva, S.; Machiels, K.; Joossens, M.; Falony, G.; Ferrante, M.; Van Assche, G.A.; Van Der Merwe, S.; Matthys, C.; Raes, J.; et al. Therapeutic Manipulation of the Gut Microbiota Through Diet to Reduce Intestinal Inflammation: Results from the FIT Trial. Gastroenterology 2017, 152, S1. [Google Scholar] [CrossRef]
- Konijeti, G.G.; Kim, N.; Lewis, J.D.; Groven, S.; Chandrasekaran, A.; Grandhe, S.; Diamant, C.; Singh, E.; Oliveira, G.; Wang, X.; et al. Efficacy of the Autoimmune Protocol Diet for Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2017, 23, 2054–2060. [Google Scholar] [CrossRef] [PubMed]
- Chicco, F.; Magrì, S.; Cingolani, A.; Paduano, D.; Pesenti, M.; Zara, F.; Tumbarello, F.; Urru, E.; Melis, A.; Casula, L.; et al. Multidimensional Impact of Mediterranean Diet on IBD Patients. Inflamm. Bowel Dis. 2020, 27, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Albenberg, L.; Brensinger, C.M.; Wu, Q.; Gilroy, E.; Kappelman, M.D.; Sandler, R.S.; Lewis, J.D. A Diet Low in Red and Processed Meat Does Not Reduce Rate of Crohn’s Disease Flares. Gastroenterology 2019, 157, 128–136.e5. [Google Scholar] [CrossRef] [Green Version]
- Chiba, M.; Abe, T.; Tsuda, H.; Sugawara, T.; Tsuda, S.; Tozawa, H.; Fujiwara, K.; Imai, H. Lifestyle-related disease in Crohn’s disease: Relapse prevention by a semi-vegetarian diet. World J. Gastroenterol. 2010, 16, 2484–2495. [Google Scholar] [CrossRef]
- Chiba, M.; Tsuji, T.; Nakane, K.; Tsuda, S.; Ishii, H.; Ohno, H.; Watanabe, K.; Ito, M.; Komatsu, M.; Sugawara, T. Induction with Infliximab and a Plant-Based Diet as First-Line (IPF) Therapy for Crohn Disease: A Single-Group Trial. Perm. J. 2017, 21, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Brotherton, C.S.; Taylor, A.G.; Bourguinon, C.; Anderson, J.G. High fiber diet may improve... CD. Gastroenterol. Nurs. 2014, 37, 206–216. [Google Scholar] [CrossRef] [Green Version]
- Chiba, M.; Tsuji, T.; Nakane, K.; Komatsu, M. High Amount of Dietary Fiber Not Harmful But Favorable for Crohn Disease. Perm. J. 2015, 19, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morton, H.; Pedley, K.C.; Stewart, R.J.C.; Coad, J. Inflammatory bowel disease: Are symptoms and diet linked? Nutrients 2020, 12, 2975. [Google Scholar] [CrossRef]
- Nolan-Clark, D.; Tapsell, L.C.; Hu, R.; Han, D.Y.; Ferguson, L.R. Effects of Dairy Products on Crohn’s Disease Symptoms Are Influenced by Fat Content and Disease Location but not Lactose Content or Disease Activity Status in a New Zealand Population. J. Am. Diet. Assoc. 2011, 111, 1165–1172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, M.; Iwao, Y.; Sasaki, S.; Okamoto, S.; Ogata, H.; Hibi, T.; Kazuma, K. Moderate dietary temperance effectively prevents relapse of Crohn disease: A prospective study of patients in remission. Gastroenterol. Nurs. 2007, 30, 202–210. [Google Scholar] [CrossRef]
- Ajabnoor, S.M.; Forbes, A. Effect of fat composition in enteral nutrition for Crohn’s disease in adults: A systematic review. Clin. Nutr. 2017. [Google Scholar] [CrossRef] [Green Version]
- Feagan, B.G.; Sandborn, W.J.; Mittmann, U.; Bar-Meir, S.; D’Haens, G.; Rutgeerts, P. Omega-3 free fatty acids for the maintenance of remission in Crohn disease: The EPIC randomized controlled trials. JAMA 2009, 299, 1690–1697. [Google Scholar] [CrossRef] [Green Version]
- Hilsden, R.J.; Scott, C.M.; Verhoef, M.J. Complementary medicine use by patients with inflammatory bowel disease. Am. J. Gastroenterol. 1998, 93, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Herfarth, H.H.; Martin, C.F.; Sandler, R.S.; Kappelman, M.D.; Long, M.D. Prevalence of a gluten-free diet and improvement of clinical symptoms in patients with inflammatory bowel diseases. Inflamm. Bowel Dis. 2014, 20, 1194–1197. [Google Scholar] [CrossRef]
- Oxford, E.C.; Nguyen, D.D.; Sauk, J.; Korzenik, J.R.; Yajnik, V.; Friedman, S.; Ananthakrishnan, A.N. Impact of co-existent celiac disease on phenotype and natural history of Inflammatory Bowel Diseases. Am. J. Gastroenterol. 2013, 108, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biesiekierski, J.R.; Peters, S.L.; Newnham, E.D.; Rosella, O.; Muir, J.G.; Gibson, P.R. No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology 2013, 145, 320–328.e3. [Google Scholar] [CrossRef] [PubMed]
- Skodje, G.I.; Sarna, V.K.; Minelle, I.H.; Rolfsen, K.L.; Muir, J.G.; Gibson, P.R.; Veierød, M.B.; Henriksen, C.; Lundin, K.E.A. Fructan, Rather Than Gluten, Induces Symptoms in Patients With Self-Reported Non-Celiac Gluten Sensitivity. Gastroenterology 2018, 154, 529–539.e2. [Google Scholar] [CrossRef] [Green Version]
- Halmos, E.P.; Power, V.A.; Shepherd, S.J.; Gibson, P.R.; Muir, J.G. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology 2014, 146, 67–75.e5. [Google Scholar] [CrossRef]
- Cox, S.R.; Lindsay, J.O.; Fromentin, S.; Stagg, A.J.; McCarthy, N.E.; Galleron, N.; Ibraim, S.B.; Roume, H.; Levenez, F.; Pons, N.; et al. Effects of Low FODMAP Diet on Symptoms, Fecal Microbiome, and Markers of Inflammation in Patients With Quiescent Inflammatory Bowel Disease in a Randomized Trial. Gastroenterology 2020, 158, 176–188.e7. [Google Scholar] [CrossRef] [Green Version]
- Halpin, S.J.; Ford, A.C. Prevalence of symptoms meeting criteria for irritable bowel syndrome in inflammatory bowel disease: Systematic review and meta-analysis. Am. J. Gastroenterol. 2012, 107, 1474–1482. [Google Scholar] [CrossRef] [PubMed]
- Quigley, E.M.M. Overlapping irritable bowel syndrome and inflammatory bowel disease: Less to this than meets the eye? Therap. Adv. Gastroenterol. 2016, 9, 199–212. [Google Scholar] [CrossRef] [Green Version]
- Ford, A.C.; Sperber, A.D.; Corsetti, M.; Camilleri, M. Series Functional Gastrointestinal Disorders 2 Irritable bowel syndrome. Lancet 2020, 6736, 1–14. [Google Scholar] [CrossRef]
- Gearry, R.B.; Irving, P.M.; Barrett, J.S.; Nathan, D.M.; Shepherd, S.J.; Gibson, P.R. Reduction of dietary poorly absorbed short-chain carbohydrates (FODMAPs) improves abdominal symptoms in patients with inflammatory bowel disease-a pilot study. J. Crohn’s Colitis 2009, 3, 8–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charlebois, A.; Rosenfeld, G.; Bressler, B. The Impact of Dietary Interventions on the Symptoms of Inflammatory Bowel Disease: A Systematic Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1370–1378. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.R.; Prince, A.C.; Myers, C.E.; Irving, P.M.; Lindsay, J.O.; Lomer, M.C.; Whelan, K. Fermentable carbohydrates [FODMAPs] exacerbate functional gastrointestinal symptoms in patients with inflammatory bowel disease: A randomised, double-blind, placebo-controlled, cross-over, re-challenge trial. J. Crohn’s Colitis 2017, 11, 1420–1429. [Google Scholar] [CrossRef] [Green Version]
- Bodini, G.; Zanella, C.; Crespi, M.; Lo Pumo, S.; Demarzo, M.G.; Savarino, E.; Savarino, V.; Giannini, E.G. A randomized, 6-wk trial of a low FODMAP diet in patients with inflammatory bowel disease. Nutrition 2019, 67–68, 110542. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Lomer, M.C.E.; Anderson, J.L.; Barrett, J.S.; Muir, J.G.; Irving, P.M.; Whelan, K. Fermentable carbohydrate restriction reduces luminal bifidobacteria and gastrointestinal symptoms in patients with irritable bowel syndrome. J. Nutr. 2012, 142, 1510–1518. [Google Scholar] [CrossRef]
- Halmos, E.P.; Christophersen, C.T.; Bird, A.R.; Shepherd, S.J.; Gibson, P.R.; Muir, J.G. Diets that differ in their FODMAP content alter the colonic luminal microenvironment. Gut 2015, 64, 93–100. [Google Scholar] [CrossRef]
- Joossens, M.; Huys, G.; Cnockaert, M.; De Preter, V.; Verbeke, K.; Rutgeerts, P.; Vandamme, P.; Vermeire, S. Dysbiosis of the faecal microbiota in patients with Crohn’s disease and their unaffected relatives. Gut 2011, 60, 631–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Png, C.W.; Lindén, S.K.; Gilshenan, K.S.; Zoetendal, E.G.; McSweeney, C.S.; Sly, L.I.; McGuckin, M.A.; Florin, T.H.J. Mucolytic bacteria with increased prevalence in IBD mucosa augment in vitro utilization of mucin by other bacteria. Am. J. Gastroenterol. 2010, 105, 2420–2428. [Google Scholar] [CrossRef]
- Stange, E.F.; Schroeder, B.O. Microbiota and mucosal defense in IBD: An update. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 963–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartel, G.; Weiss, I.; Turetschek, K.; Schima, W.; Püspök, A.; Waldhoer, T.; Gasche, C. Ingested matter affects intestinal lesions in Crohn’s disease. Inflamm. Bowel Dis. 2008, 14, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Lomer, M.C.E.; Grainger, S.L.; Ede, R.; Catterall, A.P.; Greenfield, S.M.; Cowan, R.E.; Vicary, F.R.; Jenkins, A.P.; Fidler, H.; Harvey, R.S.; et al. Lack of efficacy of a reduced microparticle diet in a multi-centred trial of patients with active Crohn’s disease. Eur. J. Gastroenterol. Hepatol. 2005, 17, 377–384. [Google Scholar] [CrossRef]
- Komperød, M.J.; Sommer, C.; Mellin-Olsen, T.; Iversen, P.O.; Røseth, A.G.; Valeur, J. Persistent symptoms in patients with Crohn’s disease in remission: An exploratory study on the role of diet. Scand. J. Gastroenterol. 2018, 53, 573–578. [Google Scholar] [CrossRef] [PubMed]
| Diet | Intervention | Personalized |
|---|---|---|
| EEN | Liquid formula-based diet delivered to the gastro-intestinal tract orally or through nasogastric feeding. | No |
| PEN | Whole food diet supplemented with liquid formula-based diet for a prespecified percentage of calories. | No |
| SCD | Restriction of complex carbohydrates and elimination of refined sugar. | No |
| IgG-guided exclusion | Exclusion of foods with high serum anti-IgG titers. | Yes |
| CDED | Exclusion of gluten and gluten-free baked goods, dairy products, animal fat, processed meats, emulsifiers, canned goods, and all packaged products with a due date. Restrictions can be loosened after 6 weeks. | No |
| CD-TREAT | Exclusion of gluten, lactose, and alcohol and matching of macronutrients, vitamins, minerals, and fibre with ordinary foods and multivitamin tablets, guided by personal preference. | Yes |
| IBD-AID | Whole foods diet consisting of lean meats, poultry, fish, omega-3 fatty acids, eggs, particular sources of carbohydrate, select fruits and vegetables, nut and legume flours, limited aged cheeses (made with active cultures and enzymes), fresh cultured yogurt, kefir, miso and other cultured products, and honey. Probiotics are suggested and dietetic advice is necessary for the symptomatology-based texture adaptations through the course of the diet. | Yes |
| FIT | Semi-vegetarian diet characterized by the exclusion of added sugars, processed foods, and emulsifiers, increased consumption of fibre, and decreased consumption of meat and fish. | No |
| AIP | Avoidance of grains, legumes, nightshades, dairy, eggs, coffee, alcohol, nuts and seeds, refined/processed sugars, oils, and food additives with personalized reintroduction combined with life-style advice. | Yes |
| Mediterranean | Diet promoting consumption of vegetables, fruits, breads and cereals, olive oil, legumes, fish/seafood, eggs, poultry, dairy foods, and low consumption of red meat and sweets. | No |
| Semi-Vegetarian | Low consumption of red or processed meat (not more than 1 serving per month). | No |
| Lacto-Ovo-(Semi)vegetarian | Consumption of eggs, milk, vegetables, legumes, fruits, rice, soup, potatoes and plain yoghurt. (Only one serving of meat per 2 weeks, 1 serving of fish per week.) | No |
| Gluten-free | Strict exclusion of gluten in the diet. | No |
| Low-FODMAP | Diet limiting fructose, lactose, fructans, galactans and polyols, thereby excluding poorly absorbed short-chain carbohydrates. | No |
| Low-Microparticle | Limiting of foods with microparticles TiO2 and/or Psil. | No |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wellens, J.; Vermeire, S.; Sabino, J. Let Food Be Thy Medicine—Its Role in Crohn’s Disease. Nutrients 2021, 13, 832. https://doi.org/10.3390/nu13030832
Wellens J, Vermeire S, Sabino J. Let Food Be Thy Medicine—Its Role in Crohn’s Disease. Nutrients. 2021; 13(3):832. https://doi.org/10.3390/nu13030832
Chicago/Turabian StyleWellens, Judith, Séverine Vermeire, and João Sabino. 2021. "Let Food Be Thy Medicine—Its Role in Crohn’s Disease" Nutrients 13, no. 3: 832. https://doi.org/10.3390/nu13030832