Exploratory Efficacy of Calcium-Vitamin D Milk Fortification and Periodontal Therapy on Maternal Oral Health and Metabolic and Inflammatory Profile
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Study Population and Eligibility Criteria
2.3. Intervention
2.4. Data Collection
2.5. Oral Health Examination
2.6. Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benjamin, R.M. Oral health: The silent epidemic. Public Health Rep. 2010, 125, 158–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazir, M.; Al-Ansari, A.; Al-Khalifa, K.; Alhareky, M.; Gaffar, B.; Almas, K. Global prevalence of periodontal disease and lack of its surveillance. Sci. World J. 2020, 28, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-De-Albornoz, A.; Figuero, E.; Herrera, D.; Cuesta, P.; Bascones-Martínez, A. Gingival changes during pregnancy: III. Impact of clinical, microbiological, immunological and socio-demographic factors on gingival inflammation. J. Clin. Periodontol. 2012, 39, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Figuero, E.; Carrillo-De-Albornoz, A.; Martín, C.; Tobías, A.; Herrera, D. Effect of pregnancy on gingival inflammation in systemically healthy women: A systematic review. J. Clin. Periodontol. 2013, 40, 457–473. [Google Scholar] [CrossRef]
- Laine, M.A. Effect of pregnancy on periodontal and dental health. Acta Odontol. Scand. 2002, 60, 257–264. [Google Scholar] [CrossRef]
- Chokwiriyachit, A.; Dasanayake, A.P.; Suwannarong, W.; Hormdee, D.; Sumanonta, G.; Prasertchareonsuk, W.; Wara-Aswapati, N.; Combellick, J.; Pitiphat, W. Periodontitis and gestational diabetes mellitus in non-smoking females. J. Periodontol. 2013, 84, 857–862. [Google Scholar] [CrossRef]
- Nguyen, A.T.M.; Akhter, R.; Garde, S.; Scott, C.; Twigg, S.M.; Colagiuri, S.; Ajwani, S.; Eberhard, J. The association of periodontal disease with the complications of diabetes mellitus. A systematic review. Diabetes Res. Clin. Pract. 2020, 108244. [Google Scholar] [CrossRef]
- Chambrone, L.; Guglielmetti, M.R.; Pannuti, C.M.; Chambrone, L.A. Evidence grade associating periodontitis to preterm birth and/or low birth weight: I. A systematic review of prospective cohort studies. J. Clin. Periodontol. 2011, 38, 795–808. [Google Scholar] [CrossRef]
- Da Silva, G.M.; Coutinho, S.B.; Piscoya, M.D.B.V.; Ximenes, R.A.A.; Jamelli, S.R. Periodontitis as a risk factor for preeclampsia. J. Periodontol. 2012, 83, 1388–1396. [Google Scholar] [CrossRef]
- Bi, W.G.; Emami, E.; Luo, Z.-C.; Santamaria, C.; Wei, S.Q. Effect of periodontal treatment in pregnancy on perinatal outcomes: A systematic review and meta-analysis. J. Matern. Neonatal Med. 2019, 21, 1–10. [Google Scholar] [CrossRef]
- Da Silva, H.E.C.; Stefani, C.M.; Melo, N.D.S.; Lima, A.D.A.D.; Rösing, C.K.; Porporatti, A.L.; Canto, G.D.L. Effect of intra-pregnancy nonsurgical periodontal therapy on inflammatory biomarkers and adverse pregnancy outcomes: A systematic review with meta-analysis. Syst. Rev. 2017, 6, 197. [Google Scholar] [CrossRef] [Green Version]
- Govindasamy, R.; Periyasamy, S.; Narayanan, M.; Balaji, V.R.; Dhanasekaran, M.; Karthikeyan, B. The influence of nonsurgical periodontal therapy on the occurrence of adverse pregnancy outcomes: A systematic review of the current evidence. J. Indian Soc. Periodontol. 2020, 24, 7–14. [Google Scholar] [CrossRef]
- Petersen, P.E. World Health Organization global policy for improvement of oral health-World Health Assembly 2007. Int. Dent. J. 2008, 58, 115–121. [Google Scholar] [CrossRef] [Green Version]
- Dietrich, T.; Joshipura, K.J.; Dawson-Hughes, B.; Bischoff-Ferrari, H.A. Association between serum concentrations of 25-hydroxyvitamin D3 and periodontal disease in the US population. Am. J. Clin. Nutr. 2004, 80, 108–113. [Google Scholar]
- Adegboye, A.R.; Christensen, L.B.; Holm-Pedersen, P.; Avlund, K.; Boucher, B.J.; Heitmann, B.L. Intakes of calcium, vitamin D, and dairy servings and dental plaque in older Danish adults. Nutr. J. 2013, 12, 61. [Google Scholar] [CrossRef] [Green Version]
- Adegboye, A.R.; Boucher, B.J.; Kongstad, J.; Fiehn, N.-E.; Christensen, L.B.; Heitmann, B.L. Calcium, vitamin D, casein and whey protein intakes and periodontitis among Danish adults. Public Health Nutr. 2016, 19, 503–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adegboye, A.R.A.; Christensen, L.B.; Holm-Pedersen, P.; Avlund, K.; Boucher, B.J.; Heitmann, B.L. Intake of dairy products in relation to periodontitis in older Danish adults. Nutrients 2012, 4, 1219–1229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adegboye, A.R.A.; Twetman, S.; Christensen, L.B.; Heitmann, B.L. Intake of dairy calcium and tooth loss among adult Danish men and women. Nutrition 2012, 28, 779–784. [Google Scholar] [CrossRef]
- Agrawal, A.A.; Kolte, A.P.; Kolte, R.A.; Chari, S.; Gupta, M.; Pakhmode, R. Evaluation and comparison of serum vitamin D and calcium levels in periodontally healthy, chronic gingivitis and chronic periodontitis in patients with and without diabetes mellitus–a cross-sectional study. Acta Odontol. Scand. 2019, 77, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Tomaz, M.R.; Macit, M.S.; Niza, M.I.; Jeavons, C.A.; Heitmann, B.L.; Adegboye, A.R.A. The effect of Vitamin D and/or Calcium supplementation on Periodontitis: Systematic review. Int. J. Food Nutr. Public Health 2019, 11, 65–81. [Google Scholar]
- Asemi, Z.; Tabassi, Z.; Heidarzadeh, Z.; Khorammian, H.; Sabihi, S.-S.; Samimi, M. Effect of calcium-vitamin D supplementation on metabolic profiles in pregnant women at risk for pre-eclampsia: A randomized placebo-controlled trial. Pakistan J. Biol. Sci. 2012, 15, 316–324. [Google Scholar] [CrossRef]
- Asemi, Z.; Samimi, M.; Siavashani, M.A.; Mazloomi, M.; Tabassi, Z.; Karamali, M.; Jamilian, M. Calcium-Vitamin D co-supplementation affects metabolic profiles, but not pregnancy outcomes, in healthy pregnant women. Int. J. Prev. Med. 2016, 7, 49. [Google Scholar] [PubMed]
- Cormick, G.; Betrán, A.P.; Romero, I.B.; Lombardo, C.F.; Gülmezoglu, A.M.; Ciapponi, A.; Belizán, J.M. Global inequities in dietary calcium intake during pregnancy: A systematic review and meta-analysis. BJOG Int. J. Obstet. Gynaecol. 2019, 126, 444–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adegboye, A.R.A.; Santana, D.D.; Cocate, P.G.; Benaim, C.; Dos Santos, P.P.T.; Heitmann, B.L.; Carvalho, M.C.D.V.S.; Schlüssel, M.M.; De Castro, M.B.T.; Kac, G. Vitamin D and calcium milk fortification in pregnant women with periodontitis: A feasibility trial. Int. J. Environ. Res. Public Health 2020, 17, 8023. [Google Scholar] [CrossRef]
- Adegboye, A.R.A.; Cocate, P.G.; Benaim, C.; Carvalho, M.C.D.V.S.; Schlüssel, M.M.; De Castro, M.B.T.; Kac, G.; Heitmann, B.L. Recruitment of low-income pregnant women into a dietary and dental care intervention: Lessons from a feasibility trial. Trials 2020, 21, 244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hallingberg, B.; Turley, R.; Segrott, J.; Wight, D.; Craig, P.; Moore, L.; Murphy, S.; Robling, M.; Simpson, S.A.; Moore, G. Exploratory studies to decide whether and how to proceed with full-scale evaluations of public health interventions: A systematic review of guidance. Pilot Feasibility Stud. 2018, 4, 104. [Google Scholar] [CrossRef] [PubMed]
- Cocate, P.G.; Kac, G.; Heitmann, B.L.; Nadanovsky, P.; Carvalho, M.C.D.V.S.; Benaim, C.; Schlüssel, M.M.; De Castro, M.B.T.; Alves-Santos, N.H.; Baptista, A.F.; et al. Calcium and vitamin D supplementation and/or periodontal therapy in the treatment of periodontitis among Brazilian pregnant women: Protocol of a feasibility randomised controlled trial (the IMPROVE trial). Pilot Feasibility Stud. 2019, 5, 38. [Google Scholar] [CrossRef] [Green Version]
- Eldridge, S.M.; Chan, C.L.; Campbell, M.J.; Bond, C.M.; Hopewell, S.; Thabane, L.; Lancaster, G.A. CONSORT 2010 statement: Extension to randomised pilot and feasibility trials. BMJ 2016, 355, 5239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeung, C.A. Book review: Oral health surveys: Basic methods, 5th edition. Br. Dent. J. 2014, 217, 333. [Google Scholar] [CrossRef]
- Shah, P.B. Intention-to-treat and per-protocol analysis. Can. Med. Assoc. J. 2011, 183, 696. [Google Scholar] [CrossRef] [Green Version]
- Checchi, L.; Montevecchi, M.; Checchi, V.; Zappulla, F. The relationship between bleeding on probing and subgingival deposits. An endoscopical evaluation. Open Dent. J. 2009, 3, 154–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leal, N.P.; Jannotti, C.B. Oral Health of pregnant women attended by SUS: Practices and representations of professionals and patients. Femina 2009, 37, 413–421. [Google Scholar]
- Marla, V.; Srii, R.; Roy, D.K.; Ajmera, H. The Importance of oral health during pregnancy: A review. Med. Express 2018, 5. [Google Scholar] [CrossRef]
- Khan, F.R.; Ahmad, T.; Hussain, R.; Bhutta, Z.A. A randomized controlled trial of oral vitamin D supplementation in pregnancy to improve maternal periodontal health and birth weight. J. Int. Oral Health 2016, 8, 657–665. [Google Scholar]
- Ernst, G.D.; De Jonge, L.L.; Hofman, A.; Lindemans, J.; Russcher, H.; Steegers, E.A.; Jaddoe, V.W. C-reactive protein levels in early pregnancy, fetal growth patterns, and the risk for neonatal complications: The Generation R Study. Am. J. Obstet. Gynecol. 2011, 205, 132.e1–132.e12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sacks, G.; Seyani, L.; Lavery, S.; Trew, G. Maternal C-reactive protein levels are raised at 4 weeks gestation. Hum. Reprod. 2004, 19, 1025–1030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almaghamsi, A.; Almalki, M.H.; Buhary, B.M. Hypocalcemia in pregnancy: A clinical review update. Oman Med. J. 2018, 33, 453–462. [Google Scholar] [CrossRef]
- Ladipo, O.A. Nutrition in pregnancy: Mineral and vitamin supplements. Am. J. Clin. Nutr. 2000, 72, 280S–290S. [Google Scholar] [CrossRef] [Green Version]
- Kovacs, C.S.; Fuleihan, G.E.-H. Calcium and bone disorders during pregnancy and lactation. Endocrinol. Metab. Clin. N. Am. 2006, 35, 21–51. [Google Scholar] [CrossRef]
- Jagelavičienė, E.; Vaitkevičienė, I.; Šilingaitė, D.; Šinkūnaitė, E.; Daugėlaitė, G. The Relationship between Vitamin D and Periodontal Pathology. Medicina 2018, 54, 45. [Google Scholar] [CrossRef] [Green Version]
- Sonagra, A.D. Normal Pregnancy- A State of Insulin Resistance. J. Clin. Diagn. Res. 2014. Available online: http://jcdr.net/article_fulltext.asp?issn=0973-709x&year=2014&volume=8&issue=11&page=CC01&issn=0973-709x&id=5081 (accessed on 10 December 2020).
- Yasuhi, I.; Yamashita, H.; Maeda, K.; Nomiyama, M.; Mizunoe, T.; Tada, K.; Yorozu, M.; Ogawa, M.; Kodama, T.; Yamaguchi, K.; et al. High-intensity breastfeeding improves insulin sensitivity during early post-partum period in obese women with gestational diabetes. Diabetes Metab. Res. Rev. 2019, 35, e3127. [Google Scholar] [CrossRef]
- Murguía-Romero, M.; Jiménez-Flores, J.R.; Méndez-Cruz, A.R.; Sigrist-Flores, S.C.; Villaobos-Molina, R. Insulin and HOMA-IR in healthy young mexicans: A cut-off points proposal. Intern. Med. Open Access. 2014, 6, 1–5. [Google Scholar]
- Riskin-Mashiah, S.; Damti, A.; Younes, G.; Auslander, R. Normal fasting plasma glucose levels during pregnancy: A hospital-based study. J. Peérinat. Med. 2011, 39, 209–211. [Google Scholar] [CrossRef] [PubMed]
- Osorio-Yáñez, C.; Qiu, C.; Gelaye, B.; Enquobahrie, D.A.; Williams, M.A. Risk of gestational diabetes mellitus in relation to maternal dietary calcium intake. Public Health Nutr. 2017, 20, 1082–1089. [Google Scholar] [CrossRef] [Green Version]
- Heppe, D.H.; Van Dam, R.M.; Willemsen, S.P.; Breeijen, H.D.; Raat, H.; Hofman, A.; Ap Steegers, E.; Jaddoe, V.W.; Steegers, E.A. Maternal milk consumption, fetal growth, and the risks of neonatal complications: The Generation R Study. Am. J. Clin. Nutr. 2011, 94, 501–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsen, S.F.; Halldorsson, T.I.; Willett, W.C.; Knudsen, V.K.; Gillman, M.W.; Mikkelsen, T.B.; Olsen, J.; The NUTRIX Consortium. Milk consumption during pregnancy is associated with increased infant size at birth: Prospective cohort study. Am. J. Clin. Nutr. 2007, 86, 1104–1110. [Google Scholar] [CrossRef] [Green Version]
- Xiong, X.; Buekens, P.; Goldenberg, R.L.; Offenbacher, S.; Qian, X. Optimal timing of periodontal disease treatment for prevention of adverse pregnancy outcomes: Before or during pregnancy? Am. J. Obstet. Gynecol. 2011, 205, 111.e1–111.e6. [Google Scholar] [CrossRef] [PubMed]
- Offenbacher, S.; Beck, J.D.; Jared, H.L.; Mauriello, S.M.; Mendoza, L.C.; Couper, D.J.; Stewart, D.D.; Murtha, A.P.; Cochran, D.L.; Dudley, D.J.; et al. Effects of periodontal therapy on rate of preterm delivery: A randomized controlled trial. Obstet. Gynecol. 2009, 114, 551–559. [Google Scholar] [CrossRef] [Green Version]
- Polyzos, N.P.; Polyzos, I.P.; Zavos, A.; Valachis, A.; Mauri, D.; Papanikolaou, E.G.; Tzioras, S.; Weber, D.; Messinis, I.E. Obstetric outcomes after treatment of periodontal disease during pregnancy: Systematic review and meta-analysis. BMJ 2010, 341, c7017. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Early PT | Late PT | ||||
|---|---|---|---|---|---|
| Variables | Fortification | Placebo | Fortification | Placebo | p-Value b |
| Age (years) (mean, SD) | 27.5 (6.4) | 26.5 (6.3) | 28.7 (6.0) | 28.8 (4.3) | 0.63 |
| Schooling (years) (mean, SD) | 10.2 (2.3) | 10.5 (2.3) | 10.7 (1.8) | 10.7 (2.7) | 0.93 |
| Per-capita income (USD) c (mean, SD) | 105.9 (60.5) | 181.9 (146.6) | 162.1 (111.5) | 159.7 (106.6) | 0.23 |
| Marital status (n, %) | |||||
| Living with a partner | 13 (21.6) | 13 (21.6) | 18 (30.0) | 16 (26.6) | 0.43 |
| Other d | 4 (44.4) | 2 (22.2) | 1 (11.1) | 2 (22.2) | |
| Self-reported skin colour (n, %) | |||||
| White | 3 (37.5) | 0 (0) | 2 (25) | 3 (37.5) | 0.36 e |
| Mixed or black | 14 (22.9) | 15 (24.5) | 17 (27.8) | 15 (24.5) | |
| Gestational age (weeks) (mean, SD) | 16 (2.5) | 15 (2.5) | 17 (2.1) | 16 (2.6) | 0.60 |
| Pre-pregnancy BMI (kg/m2) (mean, SD) | 26.3 (6.5) | 27.1 (8.0) | 27.7 (4.5) | 28.0 (9.2) | 0.23 |
| Parity (number of parturitions) (n, %) | |||||
| No previous delivery | 7 (29.1) | 5 (20.8) | 7 (29.1) | 5 (20.8) | 0.86 |
| One or more previous deliveries | 10 (22.2) | 10 (22.2) | 12 (26.6) | 13 (28.8) | |
| Smoking (n, %) | |||||
| No | 16 (26.2) | 13 (21.3) | 16 (26.2) | 16 (26.2) | 0.85 e |
| Current smoker | 1 (12.5) | 2 (25.0) | 3 (37.5) | 2 (25.0) | |
| Alcohol intake (n, %) | |||||
| No | 15 (26.3) | 12 (21) | 15 (26.3) | 15 (26.3) | 0.90 e |
| Yes | 2 (16.6) | 3 (25.0) | 4 (33.3) | 3 (25.0) | |
| Pocket depth (mm) (mean, SD) | 4.3 (0.19) | 4.21 (0.21) | 4.22 (0.19) | 4.28 (0.24) | 0.51 |
| Clinical attachment loss (mm) (mean, SD) | 4.3 (0.20) | 4.24 (0.23) | 4.27 (0.19) | 4.3 (0.27) | 0.89 |
| Sites with bleeding on probing (BOP) (mean, SD) | 0.21 (0.15) | 0.20 (0.14) | 0.15 (0.11) | 0.23 (0.16) | 0.33 |
| Early PT | Late PT | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Fortification | Placebo | Fortification | Placebo | p | |||||
| n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | Value | |
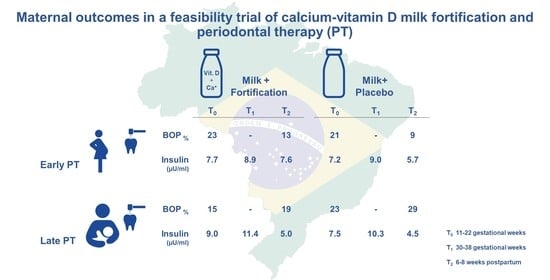
| BOP (%) | |||||||||
| T0 | 13 | 0.23 (0.16) | 9 | 0.21 (0.18) | 17 | 0.15 (0.11) | 16 | 0.23 (0.16) | |
| T2 | 0.13 (0.12) | 0.09 (0.07) | 0.19 (0.20) | 0.29 (0.18) | |||||
| T | −0.10 (0.15) | −0.11 (0.15) | 0.04 (0.19) | 0.07 (0.15) | <0.01 (0.76) | ||||
| PD (mm) | |||||||||
| T0 | 13 | 4.34 (0.20) | 9 | 4.25 (0.24) | 17 | 4.25 (0.19) | 16 | 4.27 (0.25) | |
| T2 | 4.28 (0.19) | 4.22 (0.26) | 4.20 (0.13) | 4.29 (0.17) | |||||
| T | −0.06 (0.25) | −0.03 (0.19) | −0.05 (0.21) | 0.02 (0.22) | 0.66 (0.31) | ||||
| CAL (mm) | |||||||||
| T0 | 13 | 4.34 (0.21) | 9 | 4.32 (0.27) | 17 | 4.29 (0.18) | 16 | 4.29 (0.29) | |
| T2 | 4.32 (0.19) | 4.29 (0.34) | 4.37 (0.43) | 4.29 (0.17) | |||||
| T | −0.02 (0.23) | −0.03 (0.30) | 0.08 (0.48) | 0.00 (0.27) | 0.18 (0.36) | ||||
| Calcium (mg/dL) | |||||||||
| T0 | 13 | 9.0 (0.6) | 9 | 9.3 (0.5) | 17 | 9.3 (0.7) | 15 | 9.0 (0.6) | |
| T1 | 8.7 (0.5) | 8.8 (0.6) | 8.9 (0.4) | 9.0 (0.8) | |||||
| T2 | 9.1 (0.7) | 8.7 (0.6) | 9.2 (0.6) | 9.0 (0.7) | |||||
| T | 0.1 (1.0) | −0.6 (0.7) | −0.1 (0.6) | 0.1 (0.6) | 0.53 (0.75) | ||||
| Vitamin D (ng/mL) | |||||||||
| T0 | 13 | 25.2 (9.4) | 9 | 30.1 (17.7) | 17 | 30.5 (6.3) | 16 | 31.5 (12.5) | |
| T1 | 29.9 (9.5) | 27.3 (7.8) | 31.2 (8.8) | 32.6 (10.0) | |||||
| T2 | 23.4 (10.5) | 28.8 (11.5) | 27.2 (7.1) | 28.1 (9.5) | |||||
| T | −1.8 (9.5) | −1.3 (12.1) | −3.3 (6.7) | −3.5 (5.4) | 0.43 (0.97) | ||||
| CRP (mg/L) | |||||||||
| T0 | 13 | 12.3 (8.2) | 9 | 8.9 (5.5) | 17 | 11.2 (7.5) | 16 | 8.8 (5.1) | |
| T1 | 8.3 (5.2) | 6.3 (3.5) | 12.7 (8.2) | 10.8 (8.7) | |||||
| T2 | 6.4 (4.9) | 7.6 (7.5) | 7.2 (7.4) | 4.6 (3.5) | |||||
| T | −5.9 (7.1) | −1.3 (9.5) | −4.0 (7.8) | −4.2 (4.8) | 0.90 (0.41) | ||||
| Glucose (mg/dL) | |||||||||
| T0 | 13 | 71.3 (6.4) | 9 | 73.4 (7.5) | 17 | 73.9 (7.3) | 16 | 72.7 (8.6) | |
| T1 | 77.7 (13.9) | 68.0 (8.4) | 73.7 (13.2) | 78.8 (14.5) | |||||
| T2 | 80.2 (14.6) | 75.9 (6.8) | 78.5 (11.5) | 81.3 (11.8) | |||||
| T | 8.9 (16.9) | 2.5 (8.7) | 4.6 (12.9) | 8.6 (11.8) | 0.92 (0.99) | ||||
| Insulin (µU/mL) | |||||||||
| T0 | 13 | 7.7 (4.2) | 9 | 7.2 (4.0) | 17 | 9.0 (3.8) | 16 | 7.5 (3.5) | |
| T1 | 8.9 (4.0) | 9.0 (3.5) | 11.4 (4.4) | 10.3 (3.9) | |||||
| T2 | 7.6 (5.2) | 5.7 (2.8) | 5.0 (2.5) | 4.5 (1.9) | |||||
| T | −0.1 (7.2) | −1.5 (4.5) | −4.0 (2.7) | −3.0 (2.9) | 0.02 (0.95) | ||||
| Birthweight (g) | |||||||||
| T2 | 13 | 3467.1 (327.0) | 9 | 3266.8 (457.6) | 17 | 3334.8 (385) | 16 | 3404.8 (573.6) | 0.85 (0.73) |
| Early PT | Late PT | ||||
|---|---|---|---|---|---|
| Fortification | Placebo | Fortification | Placebo | p | |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Value | |
| Calcium (mg/dL) | |||||
| T1–T0 | −0.36 (0.77) | −0.46 (0.85) | −0.38 (0.89) | 0.06 (1.21) | 0.37 (0.41) |
| T2–T1 | 0.42 (0.49) | −0.08 (0.64) | 0.29 (0.71) | −0.04 (1.3) | 0.81 (0.10) |
| T | 0.10 (1.0) | −0.60 (0.7) | −0.10 (0.6) | 0.10 (0.5) | 0.53 (0.75) |
| Vitamin D (ng/mL) | |||||
| T1–T0 | 3.98 (7.82) | −3.96 (15.6) | 0.99 (7.53) | 0.94 (8.3) | 0.84 (0.17) |
| T2–T1 | −6.49 (8.65) | 1.59 (7.28) | −3.92 (7.71) | −3.52 (5.81) | 0.69 (0.11) |
| T | −1.80 (9.5) | −1.20 (12.1) | −3.30 (6.7) | −3.50 (5.4) | 0.43 (0.97) |
| CRP (mg/L) | |||||
| T1–T0 | −3.5 (7) | −3.2 (3.9) | −0.01 (12.3) | 1.2 (8.6) | 0.09 (0.72) |
| T2–T1 | −1.9 (6.1) | 1.3 (8.2) | −5.6 (10.6) | −5.9 (9.3) | 0.04 (0.67) |
| T | −5.9 (7.1) | −1.3 (9.5) | −4.0 (7.8) | −4.2 (4.8) | 0.90 (0.41) |
| Glucose (mg/dL) | |||||
| T1–T0 | 2.94 (14.4) | −3.40 (8.2) | 0.22 (15) | 3.20 (11.8) | 0.67 (0.75) |
| T2–T1 | 2.58 (21) | 7.90 (11.4) | 4.80 (14.2) | 2.70 (10.5) | 0.79 (0.83) |
| T | 8.90 (16.9) | 2.50 (8.7) | 4.6 (12.9) | 8.60 (11.8) | 0.92 (0.99) |
| Insulin (µU/mL) | |||||
| T1–T0 | 0.3 (3.7) | 1.3 (4.8) | 2.4 (3.5) | 1.7 (4.8) | 0.21 (0.98) |
| T2–T1 | −1.6 (6.5) | −3.3 (4.3) | −6.4 (3.6) | −5.6 (4.6) | <0.01 (0.93) |
| T | 0.1 (7.2) | −1.5 (4.5) | −4.0 (2.7) | −3.0 (2.9) | 0.02 (0.95) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues Amorim Adegboye, A.; Dias Santana, D.; Teixeira dos Santos, P.P.; Guedes Cocate, P.; Benaim, C.; Trindade de Castro, M.B.; Maia Schlüssel, M.; Kac, G.; Lilienthal Heitmann, B. Exploratory Efficacy of Calcium-Vitamin D Milk Fortification and Periodontal Therapy on Maternal Oral Health and Metabolic and Inflammatory Profile. Nutrients 2021, 13, 783. https://doi.org/10.3390/nu13030783
Rodrigues Amorim Adegboye A, Dias Santana D, Teixeira dos Santos PP, Guedes Cocate P, Benaim C, Trindade de Castro MB, Maia Schlüssel M, Kac G, Lilienthal Heitmann B. Exploratory Efficacy of Calcium-Vitamin D Milk Fortification and Periodontal Therapy on Maternal Oral Health and Metabolic and Inflammatory Profile. Nutrients. 2021; 13(3):783. https://doi.org/10.3390/nu13030783
Chicago/Turabian StyleRodrigues Amorim Adegboye, Amanda, Danilo Dias Santana, Pedro Paulo Teixeira dos Santos, Paula Guedes Cocate, Camila Benaim, Maria Beatriz Trindade de Castro, Michael Maia Schlüssel, Gilberto Kac, and Berit Lilienthal Heitmann. 2021. "Exploratory Efficacy of Calcium-Vitamin D Milk Fortification and Periodontal Therapy on Maternal Oral Health and Metabolic and Inflammatory Profile" Nutrients 13, no. 3: 783. https://doi.org/10.3390/nu13030783