Chlorogenic Acid Potentiates the Anti-Inflammatory Activity of Curcumin in LPS-Stimulated THP-1 Cells
Abstract
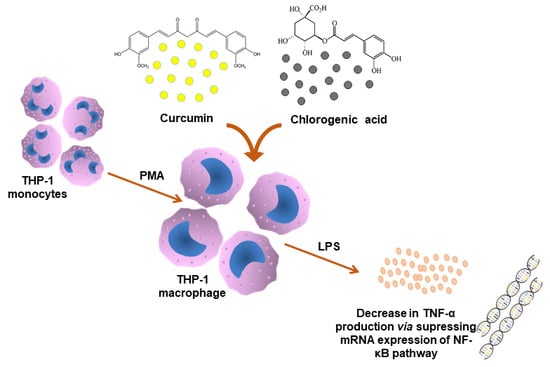
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cell Culture and Cell Treatments
2.3. Quantification of TNF-α
2.4. Cell Viability
2.5. Quantitative Real-Time PCR (qRT-PCR)
2.6. Data Analysis
3. Results
3.1. Effect of Curcumin and CGA on TNF-α and Cell Viability
3.2. Effect of Curcumin and CGA on the NF-κB Signalling Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Franceschi, C.; Campisi, J. Chronic Inflammation (Inflammaging) and Its Potential Contribution to Age-Associated Diseases. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2014, 69, S4–S9. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yue, Y.; Zheng, X.; Zhang, K.; Chen, S.; Du, Z. Curcumin, Inflammation, and Chronic Diseases: How Are They Linked? Molecules 2015, 20, 9183–9213. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-Y.; Tang, C.-Y. Strawberry, loquat, mulberry, and bitter melon juices exhibit prophylactic effects on LPS-induced inflammation using murine peritoneal macrophages. Food Chem. 2008, 107, 1587–1596. [Google Scholar] [CrossRef]
- Hewlings, S.; Kalman, D.S. Curcumin: A Review of Its’ Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Meirow, Y.; Baniyash, M. Immune biomarkers for chronic inflammation related complications in non-cancerous and cancerous diseases. Cancer Immunol. Immunother. 2017, 66, 1089–1101. [Google Scholar] [CrossRef]
- Panahi, Y.; Hosseini, M.S.; Khalili, N.; Naeimi, E.; Simental-Mendía, L.E.; Majeed, M.; Sahebkar, A. Effects of curcumin on serum cytokine concentrations in subjects with metabolic syndrome: A post-hoc analysis of a randomized controlled trial. Biomed. Pharmacother. 2016, 82, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Laine, L. Approaches to nonsteroidal anti-inflammatory drug use in the high-risk patient. Gastroenterology 2001, 120, 594–606. [Google Scholar] [CrossRef]
- Khan, R.S.; Grigor, J.; Winger, R.; Win, A. Functional food product development – Opportunities and challenges for food manufacturers. Trends Food Sci. Technol. 2013, 30, 27–37. [Google Scholar] [CrossRef]
- Mueller, M.; Hobiger, S.; Jungbauer, A. Anti-inflammatory activity of extracts from fruits, herbs and spices. Food Chem. 2010, 122, 987–996. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.S.; Van Houwelingen-Koukaliaroglou, M. Functional Foods: A Survey of Health Claims, Pros and Cons, and Current Legislation. Crit. Rev. Food Sci. Nutr. 2005, 45, 385–404. [Google Scholar] [CrossRef]
- Vicentini, A.; Liberatore, L.; Mastrocola, D. Functional foods: Trends and development of the global market. Ital. J. Food Sci. 2016, 28, 338–351. [Google Scholar]
- Astley, S.; Finglas, P. Nutrition and Health. In Reference Module in Food Science; Elsevier BV: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Calixto, J.B.; Otuki, M.F.; Santos, A.R. Anti-Inflammatory Compounds of Plant Origin. Part I. Action on Arachidonic Acid Pathway, Nitric Oxide and Nuclear Factor κ B (NF- κ B). Planta Medica 2003, 69, 973–983. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.T.; Lin, W.C.; Yu, B.; Lee, T. Antioxidant capacity of phytochemicals and their potential effects on oxidative status in animals—A review. Asian Australas. J. Anim. Sci. 2016, 30, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Sundaram, C.; Jhurani, S.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin and cancer: An “old-age” disease with an “age-old” solution. Cancer Lett. 2008, 267, 133–164. [Google Scholar] [CrossRef] [PubMed]
- Lozada-García, M.C.; Enriquez, R.G.; Teresa, R.A.; Nieto-Camacho, A.; Palacios-Espinosa, J.F.; Custodio-Galván, Z.; Soria-Arteche, O.; Pérez-Villanueva, J. Synthesis of Curcuminoids and Evaluation of Their Cytotoxic and Antioxidant Properties. Molecules 2017, 22, 633. [Google Scholar] [CrossRef]
- Noorafshan, A.; Ashkani-Esfahani, S. A review of therapeutic effects of curcumin. Curr. Pharm. Des. 2013, 19, 2032–2046. [Google Scholar]
- Aggarwal, S.; Ichikawa, H.; Takada, Y.; Sandur, S.K.; Shishodia, S.; Aggarwal, B.B. Curcumin (Diferuloylmethane) Down-Regulates Expression of Cell Proliferation and Antiapoptotic and Metastatic Gene Products through Suppression of IκBα Kinase and Akt Activation. Mol. Pharmacol. 2005, 69, 195–206. [Google Scholar] [CrossRef] [Green Version]
- Jin, C.-Y.; Lee, J.-D.; Park, C.; Choi, Y.H.; Kim, G.-Y.; Jin, J.-D.L.C.-Y. Curcumin attenuates the release of pro-inflammatory cytokines in lipopolysaccharide-stimulated BV2 microglia. Acta Pharmacol. Sin. 2007, 28, 1645–1651. [Google Scholar] [CrossRef] [Green Version]
- Chen, N.; Nie, M.; Fan, M.; Bian, Z. Anti-Inflammatory Activity of Curcumin in Macrophages Stimulated by Lipopolysaccharides from Porphyromonas gingivalis. Pharmacology 2008, 82, 264–269. [Google Scholar] [CrossRef]
- Murakami, Y.; Ishii, H.; Takada, N.; Tanaka, S.; Machino, M.; Ito, S.; Fujisawa, S. Comparative anti-inflammatory activities of curcumin and tetrahydrocurcumin based on the phenolic O-H bond dissociation enthalpy, ionization potential and quantum chemical descriptor. Anticancer. Res. 2008, 28, 699–707. [Google Scholar]
- Meng, Z.; Yan, C.; Deng, Q.; Gao, D.-F.; Niu, X.-L. Curcumin inhibits LPS-induced inflammation in rat vascular smooth muscle cells in vitro via ROS-relative TLR4-MAPK/NF-κB pathways. Acta Pharmacol. Sin. 2013, 34, 901–911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, H.; Patro, I.; Tikoo, K.; Sandhir, R. Curcumin attenuates inflammatory response and cognitive deficits in experimental model of chronic epilepsy. Neurochem. Int. 2015, 89, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Li, H.; Jia, J.; He, M. Anti-inflammatory effect of curcumin on mast cell-mediated allergic responses in ovalbumin-induced allergic rhinitis mouse. Cell. Immunol. 2015, 298, 88–95. [Google Scholar] [CrossRef]
- Khajehdehi, P.; Pakfetrat, M.; Javidnia, K.; Azad, F.; Malekmakan, L.; Nasab, M.H.; Dehghanzadeh, G. Oral supplementation of turmeric attenuates proteinuria, transforming growth factor-β and interleukin-8 levels in patients with overt type 2 diabetic nephropathy: A randomized, double-blind and placebo-controlled study. Scand. J. Urol. Nephrol. 2011, 45, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Ghanei, M.; Bashiri, S.; Hajihashemi, A.; Sahebkar, A. Short-term Curcuminoid Supplementation for Chronic Pulmonary Complications due to Sulfur Mustard Intoxication: Positive Results of a Randomized Double-blind Placebo-controlled Trial. Drug Res. 2014, 65, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of Curcumin: Problems and Promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Cui, J.; Yu, B.; Zhao, Y.; Zhu, W.; Li, H.; Lou, H.-X.; Zhai, G. Enhancement of oral absorption of curcumin by self-microemulsifying drug delivery systems. Int. J. Pharm. 2009, 371, 148–155. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Role of Chlorogenic Acids in Controlling Oxidative and Inflammatory Stress Conditions. Nutrients 2015, 8, 16. [Google Scholar] [CrossRef] [Green Version]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: A comprehensive review of the literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef]
- Palocz, O.; Pászti-Gere, E.; Gálfi, P.; Farkas, O. Chlorogenic Acid Combined with Lactobacillus plantarum 2142 Reduced LPS-Induced Intestinal Inflammation and Oxidative Stress in IPEC-J2 Cells. PLoS ONE 2016, 11, e0166642. [Google Scholar] [CrossRef]
- Han, D.; Chen, W.; Gu, X.; Shan, R.; Zou, J.; Liu, G.; Shahid, M.; Gao, J.; Han, B. Cytoprotective effect of chlorogenic acid against hydrogen peroxide-induced oxidative stress in MC3T3-E1 cells through PI3K/Akt-mediated Nrf2/HO-1 signaling pathway. Oncotarget 2017, 8, 14680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, F.; Yang, H.; Xu, Y.; Hao, H.; Li, P. A strategy for the identification of combinatorial bioactive compounds contributing to the holistic effect of herbal medicines. Sci. Rep. 2015, 5, 12361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.-W.; Chang, C.-L.; Lee, H.-C.; Chi, C.-W.; Pan, J.-P.; Yang, W.-C. Curcumin induces the apoptosis of human monocytic leukemia THP-1 cells via the activation of JNK/ERK Pathways. BMC Complement. Altern. Med. 2012, 12, 22. [Google Scholar] [CrossRef] [Green Version]
- Ma, F.; Liu, F.; Ding, L.; You, M.; Yue, H.; Zhou, Y.; Hou, Y. Anti-inflammatory effects of curcumin are associated with down regulating microRNA-155 in LPS-treated macrophages and mice. Pharm. Biol. 2017, 55, 1263–1273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2017, 97, 67–74. [Google Scholar] [CrossRef]
- Gong, X.; Su, X.; Zhan, K.; Zhao, G. The protective effect of chlorogenic acid on bovine mammary epithelial cells and neutrophil function. J. Dairy Sci. 2018, 101, 10089–10097. [Google Scholar] [CrossRef] [Green Version]
- Pavlica, S.; Gebhardt, R. Protective effects of ellagic and chlorogenic acids against oxidative stress in PC12 cells. Free. Radic. Res. 2005, 39, 1377–1390. [Google Scholar] [CrossRef]
- Zhang, Y.; Miao, L.; Zhang, H.; Wu, G.; Zhang, Z.; Lv, J. Chlorogenic acid against palmitic acid in endoplasmic reticulum stress-mediated apoptosis resulting in protective effect of primary rat hepatocytes. Lipids Health Dis. 2018, 17, 270. [Google Scholar] [CrossRef] [Green Version]
- Taram, F.; Winter, A.N.; Linseman, D.A. Neuroprotection comparison of chlorogenic acid and its metabolites against mechanistically distinct cell death-inducing agents in cultured cerebellar granule neurons. Brain Res. 2016, 1648, 69–80. [Google Scholar] [CrossRef]
- Lee, C.-W.; Won, T.-J.; Kim, H.-R.; Lee, D.; Hwang, K.W.; Park, S.-Y. Protective Effect of Chlorogenic Acid against Aβ-Induced Neurotoxicity. Biomol. Ther. 2011, 19, 181–186. [Google Scholar] [CrossRef] [Green Version]
- Shakibaei, M.; Mobasheri, A.; Buhrmann, C. Curcumin synergizes with resveratrol to stimulate the MAPK signaling pathway in human articular chondrocytes in vitro. Genes Nutr. 2010, 6, 171–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, X.; Jiang, X.; Jiang, X.; Wang, Y.; Miao, Z.; He, W.; Yang, G.; Lv, Z.; Yu, Y.; Zheng, Y. Micheliolide inhibits LPS-induced inflammatory response and protects mice from LPS challenge. Sci. Rep. 2016, 6, 23240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buhrmann, C.; Mobasheri, A.; Busch, F.; Aldinger, C.; Stahlmann, R.; Montaseri, A.; Shakibaei, M. Curcumin modulates NF-κB-mediated inflammation in human tenocytes in vitro: Role of the phosphatidylinositol 3-kinase-Akt pathway. J. Biol. Chem. 2011, 286, 28556–28566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jobin, C.; Bradham, C.A.; Russo, M.P.; Juma, B.; Narula, A.S.; Brenner, D.A.; Sartor, R.B. Curcumin blocks cytokine-mediated NF-κB activation and proinflammatory gene expression by inhibiting inhibitory factor I-κB kinase activity. J. Immunol. 1999, 163, 3474–3483. [Google Scholar]
- Surh, Y.-J.; Chun, K.-S.; Cha, H.-H.; Han, S.S.; Keum, Y.-S.; Park, K.-K.; Lee, S.S. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: Down-regulation of COX-2 and iNOS through suppression of NF-κB activation. Mutat. Res. Mol. Mech. Mutagen. 2001, 480, 243–268. [Google Scholar] [CrossRef]
- Shen, W.; Qi, R.; Zhang, J.; Wang, Z.; Wang, H.; Hu, C.; Zhao, Y.; Bie, M.; Wang, Y.; Fu, Y.; et al. Chlorogenic acid inhibits LPS-induced microglial activation and improves survival of dopaminergic neurons. Brain Res. Bull. 2012, 88, 487–494. [Google Scholar] [CrossRef]
- Li, W.; Suwanwela, N.C.; Patumraj, S. Curcumin prevents reperfusion injury following ischemic stroke in rats via inhibition of NF-κB, ICAM-1, MMP-9 and caspase-3 expression. Mol. Med. Rep. 2017, 16, 4710–4720. [Google Scholar] [CrossRef] [Green Version]





| Gene | Accession Number | Primer Sequences | Product Size (BP) |
|---|---|---|---|
| IL-6 | NM_001318095.1 | F: TGGCAGAAAACAACCTGAACC | 89 |
| R: TTTCACCAGGCAAGTCTCCTCAT | |||
| IL-10 | NM_000572.2 | F: GTGATGCCCCAAGCTGAGA | 138 |
| R: CACGGCCTTGCTCTTGTTTT | |||
| TNF-α | NM_000594.2 | F: CTGCTGCACTTTGGAGTGAT | 93 |
| R: AGATGATCTGACTGCCTGGG | |||
| iNOS | NM_000625.3 | F: CATCCTCTTTGCGACAGAGAC | 118 |
| R: GCAGCTCAGCCTGTACTTATC | |||
| COX-2 | NM_000963.3 | F: TCCCTTGGGTGTCAAAGGTAAAA | 144 |
| R: AACTGATGCGTGAAGTGCTG | |||
| TLR-4 | NM_003266.3 | F: GGTCAGACGGTGATAGCGAG | 180 |
| R: TTTAGGGCCAAGTCTCCACG | |||
| IκB-α | NM_020529.2 | F: AAGTGATCCGCCAGGTGAAG | 281 |
| R: CGTGTGGCCATTGTAGTTGG | |||
| IκB-β kinase | NM_001556.3 | F: TCCGATGGCACAATCAGGAAA | 264 |
| R: GCAGACCACAGCAGTTCTCA | |||
| NF-κB | NM_003998.2 | F: TGAGTCCTGCTCCTTCCA | 103 |
| R: GCTTCGGTGTAGCCCATT | |||
| β-Actin | NM_001101.4 | F: CACTCTTCCAGCCTTCCTTC | 104 |
| R: GTACAGGTCTTTGCGGATGT |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bisht, A.; Dickens, M.; Rutherfurd-Markwick, K.; Thota, R.; Mutukumira, A.N.; Singh, H. Chlorogenic Acid Potentiates the Anti-Inflammatory Activity of Curcumin in LPS-Stimulated THP-1 Cells. Nutrients 2020, 12, 2706. https://doi.org/10.3390/nu12092706
Bisht A, Dickens M, Rutherfurd-Markwick K, Thota R, Mutukumira AN, Singh H. Chlorogenic Acid Potentiates the Anti-Inflammatory Activity of Curcumin in LPS-Stimulated THP-1 Cells. Nutrients. 2020; 12(9):2706. https://doi.org/10.3390/nu12092706
Chicago/Turabian StyleBisht, Akshay, Martin Dickens, Kay Rutherfurd-Markwick, Rohith Thota, Anthony N. Mutukumira, and Harjinder Singh. 2020. "Chlorogenic Acid Potentiates the Anti-Inflammatory Activity of Curcumin in LPS-Stimulated THP-1 Cells" Nutrients 12, no. 9: 2706. https://doi.org/10.3390/nu12092706