Ethyl Acetate Fraction of Amomum xanthioides Ameliorates Nonalcoholic Fatty Liver Disease in a High-Fat Diet Mouse Model
Abstract
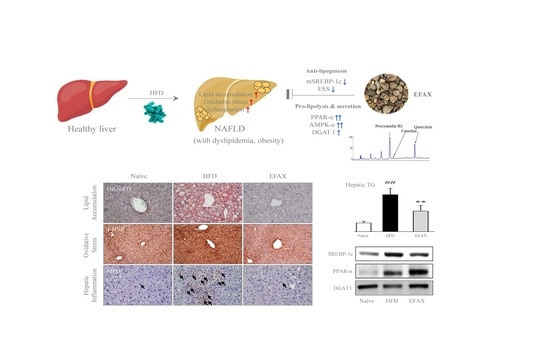
:1. Introduction
2. Materials and Methods
2.1. Preparation and Fingerprinting of A. xanthioides
2.2. Chemicals and Reagents
2.3. Animals and Experimental Design
2.4. Determination of Hepatic Lipid Levels and Serum Biochemistry
2.5. Histopathological Analysis in the Hepatic Tissue
2.6. Determination of Inflammatory Cytokines in Hepatic Tissue
2.7. Determination of Nitric Oxide (NO) and Lipid Peroxidation in Hepatic Tissue
2.8. Western Blot Analysis for Lipid Metabolic Proteins in the Hepatic Tissue
2.8.1. Fatty Acid Metabolism-Related Proteins
2.8.2. TG Synthesis-Related Proteins
2.8.3. Western Blot Performance
2.9. Statistical Analysis
3. Results
3.1. Food Intake and Body, Liver, and Adipose Tissue Weights
3.2. Effects on Hepatic Lipid Accumulation
3.3. Effects on Lipid Profiles in Serum
3.4. Effects on Molecules for Fatty Acid-Metabolism
3.5. Effects on Molecules for TG Synthesis
3.6. Effects on Hepatic Inflammation
3.7. Effects on Hepatic Oxidative Stress
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease—Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [Green Version]
- Younossi, Z.M.; Blissett, D.; Blissett, R.; Henry, L.; Stepanova, M.; Younossi, Y.; Racila, A.; Hunt, S.; Beckerman, R. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology 2016, 64, 1577–1586. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.J.; Adams, L.A.; Canbay, A.; Syn, W.-K. Extrahepatic complications of nonalcoholic fatty liver disease. Hepatology 2014, 59, 1174–1197. [Google Scholar] [CrossRef] [PubMed]
- Musso, G.; Gambino, R.; Cassader, M.; Pagano, G. Meta-analysis: Natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann. Med. 2011, 43, 617–649. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Zaza, G.; Byrne, C.D.; Lonardo, A.; Zoppini, G.; Bonora, E.; Targher, G. Nonalcoholic fatty liver disease increases risk of incident chronic kidney disease: A systematic review and meta-analysis. Metabolism 2018, 79, 64–76. [Google Scholar] [CrossRef]
- Issa, D.; Patel, V.; Sanyal, A.J. Future therapy for non-alcoholic fatty liver disease. Liver Int. 2018, 38, 56–63. [Google Scholar] [CrossRef] [Green Version]
- Romero-Gómez, M.; Zelber-Sagi, S.; Trenell, M. Treatment of NAFLD with diet, physical activity and exercise. J. Hepatol. 2017, 67, 829–846. [Google Scholar] [CrossRef] [Green Version]
- Vilar-Gomez, E.; Martinez-Perez, Y.; Calzadilla-Bertot, L.; Torres-Gonzalez, A.; Gra-Oramas, B.; Gonzalez-Fabian, L.; Friedman, S.L.; Diago, M.; Romero-Gomez, M. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology 2015, 149, 367–378. [Google Scholar] [CrossRef]
- Loomba, R.; Sanyal, A.J. The global NAFLD epidemic. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 686–690. [Google Scholar] [CrossRef]
- Pydyn, N.; Miękus, K.; Jura, J.; Kotlinowski, J. New therapeutic strategies in nonalcoholic fatty liver disease: A focus on promising drugs for nonalcoholic steatohepatitis. Pharmacol. Rep. 2020, 72, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Diehl, A.M.; Brunt, E.M.; Cusi, K.; Charlton, M.; Sanyal, A.J. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012, 55, 2005–2023. [Google Scholar] [CrossRef] [PubMed]
- EASL–EASD–EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [CrossRef] [PubMed]
- Rinella, M.E. Nonalcoholic fatty liver disease: A systematic review. JAMA 2015, 313, 2263–2273. [Google Scholar] [CrossRef]
- Miller, E.R.; Pastor-Barriuso, R.; Dalal, D.; Riemersma, R.A.; Appel, L.J.; Guallar, E. Meta-analysis: High-dosage vitamin E supplementation may increase all-cause mortality. Ann. Intern. Med. 2005, 142, 37–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, J.D.; Ferrara, A.; Peng, T.; Hedderson, M.; Bilker, W.B.; Quesenberry, C.P.; Vaughn, D.J.; Nessel, L.; Selby, J.; Strom, B.L. Risk of Bladder Cancer Among Diabetic Patients Treated With Pioglitazone. Diabetes Care 2011, 34, 916–922. [Google Scholar] [CrossRef] [Green Version]
- Colmers, I.N.; Bowker, S.L.; Majumdar, S.R.; Johnson, J.A. Use of thiazolidinediones and the risk of bladder cancer among people with type 2 diabetes: A meta-analysis. CMAJ Can. Med. Assoc. J. 2012, 184, E675–E683. [Google Scholar] [CrossRef] [Green Version]
- Thiagarajan, P.; Aithal, G.P. Drug Development for Nonalcoholic Fatty Liver Disease: Landscape and Challenges. J. Clin. Exp. Hepatol. 2019, 9, 515–521. [Google Scholar] [CrossRef] [Green Version]
- Yao, H.; Qiao, Y.-J.; Zhao, Y.-L.; Tao, X.-F.; Xu, L.-N.; Yin, L.-H.; Qi, Y.; Peng, J.-Y. Herbal medicines and nonalcoholic fatty liver disease. World J. Gastroenterol. 2016, 22, 6890–6905. [Google Scholar] [CrossRef]
- Yan, T.; Yan, N.; Wang, P.; Xia, Y.; Hao, H.; Wang, G.; Gonzalez, F.J. Herbal drug discovery for the treatment of nonalcoholic fatty liver disease. Acta Pharm. Sin. B 2020, 10, 3–18. [Google Scholar] [CrossRef]
- Suo, S.; Lai, Y.; Li, M.; Song, Q.; Cai, J.; Zhao, J.; Yang, Q.; Ung, C.O.L.; Hu, H. Phytochemicals, pharmacology, clinical application, patents, and products of Amomi fructus. Food Chem. Toxicol. 2018. [Google Scholar] [CrossRef]
- Lee, S.-B.; Kim, H.-G.; Kim, H.-S.; Lee, J.-S.; Im, H.-J.; Kim, W.-Y.; Son, C.-G. Ethyl Acetate Fraction of Amomum xanthioides Exerts Antihepatofibrotic Actions via the Regulation of Fibrogenic Cytokines in a Dimethylnitrosamine-Induced Rat Model. Evid. Based Complement. Altern. Med. ECAM 2016, 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.-G.; Han, J.-M.; Lee, J.-S.; Suk Lee, J.; Son, C.-G. Ethyl acetate fraction of Amomum xanthioides improves bile duct ligation-induced liver fibrosis of rat model via modulation of pro-fibrogenic cytokines. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carr, T.P.; Andresen, C.J.; Rudel, L.L. Enzymatic determination of triglyceride, free cholesterol, and total cholesterol in tissue lipid extracts. Clin. Biochem. 1993, 26, 39–42. [Google Scholar] [CrossRef]
- Tsikas, D. Analysis of nitrite and nitrate in biological fluids by assays based on the Griess reaction: Appraisal of the Griess reaction in the l-arginine/nitric oxide area of research. J. Chromatogr. B 2007, 851, 51–70. [Google Scholar] [CrossRef]
- Kamal, A.-A.M.; Gomaa, A.; Khafif, M.E.; Hammad, A.S. Plasma lipid peroxides among workers exposed to silica or asbestos dusts. Environ. Res. 1989, 49, 173–180. [Google Scholar] [CrossRef]
- Abd El-Kader, S.M.; El-Den Ashmawy, E.M.S. Non-alcoholic fatty liver disease: The diagnosis and management. World J. Hepatol. 2015, 7, 846–858. [Google Scholar] [CrossRef]
- Angulo, P. Nonalcoholic Fatty Liver Disease. N. Engl. J. Med. 2002, 346, 1221–1231. [Google Scholar] [CrossRef] [Green Version]
- Byrne, C.D.; Targher, G. NAFLD: A multisystem disease. J. Hepatol. 2015, 62, S47–S64. [Google Scholar] [CrossRef] [Green Version]
- Musso, G.; Gambino, R.; Tabibian, J.H.; Ekstedt, M.; Kechagias, S.; Hamaguchi, M.; Hultcrantz, R.; Hagström, H.; Yoon, S.K.; Charatcharoenwitthaya, P.; et al. Association of non-alcoholic fatty liver disease with chronic kidney disease: A systematic review and meta-analysis. PLoS Med. 2014, 11, e1001680. [Google Scholar] [CrossRef] [Green Version]
- de Araújo Souza, M.R.; de Melo Diniz, M.d.F.F.; de Medeiros-Filho, J.E.M.; de Araújo, M.S.T. Metabolic syndrome and risk factors for non-alcoholic fatty liver disease. Arq. Gastroenterol. 2012, 49, 89–96. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, B.; De Angelis-Schierbaum, G.; Egert, S.; Assmann, G.; Kratz, M. Individual Serum Triglyceride Responses to High-Fat and Low-Fat Diets Differ in Men with Modest and Severe Hypertriglyceridemia. J. Nutr. 2004, 134, 1400–1405. [Google Scholar] [CrossRef] [PubMed]
- Pieke, B.; von Eckardstein, A.; Gülbahçe, E.; Chirazi, A.; Schulte, H.; Assmann, G.; Wahrburg, U. Treatment of hypertriglyceridemia by two diets rich either in unsaturated fatty acids or in carbohydrates: Effects on lipoprotein subclasses, lipolytic enzymes, lipid transfer proteins, insulin and leptin. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 2000, 24, 1286–1296. [Google Scholar] [CrossRef] [PubMed]
- West, C.E.; Sullivan, D.R.; Katan, M.B.; Halferkamps, I.L.; van der Torre, H.W. Boys from populations with high-carbohydrate intake have higher fasting triglyceride levels than boys from populations with high-fat intake. Am. J. Epidemiol. 1990, 131, 271–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reccia, I.; Kumar, J.; Akladios, C.; Virdis, F.; Pai, M.; Habib, N.; Spalding, D. Non-alcoholic fatty liver disease: A sign of systemic disease. Metabolism 2017, 72, 94–108. [Google Scholar] [CrossRef]
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef] [Green Version]
- Harrison, S.A.; Day, C.P. Benefits of lifestyle modification in NAFLD. Gut 2007, 56, 1760–1769. [Google Scholar] [CrossRef] [Green Version]
- Chao, J.; Huo, T.-I.; Cheng, H.-Y.; Tsai, J.-C.; Liao, J.-W.; Lee, M.-S.; Qin, X.-M.; Hsieh, M.-T.; Pao, L.-H.; Peng, W.-H. Gallic acid ameliorated impaired glucose and lipid homeostasis in high fat diet-induced NAFLD mice. PLoS ONE 2014, 9, e96969. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Yang, B.; Yu, B. Paeoniflorin Protects against Nonalcoholic Fatty Liver Disease Induced by a High-Fat Diet in Mice. Biol. Pharm. Bull. 2015, 38, 1005–1011. [Google Scholar] [CrossRef] [Green Version]
- Guo, T.; Woo, S.-L.; Guo, X.; Li, H.; Zheng, J.; Botchlett, R.; Liu, M.; Pei, Y.; Xu, H.; Cai, Y.; et al. Berberine Ameliorates Hepatic Steatosis and Suppresses Liver and Adipose Tissue Inflammation in Mice with Diet-induced Obesity. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Musso, G.; Gambino, R.; Cassader, M. Recent insights into hepatic lipid metabolism in non-alcoholic fatty liver disease (NAFLD). Prog. Lipid Res. 2009, 48, 1–26. [Google Scholar] [CrossRef]
- Smith, B.K.; Marcinko, K.; Desjardins, E.M.; Lally, J.S.; Ford, R.J.; Steinberg, G.R. Treatment of nonalcoholic fatty liver disease: Role of AMPK. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E730–E740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawano, Y.; Cohen, D.E. Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. J. Gastroenterol. 2013, 48, 434–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alves-Bezerra, M.; Cohen, D.E. Triglyceride Metabolism in the Liver. In Comprehensive Physiology; Terjung, R., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 1–22. ISBN 978-0-470-65071-4. [Google Scholar]
- Hashimoto, E.; Taniai, M.; Tokushige, K. Characteristics and diagnosis of NAFLD/NASH. J. Gastroenterol. Hepatol. 2013, 28, 64–70. [Google Scholar] [CrossRef] [Green Version]
- Fan, J.-G.; Kim, S.-U.; Wong, V.W.-S. New trends on obesity and NAFLD in Asia. J. Hepatol. 2017, 67, 862–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef] [PubMed]
- Csak, T.; Ganz, M.; Pespisa, J.; Kodys, K.; Dolganiuc, A.; Szabo, G. Fatty acid and endotoxin activate inflammasomes in mouse hepatocytes that release danger signals to stimulate immune cells. Hepatology 2011, 54, 133–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klebanoff, S.J. Myeloperoxidase: Friend and foe. J. Leukoc. Biol. 2005, 77, 598–625. [Google Scholar] [CrossRef] [PubMed]
- Ekstedt, M.; Franzén, L.E.; Mathiesen, U.L.; Thorelius, L.; Holmqvist, M.; Bodemar, G.; Kechagias, S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology 2006, 44, 865–873. [Google Scholar] [CrossRef]
- Xing, Y.-W.; Lei, G.-T.; Wu, Q.-H.; Jiang, Y.; Huang, M.-X. Procyanidin B2 protects against diet-induced obesity and non-alcoholic fatty liver disease via the modulation of the gut microbiota in rabbits. World J. Gastroenterol. 2019, 25, 955–966. [Google Scholar] [CrossRef]
- Feng, J.; Wang, C.; Liu, T.; Li, J.; Wu, L.; Yu, Q.; Li, S.; Zhou, Y.; Zhang, J.; Chen, J.; et al. Procyanidin B2 inhibits the activation of hepatic stellate cells and angiogenesis via the Hedgehog pathway during liver fibrosis. J. Cell. Mol. Med. 2019, 23, 6479–6493. [Google Scholar] [CrossRef] [Green Version]
- Mahmoodi, M.; Hosseini, R.; Kazemi, A.; Ofori-Asenso, R.; Mazidi, M.; Mazloomi, S.M. Effects of green tea or green tea catechin on liver enzymes in healthy individuals and people with nonalcoholic fatty liver disease: A systematic review and meta-analysis of randomized clinical trials. Phytother. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Sakata, R.; Nakamura, T.; Torimura, T.; Ueno, T.; Sata, M. Green tea with high-density catechins improves liver function and fat infiltration in non-alcoholic fatty liver disease (NAFLD) patients: A double-blind placebo-controlled study. Int. J. Mol. Med. 2013, 32, 989–994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caldwell, S. NASH Therapy: Omega 3 supplementation, vitamin E, insulin sensitizers and statin drugs. Clin. Mol. Hepatol. 2017, 23, 103–108. [Google Scholar] [CrossRef] [Green Version]
- Lavine, J.E.; Schwimmer, J.B.; Molleston, J.P.; Murray, K.F.; Rosenthal, P.; Abrams, S.H.; Scheimann, A.O.; Sanyal, A.J.; Chalasani, N.; Tonascia, J.; et al. Effect of Vitamin E or Metformin for Treatment of Nonalcoholic Fatty Liver Disease in Children and Adolescents. JAMA 2011, 305, 1659–1668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadi, H.E.; Vettor, R.; Rossato, M. Vitamin E as a Treatment for Nonalcoholic Fatty Liver Disease: Reality or Myth? Antioxidants 2018, 7, 12. [Google Scholar] [CrossRef] [Green Version]
- Sakr, H.F.; Abbas, A.M.; Haidara, M.A. Swimming, but not vitamin E, ameliorates prothrombotic state and hypofibrinolysis in a rat model of nonalcoholic fatty liver disease. J. Basic Clin. Physiol. Pharmacol. 2018, 29, 61–71. [Google Scholar] [CrossRef]
- Şekeroğlu, V.; Aydın, B.; Atlı Şekeroğlu, Z.; Özdener Kömpe, Y. Hepatoprotective effects of capsaicin and alpha-tocopherol on mitochondrial function in mice fed a high-fat diet. Biomed. Pharmacother. 2018, 98, 821–825. [Google Scholar] [CrossRef]
- Leung, C.; Rivera, L.; Furness, J.B.; Angus, P.W. The role of the gut microbiota in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 412–425. [Google Scholar] [CrossRef]






| Group | High-Fat Diet | ||||||
|---|---|---|---|---|---|---|---|
| Naive | Control | EFAX 25 | EFAX 50 | EFAX 100 | Vitamin E | ||
| Adipose tissue weight | Epididymal (g) | 0.79 ± 0.30 | 3.16 ± 0.13 ## | 2.45 ± 0.40 * | 2.60 ± 0.43 | 2.47 ± 0.35 * | 2.99 ± 0.51 |
| Retroperitoneal (g) | 0.26 ± 0.10 | 1.40 ± 0.10 ## | 1.10 ± 0.21 | 1.12 ± 0.23 | 0.96 ± 0.18 * | 1.31 ± 0.33 | |
| Visceral (g) | 0.54 ± 0.09 | 1.20 ± 0.10 ## | 0.85 ± 0.17 * | 0.92 ± 0.18 | 0.85 ± 0.12 * | 1.22 ± 0.34 | |
| Total (g) | 1.60 ± 0.40 | 5.76 ± 0.28 ## | 4.40 ± 0.73 * | 4.64 ± 0.82 | 4.29 ± 0.60 * | 5.52 ± 1.15 | |
| Total cholesterol (mg/dL) | 89.9 ± 9.1 | 184.1 ± 15.3 ## | 157.4 ± 4.8 ** | 138.7 ± 14.8 ** | 156.1 ± 8.5 ** | 183.6 ± 9.9 | |
| LDL-C (mg/dL) | 7.9 ± 2.8 | 22.1 ± 1.6 ## | 16.3 ± 0.9 ** | 15.2 ± 2.4 ** | 16.0 ± 1.6 ** | 23.0 ± 2.9 | |
| HDL-C (mg/dL) | 90.0 ± 6.41 | 126.4 ± 8.7 ## | 124.3 ± 7.2 | 118.5 ± 9.5 | 117.3 ± 9.1 | 126.6 ± 8.2 | |
| Triglyceride (mg/dL) | 80.0 ± 15.0 | 44.2 ±14.9 ## | 37.7 ± 3.4 | 36.2 ± 6.3 | 40.9 ± 6.5 | 45.3 ± 11.4 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Im, H.-J.; Hwang, S.-J.; Lee, J.-S.; Lee, S.-B.; Kang, J.-Y.; Son, C.-G. Ethyl Acetate Fraction of Amomum xanthioides Ameliorates Nonalcoholic Fatty Liver Disease in a High-Fat Diet Mouse Model. Nutrients 2020, 12, 2433. https://doi.org/10.3390/nu12082433
Im H-J, Hwang S-J, Lee J-S, Lee S-B, Kang J-Y, Son C-G. Ethyl Acetate Fraction of Amomum xanthioides Ameliorates Nonalcoholic Fatty Liver Disease in a High-Fat Diet Mouse Model. Nutrients. 2020; 12(8):2433. https://doi.org/10.3390/nu12082433
Chicago/Turabian StyleIm, Hwi-Jin, Seung-Ju Hwang, Jin-Seok Lee, Sung-Bae Lee, Ji-Yun Kang, and Chang-Gue Son. 2020. "Ethyl Acetate Fraction of Amomum xanthioides Ameliorates Nonalcoholic Fatty Liver Disease in a High-Fat Diet Mouse Model" Nutrients 12, no. 8: 2433. https://doi.org/10.3390/nu12082433