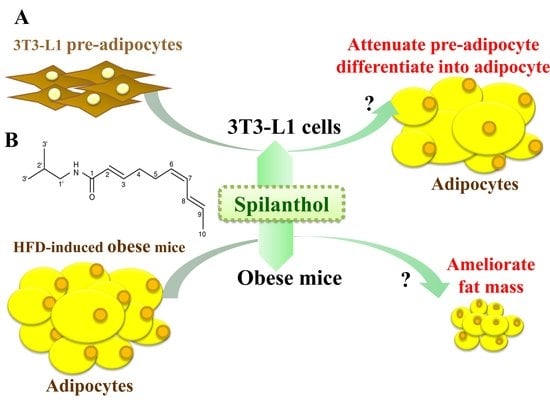
Spilanthol from Traditionally Used Spilanthes acmella Enhances AMPK and Ameliorates Obesity in Mice Fed High-Fat Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Spilanthol and Cell Culture
2.2. Adipocyte Differentiation
2.3. Animals and Spilanthol Administration
2.4. Oil Red O Staining
2.5. Fluorescence Staining
2.6. Biochemical Analysis
2.7. Histopathological Examination
2.8. Western Blot Analysis
2.9. Statistical Analysis
3. Results
3.1. Spilanthol Inhibits Lipid Accumulation in 3T3-L1 Cells
3.2. Spilanthol Regulates Lipogenic Pathway Enzymes in 3T3-L1 Cells
3.3. Spilanthol Reduces the Inflammatory Response by Downregulating MAPK Signaling Pathways in 3T3-L1 Pre-Adipocytes
3.4. Spilanthol Ameliorates Adiposity and Visceral Adipocyte Tissue in HFD-Induced Obese Mice
3.5. Spilanthol Ameliorates Hepatic Lipid Accumulation, Improves TC and HDL-c Levels, and Attenuates Serum Leptin in HFD-Induced Obese Mice
3.6. Spilanthol Activates AMPK Signaling to Regulate Lipogenesis and Adipogenesis-Related Transcription Factors in HFD-Induced Obese Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Seong, J.; Kang, J.Y.; Sun, J.S.; Kim, K.W. Hypothalamic inflammation and obesity: A mechanistic review. Arch. Pharm. Res. 2019, 5. [Google Scholar] [CrossRef]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef]
- Hirabara, S.M.; Gorjão, R.; Vinolo, M.A.; Rodrigues, A.C.; Nachbar, R.T.; Curi, R. Molecular targets related to inflammation and insulin resistance and potential interventions. J. Biomed. Biotechnol. 2012, 379024. [Google Scholar] [CrossRef]
- Tateya, S.; Kim, F.; Tamori, Y. Recent advances in obesity-induced inflammation and insulin resistance. Front. Endocrinol. 2013, 4, 93. [Google Scholar] [CrossRef] [PubMed]
- Seth, S.; Martin, B.S.; Qasim, A.; Muredach, P.; Reilly, M.B. Leptin resistance: A Possible interface of inflammation and metabolism in obesity-related cardiovascular disease. J. Am. Coll. Cardiol. 2008, 7, 1201–1210. [Google Scholar]
- Hsieh, P.S.; Jin, J.S.; Chiang, C.F. COX-2-mediated inflammation in fat is crucial for obesity-linked insulin resistance and fatty liver. Obesity 2009, 17, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Koh, E.J.; Kim, K.J.; Choi, J.; Jeon, H.J.; Seo, M.J.; Lee, B.Y. Ginsenoside Rg1 suppresses early stage of adipocyte development via activation of C/EBP homologous protein-10 in 3T3-L1 and attenuates fat accumulation in high fat diet-induced obese zebrafish. J. Ginseng. Res. 2017, 41, 23–30. [Google Scholar] [CrossRef]
- Park, Y.K.; Obiang-Obounou, B.W.; Lee, J.; Lee, T.Y.; Bae, M.A.; Hwang, K.S.; Lee, K.B.; Choi, J.S.; Jang, B.C. Anti-adipogenic effects on 3T3-L1 cells and zebrafish by Tanshinone IIA. Int. J. Mol. Sci. 2017, 18, 2065. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Chang, W.T.; Wu, S.J.; Xu, P.Y.; Ting, N.C.; Liou, C.J. Phloretin and phlorizin promote lipolysis and inhibit inflammation in mouse 3T3-L1 cells and in macrophage-adipocyte co-cultures. Mol. Nutr. Food Res. 2013, 57, 1803–1813. [Google Scholar] [CrossRef]
- Huang, W.C.; Chen, Y.L.; Liu, H.C.; Wu, S.J.; Liou, C.J. Ginkgolide C reduced oleic acid-induced lipid accumulation in HepG2 cells. Saudi. Pharm. J. 2018, 26, 1178–1184. [Google Scholar] [CrossRef] [PubMed]
- Trepiana, J.; Milton-Laskibar, I.; Gómez-Zorita, S.; Eseberri, I.; González, M.; Fernández-Quintela, A.; Portillo, M.P. Involvement of 50AMP-Activated Protein Kinase (AMPK) in the effects of resveratrol on liver steatosis. Int. J. Mol. Sci. 2018, 19, 3473. [Google Scholar] [CrossRef]
- Suchankova, G.; Nelson, L.E.; Gerhart-Hines, Z.; Kelly, M.; Gauthier, M.S.; Saha, A.K.; Ido, Y.; Puigserver, P.; Ruderman, N.B. Concurrent regulation of AMP-activated protein kinase and SIRT1 in mammalian cells. Biochem. Biophys. Res. Commun. 2009, 378, 836–841. [Google Scholar] [CrossRef]
- Cantó, C.; Auwerx, J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr. Opin. Lipidol. 2009, 20, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Liang, X.; Rogers, C.Q.; Rideout, D.; You, M. Involvement of adiponectin-SIRT1-AMPK signaling in the protective action of rosiglitazone against alcoholic fatty liver in mice. Am. J. Physiol. Gastrointest. Liver. Physiol. 2010, 298, G364–G374. [Google Scholar] [CrossRef]
- Joseph, B.; George, J.; Jeevitha, M.V. The role of Acmella Olerecea in Medicine—A review. World J. Pharm. Res. 2017, 2, 2781–2792. [Google Scholar]
- Tanwer, B.S.; Choudhary, R.K.; Vijayvergia, R. In vitro and in vivo comparative study of primary metabolites and antioxidant activity in Spilanthes acmella Murr. Int. J. Biotechnol. Biochem. 2010, 6, 819–825. [Google Scholar]
- Sahu, J.; Jain, K.; Jain, B.; Sahu, R.K. A review on phytopharmacology and micro propagation of Spilanthes acmella. Pharmacologyonline 2011, 2, 1105–1110. [Google Scholar]
- Yadav, R.; Kharya, M.D.; Yadav, N.; Savadi, R. Diuretic activity of Spilanthes acmella Murr. Leaves extract in rats. Int. J. Pharm. Pharm. Sci. 2011, 1, 57–61. [Google Scholar]
- Chakraborty, A.; Devi, B.R.K.; Sanjebam, R.; Khumbong, S.; Thokchom, I.S. Priliminary studies on local anesthetic and antipyretic activities of Spilanthes acmella Murr. in experimental animals models. Indian. J. Pharmacol. 2010, 42, 277–279. [Google Scholar] [CrossRef]
- Wu, L.C.; Fan, N.C.; Lin, M.H.; Chu, I.R.; Huang, S.J.; Hu, C.Y. Anti-inflammatory effect of spilanthol from Spilanthes acmella on murine macrophage by down-regulating LPS-induced inflammatory mediators. J. Agric. Food. Chem. 2008, 56, 2341–2349. [Google Scholar] [CrossRef]
- Leng, T.C.; Ping, N.S.; Lim, B.P.; Keng, C.L. Detection of bioactive compounds from Spilanthes acmella (L.) plants and its various in vitro culture products. J. Med. Plant. Res. 2011, 5, 371–378. [Google Scholar]
- Nabi, N.G.; Wani, T.A.; Abidwani, M.S.; Shah, S.N. Spilanthes acmella an endangered medicinal plant—Its traditional, phytochemical and therapeutic properties—An overview. Int. J. Adv. Res. 2016, 4, 627–639. [Google Scholar]
- Paulraj, J.; Govindarajan, R.; Palpu, P. The genus Spilanthes ethnopharmacol-ogy, phytochemistry, and pharmacological properties: A review. Adv. Pharmacol. Sci. 2013. [Google Scholar] [CrossRef]
- Veryser, L.; Wynendaele, E.; Taevernier, L.; Verbeke, F.; Joshi, T.; Tatke, P.; DeSpiegeleer, B. N-alkylamides: From plant to brain. Funct. Foods. Health. Dis. 2014, 4, 264–275. [Google Scholar] [CrossRef]
- Aggarwal, B.B. Targeting inflammation-induced obesity and metabolic diseases by curcumin and other nutraceuticals. Annu. Rev. Nutr. 2010, 21, 173–199. [Google Scholar] [CrossRef] [PubMed]
- Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Ramirez-Tortosa, M. Curcumin and Health. Molecules 2016, 21, 264. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zheng, S.; Feng, Q.; Zhang, Q.; Xiao, X. Dietary capsaicin and its anti-obesity potency: From mechanism to clinical implications. Biosci. Rep. 2017, 37. [Google Scholar] [CrossRef] [PubMed]
- Chopra, B.; Dhingra, A.K.; Kapoor, R.P.; Prasad, D.N. Piperine and its various physicochemical and biological aspects: A review. Open Chem. J. 2016, 3, 75–96. [Google Scholar] [CrossRef]
- Barbosaa, A.F.; de Carvalhoa, M.G.; Smithb, R.E.; Sabaa-Srura, A.U.O. Spilanthol: Occurrence, extraction, chemistry and biological activities. Rev. Bra. Farmacogn. 2016, 26, 128–133. [Google Scholar] [CrossRef]
- Jeon, S.M. Regulation and function of AMPK in physiology and diseases. Exp. Mol. Med. 2016, 15, e245. [Google Scholar] [CrossRef]
- Dordevic, A.L.; Konstantopoulos, N.; Cameron-Smith, D. 3T3-L1 Preadipocytes exhibit heightened monocyte-chemoattractant protein-1 response to acute fatty acid exposure. PLoS ONE 2014, 9, e99382. [Google Scholar] [CrossRef]
- Na, H.Y.; Seol, M.H.; Kim, M.; Lee, B.C. Effect of seyoeum on obesity, insulin resistance, and nonalcoholic fatty liver disease of high-fat diet-fed C57BL/6 mice. Evid. Based Complement. Altern. Med. 2017, 2017, 4658543. [Google Scholar] [CrossRef] [PubMed]
- Seyedan, A.; Mohamed, Z.; Alshagga, M.A.; Koosha, S.; Alshawsh, M.A. Cynometra cauliflora Linn. Attenuates metabolic abnormalities in high-fat diet-induced obese mice. J. Ethnopharmacol. 2019, 23, 173–182. [Google Scholar] [CrossRef]
- Crujeiras, A.B.; Carreira, M.C.; Cabia, B.; Andrade, S.; Amil, M.; Casanueva, F.F. Leptin resistance in obesity: An epigenetic landscape. Life. Sci. 2015, 140, 57–63. [Google Scholar] [CrossRef]
- Seoane-Collazo, P.; Ferno, J.; Gonzalez, F.; Dieguez, C.; Leis, R.; Nogueiras, R. Hypothalamic-autonomic control of energy homeostasis. Endocrine 2015, 50, 276–291. [Google Scholar] [CrossRef] [PubMed]
- Vasilenko, M.A.; Kirienkova, E.V.; Skuratovskaia, D.A.; Zatolokin, P.A.; Mironyuk, N.I.; Litvinova, L.S. The role of production of adipsin and leptin in the development of insulin resistance in patients with abdominal obesity. Dokl. Biochem. Biophys. 2017, 475, 271–276. [Google Scholar] [CrossRef]
- Lodhi, I.J.; Yin, L.; Jensen-Urstad, A.P.L.; Funai, K.; Coleman, T.; Baird, J.H. Inhibiting adipose tissue lipogenesis reprograms thermogenesis and PPARγ activation to decrease diet-induced obesity. Cell Metab. 2012, 8, 189–201. [Google Scholar] [CrossRef]
- Jung, U.J.; Choi, M.S. Obesity and its metabolic complications: The role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2014, 15, 6184–6223. [Google Scholar] [CrossRef] [PubMed]
- Veryser, L.; Taevernier, L.; Joshi, T.; Tatke, P.; Wynendaele, E. Mucosal and blood-brain barrier transport kinetics of the plant N-alkylamide spilanthol using in vitro and in vivo models. BMC Complem. Altern. Med. 2016, 16, 177. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Cha, S.H.; Chohnan, S.; Lane, M.D. Hypothalamic malonyl-CoA as a mediator of feeding behavior. Proc. Natl. Acad. Sci. 2003, 100, 12624–12629. [Google Scholar] [CrossRef] [PubMed]
- Odle, A.K.; Haney, A.; Allensworth-James, M.; Akhter, N.; Childs, G.V. Adipocyte versus pituitary leptin in the regulation of pituitary hormones: Somatotropes develop normally in the absence of circulating leptin. Endocrinology 2014, 155, 4316–4328. [Google Scholar] [CrossRef] [PubMed]
- Barateiro, A.; Mahú, I.; Domingos, A.I. Leptin resistance and the neuro-adipose connection. Front. Endocrinol. 2017, 8, 45. [Google Scholar] [CrossRef] [PubMed]










© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, W.-C.; Peng, H.-L.; Hu, S.; Wu, S.-J. Spilanthol from Traditionally Used Spilanthes acmella Enhances AMPK and Ameliorates Obesity in Mice Fed High-Fat Diet. Nutrients 2019, 11, 991. https://doi.org/10.3390/nu11050991
Huang W-C, Peng H-L, Hu S, Wu S-J. Spilanthol from Traditionally Used Spilanthes acmella Enhances AMPK and Ameliorates Obesity in Mice Fed High-Fat Diet. Nutrients. 2019; 11(5):991. https://doi.org/10.3390/nu11050991
Chicago/Turabian StyleHuang, Wen-Chung, Hui-Ling Peng, Sindy Hu, and Shu-Ju Wu. 2019. "Spilanthol from Traditionally Used Spilanthes acmella Enhances AMPK and Ameliorates Obesity in Mice Fed High-Fat Diet" Nutrients 11, no. 5: 991. https://doi.org/10.3390/nu11050991