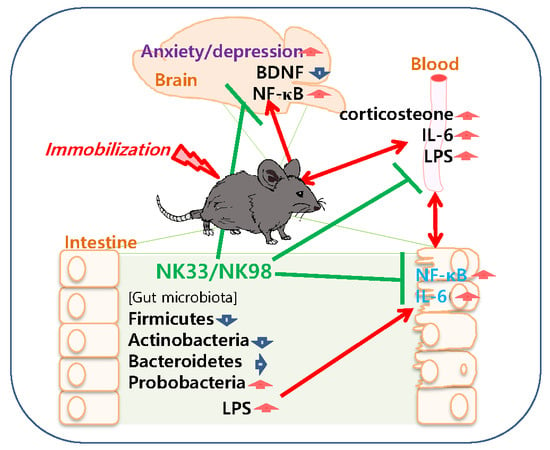
The Preventive and Curative Effects of Lactobacillus reuteri NK33 and Bifidobacterium adolescentis NK98 on Immobilization Stress-Induced Anxiety/Depression and Colitis in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Culture of Lactobacillus reuteri NK33 and Bifidobacterium adolescentis NK98 and Their Dosage Regimen
2.3. Culture of BV2 and SH-SY5Y Cells
2.4. Animals
2.5. Preparation of Mice with Anxiety/Depression and Colitis
2.6. Behavioral Tasks
2.7. Immunobloting and ELISA
2.8. Immunofluorescence Assay
2.9. Quantitative Polymerase Chain Reaction (qPCR)
2.10. Limulus Amoebocyte Lysate Assay
2.11. Statistical Analysis
3. Results
3.1. The Curative Effects of NK33 and NK98 against IS-Induced Anxiety/Depression and Colitis in Mice
3.2. The Preventive Effects of NK33 and NK98 on IS-Induced Anxiety/Depression in Mice
3.3. NK33 and NK98 Induced BDNF Expression and CREB Phosphorylation in LPS-Stimulated SH-SY5Y Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Baxter, A.J.; Scott, K.M.; Ferrari, A.J.; Norman, R.E.; Vos, T.; Whiteford, H.A. Challenging the myth of an “epidemic” of common mental disorders: Trends in the global prevalence of anxiety and depression between 1990 and 2010. Depress Anxiety 2014, 31, 506–516. [Google Scholar] [CrossRef]
- Bandelow, B.; Michaelis, S. Epidemiology of anxiety disorders in the 21st century. Dialogues Clin. Neurosci. 2015, 17, 327–335. [Google Scholar] [PubMed]
- Weger, M.; Sandi, C. High anxiety trait: A vulnerable phenotype for stress-induced depression. Neurosci. Biobehav. Rev. 2018, 87, 27–37. [Google Scholar] [CrossRef]
- Jaggi, A.S.; Bhatia, N.; Kumar, N.; Singh, N.; Anand, P.; Dhawan, R. A review on animal models for screening potential anti-stress agents. J. Neurol. Sci. 2011, 32, 993–1005. [Google Scholar] [CrossRef] [PubMed]
- Sutanto, D.; Kloet, E.R. The use of various animal models in the study of stress and stress-related phenomena. Lab. Anim. 1994, 28, 293–306. [Google Scholar] [CrossRef] [Green Version]
- Haddad, J.J.; Saade, N.E.; Safieh-Garabedian, B. Cytokines and neuro-immune-endocrine interactions: A role for the hypothalamic-pituitary-adrenal revolving axis. J. Neuroimmunol. 2002, 133, 1–19. [Google Scholar] [CrossRef]
- Foster, J.A. Gut microbiome and behavior: Focus on neuroimmune interactions. Int. Rev. Neurobiol. 2016, 131, 49–65. [Google Scholar] [PubMed]
- Xie, Z.M.; Wang, X.M.; Xu, N.; Wang, J.; Pan, W.; Tang, X.H.; Zhou, Z.Q.; Hashimoto, K.; Yang, J.J. Alterations in the inflammatory cytokines and brain-derived neurotrophic factor contribute to depression-like phenotype after spared nerve injury: Improvement by ketamine. Sci. Rep. 2017, 7, 3124. [Google Scholar] [CrossRef]
- Şahin, T.D.; Karson, A.; Balcı, F.; Yazır, Y.; Bayramgürler, D.; Utkan, T. TNF-α inhibition prevents cognitive decline and maintains hippocampal BDNF levels in the unpredictable chronic mild stress rat model of depression. Behav. Brain Res. 2015, 292, 233–240. [Google Scholar] [CrossRef]
- Libertucci, J.; Young, V.B. The role of the microbiota in infectious diseases. Nat. Microbiol. 2019, 4, 35–45. [Google Scholar] [CrossRef]
- Broecker, F.; Russo, G.; Klumpp, J.; Moelling, K. Stable core virome despite variable microbiome after fecal transfer. Gut Microbes 2017, 8, 214–220. [Google Scholar] [CrossRef]
- Flint, H.J.; Scott, K.P.; Louis, P.; Duncan, S.H. The role of the gut microbiota in nutrition and health. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 577–589. [Google Scholar] [CrossRef]
- Bäckhed, F.; Ley, R.E.; Sonnenburg, J.L.; Peterson, D.A.; Gordon, J.I. Host-bacterial mutualism in the human intestine. Science 2005, 307, 1915–1920. [Google Scholar] [CrossRef]
- Gill, S.R.; Pop, M.; Deboy, R.T.; Eckburg, P.B.; Turnbaugh, P.J.; Samuel, B.S.; Gordon, J.I.; Relman, D.A.; Fraser-Liggett, C.M.; Nelson, K.E. Metagenomic analysis of the human distal gut microbiome. Science 2006, 312, 1355–1359. [Google Scholar] [CrossRef]
- Chow, J.; Lee, S.M.; Shen, Y.; Khosravi, A.; Mazmanian, S.K. Host-bacterial symbiosis in health and disease. Adv. Immunol. 2010, 107, 243–274. [Google Scholar]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar]
- Mackos, A.R.; Varaljay, V.A.; Maltz, R.; Gur, T.L.; Bailey, M.T. Role of the intestinal microbiota in host responses to stressor exposure. Int. Rev. Neurobiol. 2016, 131, 1–19. [Google Scholar]
- Crumeyrolle-Arias, M.; Jaglin, M.; Bruneau, A.; Vancassel, S.; Cardona, A.; Daugé, V.; Naudon, L.; Rabot, S. Absence of the gut microbiota enhances anxiety-like behavior and neuroendocrine response to acute stress in rats. Psychoneuroendocrinology 2014, 42, 207–217. [Google Scholar] [CrossRef]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.N.; Kubo, C.; Koga, Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 2004, 558, 263–275. [Google Scholar] [CrossRef]
- Belda, X.; Fuentes, S.; Daviu, N.; Nadal, R.; Armario, A. Stress-induced sensitization: The hypothalamic-pituitary-adrenal axis and beyond. Stress 2015, 18, 269–279. [Google Scholar] [CrossRef]
- Malan-Muller, S.; Valles-Colomer, M.; Raes, J.; Lowry, C.A.; Seedat, S.; Hemmings, S.M.J. The gut microbiome and mental health: Implications for anxiety- and trauma-related disorders. OMICS 2018, 22, 90–107. [Google Scholar] [CrossRef]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Mechanisms of action of probiotics. Adv. Nutr. 2019, 10 (Suppl. 1), S49–S66. [Google Scholar] [CrossRef]
- Liu, X.; Cao, S.; Zhang, X. Modulation of gut microbiota-brain axis by probiotics, prebiotics, and diet. J. Agric. Food Chem. 2015, 63, 7885–7895. [Google Scholar] [CrossRef]
- Davis, D.J.; Doerr, H.M.; Grzelak, A.K.; Busi, S.B.; Jasarevic, E.; Ericsson, A.C.; Bryda, E.C. Lactobacillus plantarum attenuates anxiety-related behavior and protects against stress-induced dysbiosis in adult zebrafish. Sci. Rep. 2016, 6, 33726. [Google Scholar] [CrossRef] [Green Version]
- Jang, H.M.; Lee, H.J.; Jang, S.E.; Han, M.J.; Kim, D.H. Evidence for interplay among antibacterial-induced gut microbiota disturbance, neuro-inflammation, and anxiety in mice. Mucosal Immunol. 2018, 11, 1386–1397. [Google Scholar] [CrossRef]
- Jang, H.M.; Jang, S.E.; Han, M.J.; Kim, D.H. Anxiolytic-like effect of Bifidobacterium adolescentis IM38 in mice with or without immobilisation stress. Benef. Microbes 2018, 9, 123–132. [Google Scholar] [CrossRef]
- Lee, H.J.; Lim, S.M.; Ko, D.B.; Jeong, J.J.; Hwang, Y.H.; Kim, D.H. Soyasapogenol B and genistein attenuate lipopolysaccharide-induced memory impairment in mice by the modulation of NF-κB-Mediated BDNF expression. J. Agric. Food Chem. 2017, 65, 6877–6885. [Google Scholar] [CrossRef]
- Chu, X.; Zhou, Y.; Hu, Z.; Lou, J.; Song, W.; Li, J.; Liang, X.; Chen, C.; Wang, S.; Yang, B.; et al. 24-Hour-restraint stress induces long-term depressive-like phenotypes in mice. Sci. Rep. 2016, 9, 6:32935. [Google Scholar] [CrossRef]
- Dunn, A.J.; Swiergiel, A.H. Effects of interleukin-1 and endotoxin in the forced swim and tail suspension tests in mice. Pharmacol. Biochem. Behav. 2005, 81, 688–693. [Google Scholar] [CrossRef] [Green Version]
- Jang, S.E.; Lim, S.M.; Jeong, J.J.; Jang, H.M.; Lee, H.J.; Han, M.J.; Kim, D.H. Gastrointestinal inflammation by gut microbiota disturbance induces memory impairment in mice. Mucosal Immunol. 2018, 11, 369–379. [Google Scholar] [CrossRef]
- Kim, K.A.; Gu, W.; Lee, I.A.; Joh, E.H.; Kim, D.H. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS ONE 2012, 7, e47713. [Google Scholar] [CrossRef]
- Ait-Belgnaoui, A.; Durand, H.; Cartier, C.; Chaumaz, G.; Eutamene, H.; Ferrier, L.; Houdeau, E.; Fioramonti, J.; Bueno, L.; Theodorou, V. Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology 2012, 37, 1885–1895. [Google Scholar] [CrossRef]
- Bercik, P.; Collins, S.M. The effects of inflammation, infection and antibiotics on the microbiota-gut-brain axis. Adv. Exp. Med. Biol. 2014, 817, 279–289. [Google Scholar]
- Serrats, J.; Grigoleit, J.S.; Alvarez-Salas, E.; Sawchenko, P.E. Pro-inflammatory immune-to-brain signaling is involved in neuroendocrine responses to acute emotional stress. Brain Behav. Immun. 2017, 62, 53–63. [Google Scholar] [CrossRef]
- Jang, H.M.; Lee, K.E.; Lee, H.J.; Kim, D.H. Immobilization stress-induced Escherichia coli causes anxiety by inducing NF-κB activation through gut microbiota disturbance. Sci. Rep. 2018, 8, 13897. [Google Scholar] [CrossRef]
- Campos, A.C.; Rocha, N.P.; Nicoli, J.R.; Vieira, L.Q.; Teixeira, M.M.; Teixeira, A.L. Absence of gut microbiota influences lipopolysaccharide-induced behavioral changes in mice. Behav. Brain Res. 2016, 312, 186–194. [Google Scholar] [CrossRef]
- Jeong, M.Y.; Jang, H.M.; Kim, D.H. High-fat diet causes psychiatric disorders in mice by increasing Proteobacteria population. Neurosci. Lett. 2019, 698, 51–57. [Google Scholar] [CrossRef]
- Tanaka, S.; Ide, M.; Shibutani, T.; Ohtaki, H.; Numazawa, S.; Shioda, S.; Yoshida, T. Lipopolysaccharide-induced microglial activation induces learning and memory deficits without neuronal cell death in rats. J. Neurosci. Res. 2006, 83, 557–566. [Google Scholar] [CrossRef]
- Oh, H.A.; Kim, D.E.; Choi, H.J.; Kim, N.J.; Kim, D.H. Anti-stress effects of 20(S)-protopanaxadiol and 20(S)-protopanaxatriol in immobilized mice. Biol. Pharm. Bull. 2015, 38, 331–335. [Google Scholar] [CrossRef]
- Amitai, M.; Taler, M.; Carmel, M.; Michaelovsky, E.; Eilat, T.; Yablonski, M.; Orpaz, N.; Chen, A.; Apter, A.; Weizman, A.; et al. The relationship between plasma cytokine levels and response to selective serotonin reuptake inhibitor treatment in children and adolescents with depression and/or anxiety disorders. J. Child Adolesc. Psychopharmacol. 2016, 26, 727–732. [Google Scholar] [CrossRef]
- Guo, L.; Ren, L.; Zhang, C. Relationship between depression and inflammatory factors and brain-derived neurotrophic factor in patients with perimenopause syndrome. Exp. Ther. Med. 2018, 15, 4436–4440. [Google Scholar] [CrossRef] [Green Version]
- Ruzek, M.C.; Miller, A.H.; Opal, S.M.; Pearce, B.D.; Biron, C.A. Characterization of early cytokine responses and an interleukin (IL)-6-dependent pathway of endogenous glucocorticoid induction during murine cytomegalovirus infection. J. Exp. Med. 1997, 185, 1185–1192. [Google Scholar] [CrossRef]
- Bercik, P.; Park, A.J.; Sinclair, D.; Khoshdel, A.; Lu, J.; Huang, X.; Deng, Y.; Blennerhassett, P.A.; Fahnestock, M.; Moine, D.; et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol. Motil. 2011, 23, 1132–1139. [Google Scholar] [CrossRef]
- Desbonnet, L.; Garrett, L.; Clarke, G.; Kiely, B.; Cryan, J.F.; Dinan, T.G. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience 2010, 170, 1179–1188. [Google Scholar] [CrossRef]
- Slykerman, R.F.; Hood, F.; Wickens, K.; Thompson, J.M.D.; Barthow, C.; Murphy, R.; Kang, J.; Rowden, J.; Stone, P.; Crane, J.; et al. Probiotic in Pregnancy Study Group. Effect of Lactobacillus rhamnosus HN001 in pregnancy on postpartum symptoms of depression and anxiety: A randomised double-blind placebo-controlled trial. EBioMedicine 2017, 24, 159–165. [Google Scholar] [CrossRef]
- Liang, S.; Wang, T.; Hu, X.; Luo, J.; Li, W.; Wu, X.; Duan, Y.; Jin, F. Administration of Lactobacillus helveticus NS8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience 2015, 310, 561–577. [Google Scholar] [CrossRef]
- Foster, J.A.; McVey Neufeld, K.A. Gut-brain axis: How the microbiome influences anxiety and depression. Trends Neurosci. 2013, 36, 305–312. [Google Scholar] [CrossRef]
- Petra, A.I.; Panagiotidou, S.; Hatziagelaki, E.; Stewart, J.M.; Conti, P.; Theoharides, T.C. Gut-microbiota-brain axis and its effect on neuropsychiatric disorders with suspected immune dysregulation. Clin. Ther. 2015, 37, 984–995. [Google Scholar] [CrossRef]






| Phylum | Primer Sequence | |
|---|---|---|
| Forward | Reverse | |
| Firmicutes | 5′-GGAGYATGTGGTTTAATTCGAAGCA-3′ | 5′-AGCTGACGACAACCATGCAC-3′ |
| Bacteroidetes | 5′-GTTTAATTCGATGATACGCGAG-3′ | 5′-TTAASCCGACACCTCACGG-3′ |
| Actinobacteria | 5′-TGTAGCGGTGGAATGCGC-3′ | 5′-AATTAAGCCACATGCTCCGCT-3′ |
| δ/γ-Proteobacteria | 5′-GCTAACGCATTAAGTRYCCCG-3′ | 5′-GCCATGCRGCACCTGTCT-3′ |
| 16S rRNA | 5′-TCGTCGGCAGCGTCAGATGTGTATAA GAGACAGGTGCCAGCMGCCGCGGTAA-3′ | 5′-GTCTCGTGGGCTCGGAGATGTGTATA AGAGACAGGGACTACHVGGGTWTCTAAT-3′ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, H.-M.; Lee, K.-E.; Kim, D.-H. The Preventive and Curative Effects of Lactobacillus reuteri NK33 and Bifidobacterium adolescentis NK98 on Immobilization Stress-Induced Anxiety/Depression and Colitis in Mice. Nutrients 2019, 11, 819. https://doi.org/10.3390/nu11040819
Jang H-M, Lee K-E, Kim D-H. The Preventive and Curative Effects of Lactobacillus reuteri NK33 and Bifidobacterium adolescentis NK98 on Immobilization Stress-Induced Anxiety/Depression and Colitis in Mice. Nutrients. 2019; 11(4):819. https://doi.org/10.3390/nu11040819
Chicago/Turabian StyleJang, Hyo-Min, Kyung-Eon Lee, and Dong-Hyun Kim. 2019. "The Preventive and Curative Effects of Lactobacillus reuteri NK33 and Bifidobacterium adolescentis NK98 on Immobilization Stress-Induced Anxiety/Depression and Colitis in Mice" Nutrients 11, no. 4: 819. https://doi.org/10.3390/nu11040819