HM-Chromanone Isolated from Portulaca Oleracea L. Protects INS-1 Pancreatic β Cells against Glucotoxicity-Induced Apoptosis
Abstract
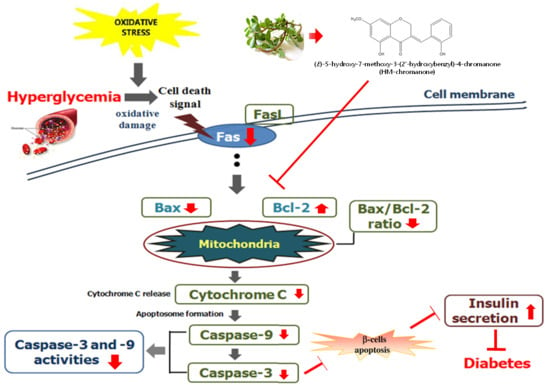
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction and Isolation
2.3. Cell Culture
2.4. Cell Viability Assay
2.5. Assay of Intracellular ROS Levels
2.6. Assay for Measurement of Lipid Peroxidation
2.7. Assay to Measure Nitric Oxide (NO) Levels
2.8. Glucose-Stimulated Insulin Secretion (GSIS)
2.9. Western Blot Analysis
2.10. Flow Cytometric Assessment of Apoptosis
2.11. Statistical Analysis
3. Results
3.1. (E)-5-Hydroxy-7-Methoxy-3-(2′-Hydroxybenzyl)-4-Chromanone
3.2. Effect of HM-Chromanone on Cell Viability
3.3. Effect of HM-Chromanone on Intracellular Levels of Reactive Oxygen Species (ROS)
3.4. Effect of HM-Chromanone on Generation of Thiobarbituric Acid Reactive Substances (TBARS)
3.5. Effect of HM-Chromanone on the Level of Nitric Oxide (NO)
3.6. Effect of HM-Chromanone on Insulin Secretion
3.7. Effect of HM-Chromanone on Apoptosis-Related Protein Expression
3.8. Identification of the Type of Cell Death By Annexin-V/PI Staining
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Butler, A.E.; Janson, J.; Bonner-Weir, S.; Ritzel, R.; Rizza, R.A.; Butler, P.C. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 2003, 52, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.E. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of Type 2 diabetes. Diabetologia 2003, 46, 3–19. [Google Scholar] [CrossRef] [PubMed]
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998, 352, 837–853. [Google Scholar] [CrossRef]
- Rashid, M.A.; Lee, S.; Tak, E. Carbonyl reductase 1 protects pancreatic β-cells against oxidative stress-induced apoptosis in glucotoxicity and glucolipotoxicity. Radic. Biol. Med. 2010, 49, 1522–1533. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.A.; Hong, Y.S. Mechanism of the insulin secretory defect by chronically elevated glucose levels in pancreatic islets: Depletion of insulin content due to hyperstimulation by glucose. J. Kor. Diabetes Asso. 2000, 24, 1–9. [Google Scholar]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef]
- Baynes, J.W.; Thorpe, S.R. Role of oxidative stress in diabetic complications: A new perspective on an old paradigm. Diabetes 1999, 48, 1–9. [Google Scholar] [CrossRef]
- Robertson, R.; Zhou, H.; Zhang, T.; Harmon, J.S. Chronic oxidative stress as a mechanism for glucose toxicity of the beta cell in type 2 diabetes. Cell. Biochem. Biophys. 2007, 48, 139–146. [Google Scholar] [CrossRef]
- Zhou, Y.X.; Xin, H.L.; Rahman, K.; Wang, S.J.; Peng, C.; Zhang, H. Portulaca oleracea L.: A review of phytochemistry and pharmacological effects. Biomed. Res. Int. 2015. [Google Scholar] [CrossRef]
- Zhu, H.B.; Wang, Y.Z.; Liu, Y.X.; Xia, Y.L.; Tang, T. Analysis of flavonoids in Portulaca oleracea L. by UV-vis spectrophotometry with comparative study on different extraction technologies. Food Anal. Methods 2010, 3, 90–97. [Google Scholar] [CrossRef]
- Xiang, L.; Xing, D.; Wang, W.; Wang, R.; Ding, Y.; Du, L. Alkaloids from Portulaca oleracea L. Phytochemistry 2005, 66, 2595–2601. [Google Scholar] [CrossRef]
- Kamal, U.; Abdul, S.J.; Eaqub, A.; Mohd, R.I. Evaluation of Antioxidant properties and mineral composition of purslane (portulaca oleracea) at different growth stages. Int. J. Mol. Sci. 2012, 13, 10257–10267. [Google Scholar]
- Abdel Moneim, A.E. The neuroprotective effects of purslane (Portulaca oleracea) on rotenone-induced biochemical changes and apoptosis in brain of rat. Neurol. Disord. Drug Targets 2013, 12, 830–841. [Google Scholar] [CrossRef]
- Park, J.E.; Han, J.S. Portulaca oleracea L. extract lowers postprandial hyperglycemia by inhibiting. J. Life Sci. 2018, 28, 421–428. [Google Scholar]
- Roy, S.K.; Agrahari, U.C.; Gautam, R.; Srivastava, A.; Jachak, S.M. Isointricatinol, a new antioxidant homoisoflavonoid from the roots of Caesalpinia digyna Rottler. Nat. Prod. Res. 2012, 26, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Mei, W.L.; Wu, J.; Zhao, Y.X.; Peng, M.; Dai, H.F. A new cytotoxic homoisoflavonoid from Dracaena cambodiana. J. Asian Nat. Prod. Res. 2009, 11, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Park, J.Y.; Seo, Y.; Han, J.S. A new chromanone isolated from Portulaca oleracea L. increases glucose uptake by stimulating GLUT4 translocation to the plasma membrane in 3T3-L1 adipocytes. Int. J. Biol. Macromol. 2018, 123, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 2013, 39, 44–84. [Google Scholar] [CrossRef]
- Lin, L.G.; Liu, Q.Y.; Ye, Y. Naturally occurring homoisoflavonoids and their pharmacological activities. Planta. Medica. 2014, 80, 1053–1066. [Google Scholar] [CrossRef]
- Ihara, Y.; Toyokuni, S.; Uchida, K.; Odaka, H.; Tanaka, T.; Ikeda, H.; Hiai, H.; Seino, Y.; Yamada, Y. Hyperglycemia causes oxidative stress in pancreatic beta-cells of GK rats, a model of type 2 diabetes. Diabetes 1999, 48, 927–932. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Pi, J.; Zhang, Q.; Fu, J.; Woods, C.G.; Hou, Y.; Corkey, B.E.; Collins, S.; Andersen, M.E. ROS signaling, oxidative stress and Nrf2 in pancreatic beta-cell function. Toxicol. Appl. Pharmacol. 2010, 244, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Calvo, M.I. Three new homoisoflavanones from the bulbs of Ledebouria floribunda. Fitoterapia 2009, 80, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Uddin, G.M.; Kim, C.Y.; Chung, D.; Kim, K.A.; Jung, S.H. One-step isolation of sappanol and brazilin from Caesalpinia sappan and their effects on oxidative stress-induced retinal death. BMB Rep. 2015, 48, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Namdar, R.; Nafisi, S. Study on the Interaction of Homoisoflavonoids with Nucleic Acids Comparative Study by Spectroscopic Methods; LAP LAMBERT Academic Publishing: Riga, Latvia, 2013; ISBN 978-3-659-49924-1. [Google Scholar]
- Ma, C.; Li, G.; Zhang, J.; Zheng, Q.; Fan, X.; Wang, Z. An efficient combination of supercritical fluid extraction and high-speed counter-current chromatography to extract and purify homoisoflavonoids from Ophiopogon japonicus (Thunb.) Ker-Gawler. J. Sep. Sci. 2009, 32, 1949–1956. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure–antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Cao, G.; Alessio, H.M.; Cutler, R.G. Oxygen-radical absorbance capacity assay for antioxidants. Free Radic. Biol. Med. 1993, 14, 303–311. [Google Scholar] [CrossRef]
- Pi, J.; Bai, Y.; Zhang, Q.; Wong, V.; Floering, L.M.; Daniel, K.; Collins, S. Reactive oxygen species as a signal in glucose-stimulated insulin secretion. Diabetes 2007, 56, 1783–1791. [Google Scholar] [CrossRef]
- Chikezie, P.C.; Ojiako, O.A.; Ogbuji, A.C. Oxidative stress in diabetes mellitus. Int. J. Biol. Chem. 2015, 9, 92–109. [Google Scholar] [CrossRef]
- Kakkar, R.; Kalra, J.; Mantha, S.V.; Prasad, K. Lipid peroxidation and activity of antioxidant enzymes in diabetic rats. Mol. Cell Biochem. 1995, 151, 113–119. [Google Scholar] [CrossRef]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant and prooxidant behavior of flavonoids: Structure-activity relationships. Free Radical Biol. Med. 1997, 22, 749–760. [Google Scholar] [CrossRef]
- Safari, M.R.; Sheikh, N. Effects of flavonoids on the susceptibility of low-density lipoprotein to oxidative modification. Prostaglandins Leukot Essent Fatty Acids 2003, 69, 73–77. [Google Scholar] [CrossRef]
- Bors, W.; Heller, W.; Michel, C.; Saran, M. Flavonoids as antioxidants: Determination of radical-scavenging efficiencies. Methods Enzymol. 1990, 186, 343–355. [Google Scholar] [PubMed]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2012, 35, 564–571. [Google Scholar]
- Angelo, A.; Mattia, A.; Lisa, M.; Saulade, K.; Gian, P.F. Endothelial dysfunction in diabetes the role of reparatory mechanisms. Diabetes Care 2011, 34, S285–S290. [Google Scholar]
- Pal, P.; Joseph, S.B.; Lucas, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar]
- Mandrup-Poulsen, T.; Helqvist, S.; Wogensen, L.D.; Mølvig, J.; Pociot, F.; Johannesen, J.; Nerup, J. Cytokine and free radicals as effector molecules in the destruction of pancreatic beta cells. Curr. Top. Microbiol. Immunol. 1990, 164, 169–193. [Google Scholar]
- Robinson, M.J.; Cobb, M.H. Mitogen-activated protein kinase pathways. Curr. Opin. Cell Biol. 1997, 9, 180–186. [Google Scholar] [CrossRef]
- Kuwana, T.; Newmeyer, D.D. Bcl-2-family proteins and the role of mitochondria in apoptosis. Curr. Opin. Cell Biol. 2003, 15, 691–699. [Google Scholar] [CrossRef]
- Desagher, S.; Martinou, J.C. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 2000, 10, 369–377. [Google Scholar] [CrossRef]
- Nickells, R.W. Apoptosis of retinal ganglion cells in glaucoma: An update of the molecular pathways involved in cell death. Surv. Ophthalmol. 1999, 43, S151–S161. [Google Scholar] [CrossRef]
- Porter, A.G.; Jänicke, R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Diff. 1999, 6, 99. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.R.; Kim, J.S.; Kim, M.S.; Jang, J.Y.; Oh, C.H.; Kang, D.H.; Qian, Z.J.; Jung, W.K.; Choi, I.W.; He, S.J. Induction of apoptosis by the tropical seaweed Pylaiella littoralis in HT-29 cells via the mitochondrial and MAPK pathways. Ocean Sci. 2013, 48, 339–348. [Google Scholar] [CrossRef]
- Kim, K.H.; Kim, Y.W.; Kim, H.B.; Lee, B.J.; Lee, D.S. Anti-apoptotic activity of laminarin polysaccharides and their enzymatically hydrolyzed oligosaccharides from Laminaria japonica. Biotechnol Lett. 2006, 28, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Mouria, M.; Gukovskaya, A.S.; Jung, Y.; Buechler, P.; Hines, O.J.; Reber, H.A.; Pandol, S.J. Food-derived polyphenols inhibit pancreatic cancer growth through mitochondrial cytochrome C release and apoptosis. Int. J. Cancer 2002, 98, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Kang, K.A.; Kang, S.S.; Park, J.W.; Hyun, J.W. Morin (29,3,49,5,7-pentahydroxyflavone) protected cells against c-radiation-induced oxidative stress. Basic Clin. Pharmacol Toxicol 2011, 108, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Lecoeur, H.; Ledru, E.; Prévost, M.C.; Gougeon, M.L. Strategies for phenotyping apoptotic peripheral human lymphocytes comparing ISNT, annexin-V and 7-AAD cytofluorometric staining methods. J. Immuno. Met. 1997, 209, 111–123. [Google Scholar] [CrossRef]
- Kaneto, H.; Fujii, J.; Seo, H.G.; Suzuki, K.; Matsuko, T.; Masahiro, N.; Tatsumi, H.; Yamasaki, Y.; Kamada, T.; Taniguchi, N. Apoptotic cell death triggered by nitric oxide in pancreatic β-cells. Diabetes 1995, 44, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Federici, M.; Hribal, M.; Perego, L.; Ranalli, M.; Caradonna, Z.; Perego, C.; Usellini, L.; Nano, R.; Bonini, P.; Bertuzzi, F.; et al. High glucose causes apoptosis in cultured human pancreatic islets of Langerhans: A potential role for regulation of specific Bcl family genes toward an apoptotic cell death program. Diabetes 2001, 50, 1290–1301. [Google Scholar] [CrossRef]
- Maedler, K.; Spinas, G.A.; Lehmann, R.; Sergeev, P.; Weber, M.; Fontana, A.; Kaiser, N.; Donath, M.Y. Glucose induces beta-cell apoptosis via upregulation of the Fas-receptor in human islets. Diabetes 2001, 50, 1683–1690. [Google Scholar] [CrossRef]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.E.; Seo, Y.; Han, J.S. HM-Chromanone Isolated from Portulaca Oleracea L. Protects INS-1 Pancreatic β Cells against Glucotoxicity-Induced Apoptosis. Nutrients 2019, 11, 404. https://doi.org/10.3390/nu11020404
Park JE, Seo Y, Han JS. HM-Chromanone Isolated from Portulaca Oleracea L. Protects INS-1 Pancreatic β Cells against Glucotoxicity-Induced Apoptosis. Nutrients. 2019; 11(2):404. https://doi.org/10.3390/nu11020404
Chicago/Turabian StylePark, Jae Eun, Youngwan Seo, and Ji Sook Han. 2019. "HM-Chromanone Isolated from Portulaca Oleracea L. Protects INS-1 Pancreatic β Cells against Glucotoxicity-Induced Apoptosis" Nutrients 11, no. 2: 404. https://doi.org/10.3390/nu11020404