Ionic Liquid-Polymer Nanoparticle Hybrid Systems as New Tools to Deliver Poorly Soluble Drugs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of ILs
2.3. Production of the IL-Polymer Nanoparticle Hybrid Systems
2.4. Particle Size, Polydispersity Index and Zeta Potential
2.5. Association Efficiency (AE) of Rutin
2.6. Freeze-Drying of the IL-Polymer Nanoparticle Hybrid Systems
2.7. Reconstitution of the Lyophilizates and Freeze-Drying Ratio
2.8. Fourier Transform Infrared Spectroscopy (FTIR)
2.9. Differential Scanning Calorimetry (DSC)
2.10. Scanning Electron Microscopy (SEM)
2.11. In Vitro Release Study
2.12. Permeation Study
2.13. MTT Cytotoxicity Studies
2.14. Statistical Analysis
3. Results and Discussion
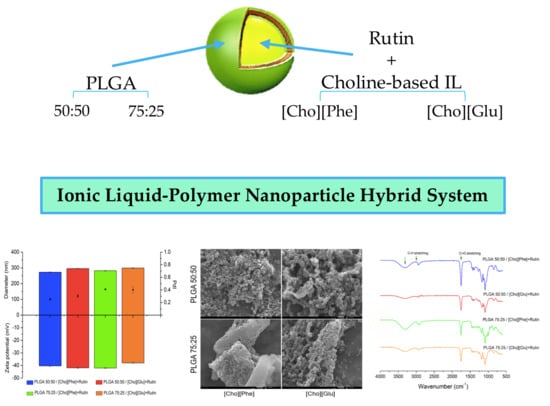
3.1. Particle Size, Polydispersity Index, and Zeta Potential
3.2. Association Efficiency (AE) of Rutin
3.3. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
3.4. Differential Scanning Calorimetry (DSC) Analysis
3.5. Scanning Electron Microscopy (SEM) Analysis
3.6. In Vitro Rutin Release Study
3.7. Permeation Study
3.8. MTT Cytotoxicity Assay
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Frizzo, C.P.; Gindri, I.M.; Tier, A.Z.; Buriol, L.; Moreira, D.N.; Martins, M.A.P. Additional Pharmaceutical Salts: Solids to Liquids by Using Ionic Liquid Design. In Ionic Liquids—New Aspects for the Future; Kadokawa, J., Ed.; InTech Open Science: London, UK, 2013; pp. 557–579. [Google Scholar]
- Mizuuchi, H.; Jaitely, V.; Murdan, S.; Florence, A.T. Room temperature ionic liquids and their mixtures: Potential pharmaceutical solvents. Eur. J. Pharm. Sci. 2008, 33, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Dobler, D.; Schmidts, T.; Klingenhoefer, I.; Runkel, F. Ionic liquids as ingredients in topical drug delivery systems. Int. J. Pharm. 2012, 441, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, W.; Jorge, T.F.; Martins, S.; Meireles, M.; Carolino, M.; Cruz, C.; Almeida, T.V.; Araújo, M.E.M. Toxicity of ionic liquids prepared from biomaterials. Chemosphere 2014, 104, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, R.; Branco, L.C.; Prudêncio, C.; Noronha, J.P.; Petrovski, Ž. Ionic liquids as active pharmaceutical ingredients. Chem. Med. Chem. 2011, 6, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Caparica, R.; Júlio, A.; Rosado, C.; Santos de Almeida, T. Applicability of ionic liquids in topical drug delivery systems: A mini review. J. Pharmacol. Clin. Res. 2018, 4, 555649–555655. [Google Scholar] [CrossRef]
- Ghandi, K. A review of ionic liquids, their limits and applications. Green Sustain. Chem. 2014, 4, 44–53. [Google Scholar] [CrossRef]
- Santos de Almeida, T.; Júlio, A.; Saraiva, N.; Fernandes, A.S.; Araújo, M.E.M.; Baby, A.R.; Rosado, C.; Mota, J.P. Choline-versus imidazole-based ionic liquids as functional ingredients in topical delivery systems: Cytotoxicity, solubility, and skin permeation studies. Drug Dev. Ind. Pharm. 2017, 43, 1858–1865. [Google Scholar] [CrossRef]
- Santos de Almeida, T.; Júlio, A.; Mota, J.P.; Rijo, P.; Reis, C.P. An emerging integration between ionic liquids ans nanotechnology: General uses and future prospects in drug delivery. Ther. Deliv. 2017, 6, 461–473. [Google Scholar] [CrossRef]
- Romero, A.; Santos, A.; Tojo, J.; Rodríguez, A. Toxicity and biodegradability of imidazolium ionic liquids. J. Hazard. Mater. 2008, 151, 268–273. [Google Scholar] [CrossRef]
- Melo, C.I.; Bogel-Łukasik, R.; Nunes da Ponte, M.; Bogel-Łukasik, E. Ammonium ionic liquids as green solvents for drugs. Fluid Phase Equilibria 2013, 338, 209–216. [Google Scholar] [CrossRef] [Green Version]
- Caparica, R.; Júlio, A.; Baby, A.R.; Eduarda, M.E.A.; Fernandes, A.S.; Costa, J.G.; Santos de Almeida, T. Choline-Amino Acid Ionic Liquids as Green Functional Excipients to Enhance Drug Solubility. Pharmaceutics 2018, 10, 288. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.M.; Silva, S.S.; Reis, R.L. Biocompatible ionic liquids: Fundamental behaviours and applications. Chem. Soc. Rev. 2019, 48, 4317–4335. [Google Scholar] [CrossRef] [PubMed]
- Kubota, K.; Shibata, A.; Yamaguchi, T. The molecular assembly of the ionic liquid/aliphatic carboxylic acid/aliphatic amine as effective and safety transdermal permeation enhancers. Eur. J. Pharm. Sci. 2016, 86, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Moriel, P.; García-Suárez, E.J.; Martínez, M.; García, A.B.; Montes-Morán, M.A.; Calvino-Casilda, V.; Bañares, M.A. Synthesis, characterization, and catalytic activity of ionic liquids based on biosources. Tetrahedron Lett. 2010, 51, 4877–4881. [Google Scholar] [CrossRef]
- Sowmiah, S.; Srinivasadesikan, V.; Tseng, M.; Chu, Y. On the Chemical Stabilities of Ionic Liquids. Molecules 2009, 14, 3780–3813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, W.; Tak, H.C.; Haumann, M.; Riisager, A. Ionic-liquid-supported synthesis: A novel liquid-phase strategy for organic synthesis. Acc. Chem. Res. 2006, 39, 897–908. [Google Scholar] [CrossRef] [PubMed]
- Vrikkis, R.M.; Fraser, K.J.; Fujita, K.; Macfarlane, D.R.; Elliott, G.D. Biocompatible ionic liquids: A new approach for stabilizing proteins in liquid formulation. J. Biomech. Eng. 2009, 131, 074514. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zheng, L.; Bai, X.; Li, N.; Yu, L. Fabrication of silica nanoparticles and hollow spheres using ionic liquid microemulsion droplets as templates. Colloids Surf. A Physicochem. Eng. Asp. 2009, 346, 229–236. [Google Scholar] [CrossRef]
- Gadilohar, B.L.; Shankarling, G.S. Choline based ionic liquids and their applications in organic transformation. J. Mol. Liq. 2017, 227, 234–261. [Google Scholar] [CrossRef]
- Haumann, M.; Riisager, A. Hydroformylation in room temperature ionic liquids (RTILs): Catalyst and process developments. Chem. Rev. 2008, 108, 1474–1497. [Google Scholar] [CrossRef]
- Huddleston, J.G.; Willauer, H.D.; Swatloski, R.P.; Visser, A.E.; Rogers, R.D. Room temperature ionic liquids as novel media for ‘clean’ liquid—Liquid extraction. Chem. Commun. 1998, 16, 1765–1766. [Google Scholar] [CrossRef]
- Han, X.; Armstrong, D.W. Ionic liquids in separations. Acc. Chem. Res. 2007, 40, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Kissoudi, M.; Samanidou, V. Recent advances in applications of ionic liquids in miniaturized microextraction techniques. Molecules 2018, 23, 1437. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, D.R.; Forsyth, M.; Howlett, P.C.; Pringle, J.M.; Sun, J.; Annat, G.; Neil, W.; Izgorodina, E.I. Ionic liquids in electrochemical devices and processes: Managing interfacial electrochemistry. Acc. Chem. Res. 2007, 40, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Hapiot, P.; Lagrost, C. Electrochemical reactivity in room-temperature ionic liquids. Chem. Rev. 2008, 108, 2238–2264. [Google Scholar] [CrossRef] [PubMed]
- Pringle, J.M.; MacFarlane, D.R.; Seddon, K.R.; Kar, M.; Plechkova, N.V. Ionic Liquids—Further Progress on the Fundamental Issues. Aust. J. Chem. 2018, 72, 3–10. [Google Scholar] [CrossRef]
- Prado, R.; Weber, C.C. Applications of Ionic Liquids. In Application, Purification, and Recovery of Ionic Liquids; Kuzmina, O., Hallet, J., Eds.; Elsevier: London, UK, 2016; Volume 1, pp. 1–58. ISBN 9780444637130. [Google Scholar]
- Palacio, M.; Bhushan, B. A review of ionic liquids for green molecular lubrication in nanotechnology. Tribol. Lett. 2010, 40, 247–268. [Google Scholar] [CrossRef]
- Biswas, A.K.; Islam, M.R.; Choudhury, Z.S.; Mostafa, A.; Kadir, M.F. Nanotechnology based approaches in cancer therapeutics. Adv. Nat. Sci. Nanosci. Nanotechnol. 2014, 5, 043001. [Google Scholar] [CrossRef]
- Adawiyah, N.; Moniruzzaman, M.; Hawatulaila, S.; Goto, M. Ionic liquids as a potential tool for drug delivery systems. Med. Chem. Comm. 2016, 7, 1881–1897. [Google Scholar] [CrossRef]
- Stoimenovski, J.; MacFarlane, D.R.; Bica, K.; Rogers, R.D. Crystalline vs. ionic liquid salt forms of active pharmaceutical ingredients: A position paper. Pharm. Res. 2010, 27, 521–526. [Google Scholar] [CrossRef]
- Hough, W.L.; Rogers, R.D. Ionic liquids then and now: From solvents to materials to active pharmaceutical ingredients. Bull. Chem. Soc. Jpn. 2007, 80, 2262–2269. [Google Scholar] [CrossRef]
- Júlio, A.; Antunes, C.; Mineiro, R.; Raposo, M.; Caparica, R.M.; Araújo, M.E.; Rosado, C.; Fonte, P.; Santos de Almeida, T. Influence of two choline-based ionic liquids on the solubility of caffeine. J. Biomed. Biopharm. Res. 2018, 15, 96–102. [Google Scholar] [CrossRef]
- Balk, A.; Holzgrabe, U.; Meinel, L. Pro et contra’ ionic liquid drugs—Challenges and opportunities for pharmaceutical translation. Eur. J. Pharm. Biopharm. 2015, 94, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Frade, R.F.; Afonso, C.A. Impact of ionic liquids in environment and humans: An overview. Hum. Exp. Toxicol. 2010, 29, 1038–1054. [Google Scholar] [CrossRef] [PubMed]
- Bawa, R. Nanopharmaceuticals: Nanopharmaceuticals. Eur. J. Nanomed. 2010, 3, 122–127. [Google Scholar] [CrossRef]
- Hare, J.I.; Lammers, T.; Ashford, M.B.; Puri, S.; Storm, G.; Barry, S.T. Challenges and strategies in anti-cancer nanomedicine development: An industry perspective. Adv. Drug Deliv. Rev. 2017, 108, 25–38. [Google Scholar] [CrossRef] [Green Version]
- Júlio, A.; Costa Lima, S.A.; Reis, S.; Santos de Almeida, T.; Fonte, P. Development of ionic liquid-polymer nanoparticle hybrid systems for delivery of poorly soluble drugs. J. Drug Deliv. Sci. Technol. 2019, 1–23. [Google Scholar] [CrossRef]
- Weissig, V.; Pettinger, T.K.; Murdock, N. Nanopharmaceuticals (part 1): Products on the market. Int. J. Nanomed. 2014, 9, 4357–4373. [Google Scholar] [CrossRef]
- Safari, J.; Zarnegar, Z. Advanced drug delivery systems: Nanotechnology of health design A review. J. Saudi Chem. Soc. 2014, 18, 85–99. [Google Scholar] [CrossRef]
- Jawahar, N.; Meyyanathan, S. Polymeric nanoparticles for drug delivery and targeting: A comprehensive review. Int. J. Health Allied Sci. 2012, 1, 217–223. [Google Scholar] [CrossRef]
- Gelperina, S.; Kisich, K.; Iseman, M.D.; Heifets, L. The potential advantages of nanoparticle drug delivery systems in chemotherapy of tuberculosis. Am. J. Respir. Crit. Care Med. 2005, 172, 1487–1490. [Google Scholar] [CrossRef]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef]
- Bohrey, S.; Chourasiya, V.; Pandey, A. Polymeric nanoparticles containing diazepam: Preparation, optimization, characterization, in-vitro drug release and release kinetic study. Nano Converg. 2016, 3, 3–7. [Google Scholar] [CrossRef]
- Wang, Y.; Li, P.; Tran, T.T.-D.; Zhang, J.; Kong, L. Manufacturing Techniques and Surface Engineering of Polymer Based Nanoparticles for Targeted Drug Delivery to Cancer. Nanomaterials 2016, 6, 26. [Google Scholar] [CrossRef]
- Fonte, P.; Reis, S.; Sarmento, B. Facts and evidences on the lyophilization of polymeric nanoparticles for drug delivery. J. Control. Release 2016, 225, 75–86. [Google Scholar] [CrossRef]
- Kretlow, J.D.; Klouda, L.; Mikos, A.G. Injectable matrices and scaffolds for drug delivery in tissue engineering. Adv. Drug Deliv. Rev. 2007, 59, 263–273. [Google Scholar] [CrossRef]
- Li, Q.; Cai, T.; Huang, Y.; Xia, X.; Cole, S.P.C.; Cai, Y. A Review of the Structure, Preparation, and Application of NLCs, PNPs, and PLNs. Nanomaterials 2017, 7, 122. [Google Scholar] [CrossRef]
- Zhang, H.; Cui, W.; Bei, J.; Wang, S. Preparation of poly (lactide-co-glycolide-co-caprolactone) nanoparticles and their degradation behaviour in aqueous solution. Polym. Degrad. Stab. 2006, 91, 1929–1936. [Google Scholar] [CrossRef]
- Fonte, P.; Araújo, F.; Seabra, V.; Reis, S.; Van De Weert, M.; Sarmento, B. Co-encapsulation of lyoprotectants improves the stability of protein-loaded PLGA nanoparticles upon lyophilization. Int. J. Pharm. 2015, 496, 850–862. [Google Scholar] [CrossRef]
- Fonte, P.; Soares, S.; Sousa, F.; Costa, A.; Seabra, V.; Reis, S.; Sarmento, B. Stability study perspective of the effect of freeze-drying using cryoprotectants on the structure of insulin loaded into PLGA nanoparticles. Biomacromolecules 2014, 15, 3753–3765. [Google Scholar] [CrossRef]
- Murakami, H.; Kobayashi, M.; Takeuchi, H.; Kawashima, Y. Preparation of poly (DL-lactide-co-glycolide) nanoparticles by modified spontaneous emulsification solvent diffusion method. Int. J. Pharm. 1999, 187, 143–152. [Google Scholar] [CrossRef]
- He, Z.; Alexandridis, P. Ionic liquid and nanoparticle hybrid systems: Emerging applications. Adv. Colloid Interface Sci. 2017, 244, 54–70. [Google Scholar] [CrossRef]
- Prabhu Charan, K.T.; Pothanagandhi, N.; Vijayakrishna, K.; Sivaramakrishna, A.; Mecerreyes, D.; Sreedhar, B. Poly (ionic liquids) as “smart” stabilizers for metal nanoparticles. Eur. Polym. J. 2014, 60, 114–122. [Google Scholar] [CrossRef]
- Ueno, K.; Watanabe, M. From Colloidal Stability in Ionic Liquids to Advanced Soft Materials Using Unique Media. Langmuir 2011, 27, 9105–9115. [Google Scholar] [CrossRef]
- Gullón, B.; Lú-Chau, T.A.; Moreira, M.T.; Lema, J.M.; Eibes, G. Rutin: A review on extraction, identification and purification methods, biological activities and approaches to enhance its bioavailability. Trends Food Sci. Technol. 2017, 67, 220–235. [Google Scholar] [CrossRef]
- Chua, L.S. A review on plant-based rutin extraction methods and its pharmacological activities. J. Ethnopharmacol. 2013, 150, 805–817. [Google Scholar] [CrossRef]
- Sharma, S.; Ali, A.; Ali, J.; Sahni, J.K.; Baboota, S. Rutin : Therapeutic potential and recent advances in drug delivery. Expert Opin. Investig. Drugs 2013, 22, 1063–1079. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A.K. The Pharmacological Potential of Rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef]
- Oliveira, C.A.; De Peres, D.D.A.; Graziola, F.; Chacra, N.A.B.; Araújo, G.L.B.; De Flórido, A.C.; Mota, J.; Rosado, C.; Velasco, M.V.R.; Rodrigues, L.M.; et al. Cutaneous biocompatible rutin-loaded gelatin-based nanoparticles increase the SPF of the association of UVA and UVB filters. Eur. J. Pharm. Sci. 2016, 81, 1–9. [Google Scholar] [CrossRef]
- Fernandes, A.S.; Serejo, J.; Gaspar, J.; Cabral, F.; Bettencourt, A.F.; Rueff, J.; Castro, M.; Costa, J.; Oliveira, N.G. Oxidative injury in V79 Chinese hamster cells: Protective role of the superoxide dismutase mimetic MnTM-4-PyP. Cell Biol. Toxicol. 2010, 26, 91–101. [Google Scholar] [CrossRef]
- Wagemaker, T.A.L.; Rijo, P.; Rodrigues, L.M.; Maia Campos, P.M.B.G.; Fernandes, A.S.; Rosado, C. Integrated approach in the assessment of skin compatibility of cosmetic formulations with green coffee oil. Int. J. Cosmet. Sci. 2015, 37, 506–510. [Google Scholar] [CrossRef]
- Biswas, K.; Rao, C.N.R. Use of ionic liquids in the synthesis of nanocrystals and nanorods of semiconducting metal chalcogenides. Chem. Eur. J. 2007, 13, 6123–6129. [Google Scholar] [CrossRef]
- Fonte, P.; Andrade, F.; Azevedo, C.; Pinto, J.; Seabra, V.; van de Weert, M.; Reis, S.; Sarmento, B. Effect of the Freezing Step in the Stability and Bioactivity of Protein-Loaded PLGA Nanoparticles Upon Lyophilization. Pharm. Res. 2016, 33, 2777–2793. [Google Scholar] [CrossRef]
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P. Biological activity of ionic liquids and their application in pharmaceutics and medicine. Chem. Rev. 2017, 117, 7132–7189. [Google Scholar] [CrossRef]
- Singh, G.; Kaur, T.; Kaur Ravinder, K.A. Recent biomedical applications and patents on biodegradable polymer-PLGA. Int. J. Pharmacol. Pharm. Sci. 2014, 1, 30–42. [Google Scholar]
- Singh, R.; Singh, S.; Banerjee, S.; Jain, N.K.; Mehra, N.K.; Kesharwani, P. Development and characterization of folate anchored Saquinavir entrapped PLGA nanoparticles for anti-tumor activity. Drug Dev. Ind. Pharm. 2015, 41, 1888–1901. [Google Scholar] [CrossRef]
- Erbetta, C.D.; Alves, R.J.; Resende, J.M.; de Souza Freitas, R.F.; de Sousa, R.G. Synthesis and characterization of poly (D, L-lactide-co-glycolide) copolymer. J. Biomater. Nanobiotechnol. 2012, 3, 208–225. [Google Scholar] [CrossRef]
- Selvaraj, K.; Chowdhury, R.; Bhattacharjee, C. Isolation and structural elucidation of flavonoids from aquatic fern Azolla microphylla and evaluation of free radical scavenging activity. Int. J. Pharm. Pharm. Sci. 2013, 5, 743–749. [Google Scholar]
- Hooresfand, Z.; Ghanbarzadeh, S.; Hamishehkar, H. Preparation and Characterization of Rutin-loaded Nanophytosomes. Pharm. Sci. 2015, 21, 145–151. [Google Scholar] [CrossRef] [Green Version]
- Kızılbey, K. Optimization of Rutin-Loaded PLGA Nanoparticles Synthesized by Single-Emulsion Solvent Evaporation Method. ACS Omega 2019, 4, 555–562. [Google Scholar] [CrossRef]
- Pirooznia, N.; Hasannia, S.; Lotfi, A.S.; Ghanei, M. Encapsulation of Alpha-1 antitrypsin in PLGA nanoparticles: In Vitro characterization as an effective aerosol formulation in pulmonary diseases. J. Nanobiotechnol. 2012, 10, 20. [Google Scholar] [CrossRef]
- Rodrigues de Azevedo, C.; von Stosch, M.; Costa, M.S.; Ramos, A.M.; Cardoso, M.M.; Danhier, F.; Préat, V.; Oliveira, R. Modeling of the burst release from PLGA micro- and nanoparticles as function of physicochemical parameters and formulation characteristics. Int. J. Pharm. 2017, 532, 229–240. [Google Scholar] [CrossRef]
- Soares, S.; Fonte, P.; Costa, A.; Andrade, J.; Seabra, V.; Ferreira, D.; Reis, S.; Sarmento, B. Effect of freeze-drying, cryoprotectants and storage conditions on the stability of secondary structure of insulin-loaded solid lipid nanoparticles. Int. J. Pharm. 2013, 456, 370–381. [Google Scholar] [CrossRef]
- Oliveira, C.A.; de Dario, M.F.; Sarruf, F.D.; Mariz, I.F.A.; Velasco, M.V.R.; Rosado, C.; Baby, A.R. Safety and efficacy evaluation of gelatin-based nanoparticles associated with UV filters. Colloids Surf. B Biointerfaces 2016, 140, 531–537. [Google Scholar] [CrossRef]
- Lewis, T.W.; Breadmore, M.C.; Innis, P.C.; Waheed, S.; Farajikhah, S.; Cabot, J.M.; Paull, B.; Nesterenko, P.N.; Kalsoom, U.; Macdonald, N.P. Enhanced physicochemical properties of polydimethylsiloxane based microfluidic devices and thin films by incorporating synthetic micro-diamond. Sci. Rep. 2017, 7, 15109. [Google Scholar] [CrossRef]
- Armbruster, C.; Schneider, M.; Schumann, S.; Gamerdinger, K.; Cuevas, M.; Rausch, S.; Baaken, G.; Guttmann, J. Characteristics of highly flexible PDMS membranes for long-term mechanostimulation of biological tissue. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 91, 700–705. [Google Scholar] [CrossRef]







| Polymer | IL | Freeze-Drying Ratio | |
|---|---|---|---|
| No Lyoprotectant | Trehalose at 3% (w/v) | ||
| PLGA 50:50 | [Cho][Phe] | 1.31 ± 0.01 | 1.01 ± 0.01 |
| [Cho][Glu] | 1.45 ± 0.05 | 1.04 ± 0.01 | |
| PLGA 75:25 | [Cho][Phe] | 1.28 ± 0.04 | 1.01 ± 0.02 |
| [Cho][Glu] | 1.50 ± 0.04 | 0.98 ± 0.02 | |
| Polymer | IL | AE (%) |
|---|---|---|
| PLGA 50:50 | [Cho][Phe] | 75.6 ± 1.0 * |
| [Cho][Glu] | 53.8 ± 2.4 | |
| PLGA 75:25 | [Cho][Phe] | 73.2 ± 0.9 * |
| [Cho][Glu] | 51.3 ± 1.3 |
| Formulation | IL | Flux (µg/cm2/h) |
|---|---|---|
| Rutin solution | [Cho][Phe] | 0.50 ± 0.09 |
| [Cho][Glu] | 0.52 ± 0.08 | |
| PLGA 50:50 | [Cho][Phe] | 0.55 ± 0.13 |
| [Cho][Glu] | 0.51 ± 0.11 | |
| PLGA 75:25 | [Cho][Phe] | 0.50 ± 0.06 |
| [Cho][Glu] | 0.49 ± 0.12 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Júlio, A.; Caparica, R.; Costa Lima, S.A.; Fernandes, A.S.; Rosado, C.; Prazeres, D.M.F.; Reis, S.; Santos de Almeida, T.; Fonte, P. Ionic Liquid-Polymer Nanoparticle Hybrid Systems as New Tools to Deliver Poorly Soluble Drugs. Nanomaterials 2019, 9, 1148. https://doi.org/10.3390/nano9081148
Júlio A, Caparica R, Costa Lima SA, Fernandes AS, Rosado C, Prazeres DMF, Reis S, Santos de Almeida T, Fonte P. Ionic Liquid-Polymer Nanoparticle Hybrid Systems as New Tools to Deliver Poorly Soluble Drugs. Nanomaterials. 2019; 9(8):1148. https://doi.org/10.3390/nano9081148
Chicago/Turabian StyleJúlio, Ana, Rita Caparica, Sofia A. Costa Lima, Ana Sofia Fernandes, Catarina Rosado, Duarte M. F. Prazeres, Salette Reis, Tânia Santos de Almeida, and Pedro Fonte. 2019. "Ionic Liquid-Polymer Nanoparticle Hybrid Systems as New Tools to Deliver Poorly Soluble Drugs" Nanomaterials 9, no. 8: 1148. https://doi.org/10.3390/nano9081148