Comparative Analysis of Properties of PVA Composites with Various Nanofillers: Pristine Clay, Organoclay, and Functionalized Graphene
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of HDA-GS
2.3. Preparation of PVA Hybrid Films
2.4. Characterization
3. Results and Discussion
3.1. FT-IR Spectroscopy
3.2. Morphology of HDA-GS
3.3. Dispersion
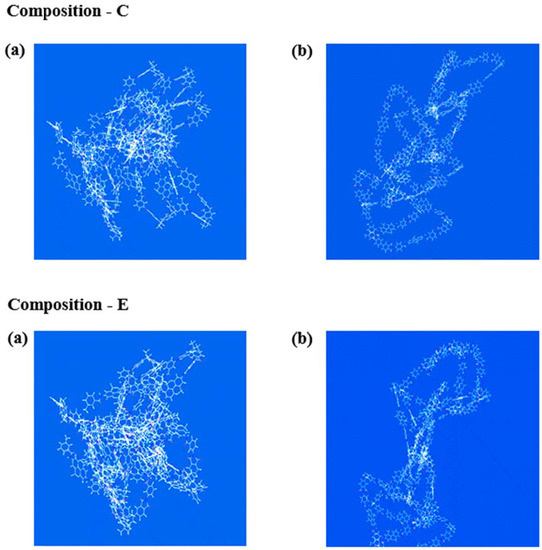
3.4. Morphologies of PVA Hybrids
3.5. Thermal Properties
3.6. Gas Permeation
4. Conclusions
Funding
Conflicts of Interest
Abbreviations
| AFM | Atomic force microscopy |
| DSC | Differential scanning calorimetry |
| FGSs | Functionalized graphene sheets |
| FT-IR | Fourier-transform infrared |
| GO | Graphene oxide |
| HDA-GSs | Hexadecylamine-functionalized graphene sheets |
| OMB | Organically modified bentonite |
| O2TRs | O2 transmission rates |
| PVA | Poly(vinyl alcohol |
| PET | Poly(ethylene terephthalate) |
| SEM | Scanning electron microscope |
| SPT | Saponite |
| TDi | Initial thermal degradation temperatures |
| TEM | Transmission electron microscopy |
| TGA | Thermogravimetric analyzer |
| Tg | Glass transition temperature |
| Tm | Melting transition temperature |
| wtR600 | Weight residue at 600 °C |
| XRD | Wide-angle X-ray diffraction |
References
- Zhou, K.; Gui, Z.; Hu, Y. Facile synthesis of LDH nanoplates as reinforcing agents in PVA nanocomposites. Polym. Adv. Technol. 2017, 28, 386–392. [Google Scholar] [CrossRef]
- Kashyap, S.; Pratihar, S.K.; Behera, S.K. Strong and ductile graphene oxide reinforced PVA nanocomposites. J. Alloys Compd. 2016, 684, 254–260. [Google Scholar] [CrossRef]
- Yahia, I.S.; Mohammed, M.I. Facile synthesis of graphene oxide/PVA nanocomposites for laser optical limiting: Band gap analysis and dielectric constants. J. Mater. Sci. Mater. Electron. 2018, 29, 8555–8563. [Google Scholar] [CrossRef]
- Rathod, S.G.; Bhajantri, R.F.; Ravindrachary, V.; Naik, J.; Kumar, D.J.M. High mechanical and pressure sensitive dielectric properties of graphene oxide dope PVA nanocomposites. RSC Adv. 2016, 6, 77977–77986. [Google Scholar] [CrossRef]
- Chang, J.-H.; Ham, M.; Kim, J.-C. Comparison of properties of poly(vinyl alcohol) nanocomposites containing two different clays. J. Nanosci. Nanotechnol. 2014, 14, 8783–8791. [Google Scholar] [CrossRef] [PubMed]
- Heo, C.; Chang, J.-H. Syntheses of functionalized grapheme sheets and their polymer nanocomposites. In Handbook of Functional Nanomaterials Volume 2—Characterization and Reliability; Niknam, Z.A., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2013; Chapter 4. [Google Scholar]
- Jan, R.; Habib, A.; Akram, M.A.; Zia, T.; Khan, A.N. Uniaxial drawing of graphene-PVA nanocomposites: Improvement in mechanical characteristics via strain-induced exfoliation of graphene. Nanoscale Res. Lett. 2016, 11, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Lagaly, G. Smectitc Calys as Ionic Macromolecules. Developments in Ionic Polymers; Elsevier Science: London, UK, 1986; Volume 2, pp. 77–140. [Google Scholar]
- Fukushima, Y.; Inagaki, S. Synthesis of an intercalated compound of montmorillonite and 6-polyamide. J. Incl. Phenom. Macrocycl. Chem. 1987, 5, 473–482. [Google Scholar] [CrossRef]
- Giannelis, E.P. Polymer layered silicate nanocomposites. Adv. Mater. 1996, 8, 29–35. [Google Scholar] [CrossRef]
- Manias, E.; Touny, A.; Wu, L.; Strawhecker, K.; Lu, B.; Chung, T.C. Polypropylene/ montmorillonite nanocomposites. Review of the synthetic routes and materials properties. Chem. Mater. 2001, 13, 3516–3523. [Google Scholar] [CrossRef]
- Cendoya, I.; Lopez, D.; Alegria, A.; Mijangos, C. Dynamic mechanical and dielectrical properties of poly(vinyl alcohol) and poly(vinyl alcohol)-based nanocomposites. J. Polym. Sci. Part B Polym. Phys. 2001, 39, 1968–1971. [Google Scholar] [CrossRef]
- Hernandez, M.C.; Suarez, N.; Martinez, L.A.; Feijoo, J.L.; Monaco, S.L.; Salazar, N. Effects of nanoscale dispersion in the dielectric properties of poly(vinyl alcohol)-bentonite nanocomposites. Phys. Rev. E 2008, 77, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Turhan, Y.; Alp, Z.G.; Alkan, M.; Dogan, M. Preparation and characterization of poly(vinyl alcohol)/modified bentonite nanocomposites. Microporous Mesoporous Mater. 2013, 174, 144–153. [Google Scholar] [CrossRef]
- Chang, J.-H.; Jang, T.G.; Ihn, K.J.; Lee, W.K.; Sur, G.S. Poly(vinyl alcohol) nanocomposites with different clays: Pristine clays and organoclays. J. Polym. Sci. 2003, 90, 3208–3214. [Google Scholar] [CrossRef]
- Gilman, J.W. Flammability and thermal stability studies of polymer layered-silicate (clay) nanocomposites. Appl. Clay Sci. 1999, 15, 31–49. [Google Scholar] [CrossRef]
- Messersmith, P.B.; Giannelis, E.P. Polymer-layered silicate nanocomposites: In situ intercalative polymerization of e-caprolactone in layered silicates. Chem. Mater. 1993, 5, 1064–1066. [Google Scholar] [CrossRef]
- Zhu, J.; Morgan, A.B.; Lamelas, F.J.; Wilkie, C.A. Fire properties of polystyrene−clay nanocomposites. Chem. Mater. 2001, 13, 3774–3780. [Google Scholar] [CrossRef]
- Davis, C.H.; Mathias, L.J.; Gilman, J.W.; Schiraldi, D.A.; Shields, J.R.; Trulove, P.; Sutto, T.E.; Delong, H.C. Effects of melt-processing conditions on the quality of poly(ethylene terephthalate) montmorillonite clay nanocomposites. J. Polym. Sci. Part B Polym. Phys. 2002, 40, 2661–2666. [Google Scholar] [CrossRef] [Green Version]
- Pinnavaia, T.J. Intercalated clay catalysts. Science 1983, 220, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Potts, J.R.; Dreyer, D.R.; Bielawski, C.W.; Ruoff, R.S. Graphene-based polymer nanocomposites. Polymer 2011, 52, 5–25. [Google Scholar] [CrossRef] [Green Version]
- Pradhan, S.K.; Nayak, B.B.; Sahay, S.S.; Mishra, B.K. Mechanical properties of graphite flakes and spherulites measured by nanoindentation. Carbon 2009, 47, 2290–2292. [Google Scholar] [CrossRef]
- Srinivas, G.; Zhu, Y.; Piner, R.; Skipper, N.; Ellerby, M.; Ruoff, R. Synthesis of graphene-like nanosheets and their hydrogen adsorption capacity. Carbon 2010, 48, 630–635. [Google Scholar] [CrossRef]
- Ansari, S.; Giannelis, E.P. Functionalized graphene sheet-poly(vinylidene fluoride) conductive nanocomposites. J. Polym. Sci. Part B Polym. Phys. 2009, 47, 888–897. [Google Scholar] [CrossRef]
- Raghu, A.V.; Lee, Y.R.; Jeong, H.M. Preparation and physical properties of waterborne polyurethane/functionalized graphene sheet nanocomposites. Macromol. Chem. Phys. 2008, 209, 2487–2493. [Google Scholar] [CrossRef]
- Pradhan, B.; Setyowati, K.; Liu, H.; Waldeck, D.H. Carbon nanotube−polymer nanocomposite infrared sensor. Nano Lett. 2008, 8, 1142–1146. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, L. Synthesis and characterization of layer-aligned poly(vinyl alcohol)/ graphene nanocomposites. Polymer 2010, 51, 3431–3435. [Google Scholar] [CrossRef]
- Usuki, A.; Kawasumi, M.; Kojima, Y.; Okada, A.; Kurauchi, T.; Kamigato, O. Synthesis of nylon 6-clay hybrid. J. Mater. Res. 1993, 8, 1174–1184. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, Y.W.; Stormer, H.L.; Kim, P.P. Experimental observation of the quantum hall effect and berry’s phase in graphene. Nature 2005, 438, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Hummers, W.; Offman, R. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S. Introduction to Spectroscopy; Harcourt: Washington, DC, USA, 2001. [Google Scholar]
- Si, Y.; Samulski, E.T. Exfoliated graphene separated by platinum nanoparticles. Chem. Mater. 2008, 20, 6792–6797. [Google Scholar] [CrossRef]
- Morgan, A.B.; Gilman, J.W. Characterization of polymer-layered silicate (clay) nanocomposites by transmission electron microscopy and X-ray diffraction: A comparative study. J. Appl. Polym. Sci. 2003, 87, 1329–1338. [Google Scholar] [CrossRef] [Green Version]
- Galgali, G.; Ramesh, C.; Lele, A. A rheological study on the kinetics of hybrid formation in polypropylene nanocomposites. Macromolecules 2001, 34, 852–858. [Google Scholar] [CrossRef]
- Heo, C.; Moon, H.G.; Yoon, C.S.; Chang, J.-H. ABS nanocomposite films based on functionalized-graphene sheets. J. Appl. Polym. Sci. 2012, 124, 4663–4670. [Google Scholar] [CrossRef]
- Choi, I.H.; Chang, J.-H. Colorless polyimide nanocomposite films containing hexafluoro-isopropylidene group. Polym. Adv. Technol. 2011, 22, 682–689. [Google Scholar] [CrossRef]
- Agag, T.; Takeichi, T. Polybenzoxazine–montmorillonite hybrid nanocomposites: Synthesis and characterization. Polymer 2000, 41, 7083–7090. [Google Scholar] [CrossRef]
- Kumar, S.; Jog, J.P.; Natarajan, U. Preparation and characterization of poly(methyl methacrylate)–clay nanocomposites via melt intercalation: The effect of organoclay on the structure and thermal properties. J. Appl. Polym. Sci. 2003, 89, 1186–1194. [Google Scholar] [CrossRef]
- LeBaron, P.C.; Wang, Z.; Pinnavaia, T.J. Polymer-layered silicate nanocomposites: An overview. Appl. Clay Sci. 1999, 15, 11–29. [Google Scholar] [CrossRef]
- Fornes, T.D.; Yoon, P.J.; Hunter, D.L.; Keskkula, H.; Paul, D.R. Effect of organoclay structure on nylon 6 nanocomposite morphology and properties. Polymer 2002, 43, 5915–5933. [Google Scholar] [CrossRef]
- Pertrvic, X.S.; Javni, L.; Waddong, A.; Banhegyi, G.L. Structure and properties of polyurethane–silica nanocomposites. J. Appl. Polym. Sci. 2000, 76, 133–151. [Google Scholar] [CrossRef]
- Sakaya, T.; Osaki, N. The potential of nanocomposite barrier technology. J. Photopolym. Sci. Technol. 2006, 19, 197–202. [Google Scholar] [CrossRef]
- Xu, B.; Zheng, Q.; Song, Y.; Shangguan, Y. Calculating barrier properties of polymer/clay nanocomposites: Effects of clay layers. Polymer 2006, 47, 2904–2910. [Google Scholar] [CrossRef]
- Jarus, D.; Hiltner, A.; Baer, E. Barrier properties of polypropylene/polyamide blends produced by microlayer coextrusion. Polymer 2002, 43, 2401–2408. [Google Scholar] [CrossRef]
- Ham, M.R.; Kim, J.-C.; Chang, J.-H. Characterization of poly (vinyl alcohol) nanocomposite films with various clays. Polymer (Korea) 2013, 37, 225–231. [Google Scholar] [CrossRef]









| Nanofiller in PVA (wt%) | SPT | OMB | HDA-GS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tg (°C) | Tm (°C) | TDi a (°C) | wtR600 b (%) | Tg (°C) | Tm (°C) | TDi (°C) | wtR600 (%) | Tg (°C) | Tm (°C) | TDi (°C) | wtR600 (%) | |
| 0 (pure PVA) | 69 | 165 | 227 | 3 | 69 | 165 | 227 | 3 | 69 | 165 | 227 | 3 |
| 3 | 70 | 172 | 248 | 6 | 70 | 170 | 240 | 6 | 69 | 166 | 236 | 7 |
| 5 | 70 | 176 | 249 | 12 | 69 | 184 | 252 | 12 | 70 | 168 | 237 | 11 |
| 7 | 69 | 168 | 240 | 12 | 70 | 180 | 251 | 12 | 71 | 173 | 245 | 12 |
| 10 | 70 | 166 | 238 | 15 | 68 | 178 | 246 | 14 | 70 | 156 | 238 | 15 |
| Nanofiller in PVA (wt%) | SPT | OMB | HDA-GS | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Thickness (μm) | O2TR a (cc/m2/day) | Pc/Pp b | Thickness (μm) | O2TR (cc/m2/day) | Pc/Pp | Thickness (μm) | O2TR (cc/m2/day) | Pc/Pp | |
| 0 (pure PVA) | 20 | 5.13 | 1.00 | 20 | 5.13 | 1.00 | 20 | 5.13 | 1.00 |
| 3 | 20 | 0.44 | 0.09 | 20 | <10−2 | ≈ 0 | 21 | 2.41 | 0.47 |
| 5 | 22 | 0.25 | 0.05 | 21 | <10−2 | ≈ 0 | 23 | 0.98 | 0.19 |
| 7 | 24 | 0.24 | 0.05 | 22 | <10−2 | ≈ 0 | 22 | 3.85 | 0.75 |
| 10 | 20 | 0.88 | 0.17 | 21 | 3.18 | 0.62 | 24 | 4.27 | 0.83 |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, J.-H. Comparative Analysis of Properties of PVA Composites with Various Nanofillers: Pristine Clay, Organoclay, and Functionalized Graphene. Nanomaterials 2019, 9, 323. https://doi.org/10.3390/nano9030323
Chang J-H. Comparative Analysis of Properties of PVA Composites with Various Nanofillers: Pristine Clay, Organoclay, and Functionalized Graphene. Nanomaterials. 2019; 9(3):323. https://doi.org/10.3390/nano9030323
Chicago/Turabian StyleChang, Jin-Hae. 2019. "Comparative Analysis of Properties of PVA Composites with Various Nanofillers: Pristine Clay, Organoclay, and Functionalized Graphene" Nanomaterials 9, no. 3: 323. https://doi.org/10.3390/nano9030323