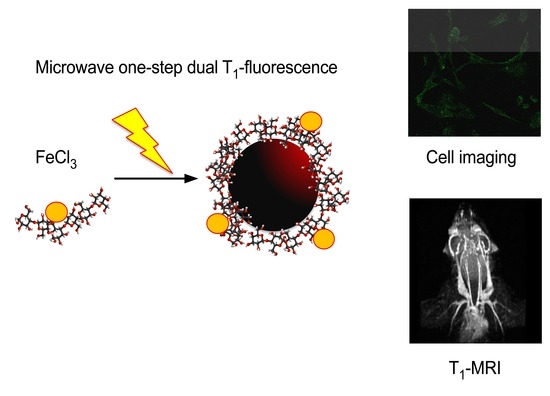
T1-MRI Fluorescent Iron Oxide Nanoparticles by Microwave Assisted Synthesis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Microwave Synthesis and Characterization of Fluorescein Isothiocyanate Carboxymethyl Dextran Iron Oxide Nanoparticles

2.2. Cell Labeling Studies

2.3. In Vivo Magnetic Resonance Angiography

3. Experimental Section
3.1. Preparation and Characterization of fdIONP
3.2. XRD
3.3. Relaxivity Studies
3.4. Cell Labeling Studies
3.4.1. Cell Culture and Media
3.4.2. fdIONPs Uptake and Cytotoxicity Assays
3.4.3. In vitro Inmunofluroescence Assay
3.5. MRI
3.6. Fluorescence Studies
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bulte, J.W.M.; Kraitchman, D.L. Iron oxide MR contrast agents for molecular and cellular imaging. NMR Biomed. 2004, 17, 484–499. [Google Scholar] [CrossRef] [PubMed]
- Babes, L.; Denizot, B.; Tanguy, G.; Le Jeune, J.J.; Jallet, P. Synthesis of Iron Oxide Nanoparticles Used as MRI Contrast Agents: A Parametric Study. J. Colloid Interface Sci. 1999, 212, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Pöselt, E.; Kloust, H.; Tromsdorf, U.; Janschel, M.; Hahn, C.; Maßlo, C.; Weller, H. Relaxivity Optimization of a PEGylated Iron-Oxide-Based Negative Magnetic Resonance Contrast Agent for T2-Weighted Spin-Echo Imaging. ACS Nano 2012, 6, 1619–1624. [Google Scholar] [CrossRef] [PubMed]
- Lartigue, L.; Innocenti, C.; Kalaivani, T.; Awwad, A.; Sanchez, D.; del Mar, M.; Guari, Y.; Larionova, J.; Guérin, C.; Montero, J.G.; et al. Water-dispersible sugar-coated iron oxide nanoparticles. An evaluation of their relaxometric and magnetic hyperthermia properties. J. Am. Chem. Soc. 2011, 133, 10459–10472. [Google Scholar] [CrossRef] [PubMed]
- Mccarthy, J.R.; Weissleder, R. Multifunctional magnetic nanoparticles for targeted imaging and therapy. 2008; 60, 1241–1251. [Google Scholar]
- Cunningham, C.H.; Arai, T.; Yang, P.C.; McConnell, M.V.; Pauly, J.M.; Conolly, S.M. Positive contrast magnetic resonance imaging of cells labeled with magnetic nanoparticles. Magn. Reson. Med. 2005, 53, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Xiao, N.; Gu, W.; Wang, H.; Deng, Y.; Shi, X.; Ye, L. T1-T2 dual-modal MRI of brain gliomas using PEGylated Gd-doped iron oxide nanoparticles. J. Colloid Interface Sci. 2014, 417, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Zhao, Y.S. Inorganic nanoparticle-based T1 and T1/T2 magnetic resonance contrast probes. Nanoscale 2012, 4, 6235–6243. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Haedicke, I.E.; Nofiele, J.; Martinez, F.; Beera, K.; Scholl, T.J.; Cheng, H.-L.M.; Zhang, X.-A. Complementary strategies for developing Gd-free high-field T1 MRI contrast agents based on Mn (III) porphyrins. J. Med. Chem. 2014, 57, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Na, H.B.; Lee, J.H.; An, K.; Park, Y.I.; Park, M.; Lee, I.S.; Nam, D.-H.; Kim, S.T.; Kim, S.-H.; Kim, S.-W.; et al. Development of a T1 contrast agent for magnetic resonance imaging using MnO nanoparticles. Angew. Chem. Int. Ed. Engl. 2007, 46, 5397–5401. [Google Scholar] [CrossRef] [PubMed]
- Bilecka, I.; Djerdj, I.; Niederberger, M. One-minute synthesis of crystalline binary and ternary metal oxide nanoparticles. Chem. Commun. 2008, 886–888. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.H.; Lee, N.; Kim, H.; An, K.; Park, Y.I.; Choi, Y.; Shin, K.; Lee, Y.; Kwon, S.G.; Na, H.B.; et al. Large-Scale Synthesis of Uniform and Extremely Small-Sized Iron Oxide Nanoparticles for High-Resolution T1 Magnetic Resonance Imaging Contrast Agents. J. Am. Chem. Soc. 2011, 133, 12624–12631. [Google Scholar] [CrossRef] [PubMed]
- Lidström, P.; Tierney, J.; Wathey, B.; Westman, J. Microwave assisted organic synthesis—A review. Tetrahedron 2001, 57, 9225–9283. [Google Scholar] [CrossRef]
- Sreeja, V.; Joy, P.A. Microwave-hydrothermal synthesis of γ-Fe2O3 nanoparticles and their magnetic properties. Mater. Res. Bull. 2007, 42, 1570–1576. [Google Scholar] [CrossRef]
- Pellico, J.; Lechuga-Vieco, A.V.; Benito, M.; García-Segura, J.M.; Fuster, V.; Ruiz-Cabello, J.; Herranz, F. Microwave-driven synthesis of bisphosphonate nanoparticles allows in vivo visualisation of atherosclerotic plaque. RSC Adv. 2014, 5, 1661–1665. [Google Scholar] [CrossRef]
- Bilecka, I.; Niederberger, M. Microwave chemistry for inorganic nanomaterials synthesis. Nanoscale 2010, 2. [Google Scholar] [CrossRef]
- Aphesteguy, J.C.; Kurlyandskaya, G.V.; de Celis, J.P.; Safronov, A.P.; Schegoleva, N.N. Magnetite nanoparticles prepared by co-precipitation method in different conditions. Mater. Chem. Phys. 2015, 161, 243–249. [Google Scholar] [CrossRef]
- Blanco-Andujar, C.; Ortega, D.; Southern, P.; Pankhurst, Q.A.; Thanh, N.T.K. High performance multi-core iron oxide nanoparticles for magnetic hyperthermia: microwave synthesis, and the role of core-to-core interactions. Nanoscale 2015, 7, 1768–1775. [Google Scholar] [CrossRef] [PubMed]
- Garzón-Manjón, A.; Solano, E.; de la Mata, M.; Guzmán, R.; Arbiol, J.; Puig, T.; Obradors, X.; Yáñez, R.; Ricart, S.; Ros, J. Induced shape controllability by tailored precursor design in thermal and microwave-assisted synthesis of Fe3O4 nanoparticles. J. Nanoparticle Res. 2015, 17. [Google Scholar] [CrossRef]
- Kalyani, S.; Sangeetha, J.; Philip, J. Microwave Assisted Synthesis of Ferrite Nanoparticles: Effect of Reaction Temperature on Particle Size and Magnetic Properties. J. Nanosci. Nanotechnol. 2015, 15, 5768–5774. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Hachtel, J.A.; Chisholm, M.F.; Pantelides, S.T.; Laromaine, A.; Roig, A. Magnetic gold nanotriangles by microwave-assisted polyol synthesis. Nanoscale 2015, 7, 14039–14046. [Google Scholar] [CrossRef] [PubMed]
- Linderoth, S.; Hendriksen, P.V.; Bo̸dker, F.; Wells, S.; Davies, K.; Charles, S.W.; Mo̸rup, S. On spin-canting in maghemite particles. J. Appl. Phys. 1994, 75. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhavesh, R.; Lechuga-Vieco, A.V.; Ruiz-Cabello, J.; Herranz, F. T1-MRI Fluorescent Iron Oxide Nanoparticles by Microwave Assisted Synthesis. Nanomaterials 2015, 5, 1880-1890. https://doi.org/10.3390/nano5041880
Bhavesh R, Lechuga-Vieco AV, Ruiz-Cabello J, Herranz F. T1-MRI Fluorescent Iron Oxide Nanoparticles by Microwave Assisted Synthesis. Nanomaterials. 2015; 5(4):1880-1890. https://doi.org/10.3390/nano5041880
Chicago/Turabian StyleBhavesh, Riju, Ana V. Lechuga-Vieco, Jesús Ruiz-Cabello, and Fernando Herranz. 2015. "T1-MRI Fluorescent Iron Oxide Nanoparticles by Microwave Assisted Synthesis" Nanomaterials 5, no. 4: 1880-1890. https://doi.org/10.3390/nano5041880