Two-Dimensional Bimetallic Phthalocyanine Covalent-Organic-Framework-Based Chemiresistive Gas Sensor for ppb-Level NO2 Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of COF-CuNiPc
2.3. Characterization
2.4. Gas Sensor Fabrication and Sensing Measurements
3. Results
3.1. Synthesis and Characterization of COFs
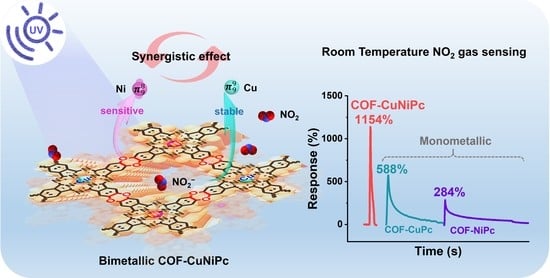
3.2. Gas-Sensing Properties of the COF-Based Devices
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Q.; Zeng, W.; Li, Y. Metal oxide gas sensors for detecting NO2 in industrial exhaust gas: Recent developments. Sens. Actuators B-Chem. 2022, 359, 131579. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, A.K.; Sohal, M.K.; Sharma, D.P.; Debnath, A.K.; Aswal, D.K.; Mahajan, A. Room temperature highly sensitive chlorine sensor based on reduced graphene oxide anchored with substituted copper phthalocyanine. Sens. Actuators B-Chem. 2021, 327, 128925. [Google Scholar] [CrossRef]
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef]
- Choi, J.Y.; Flood, J.; Stodolka, M.; Pham, H.T.B.; Park, J. From 2D to 3D: Postsynthetic pillar insertion in electrically conductive MOF. ACS Nano 2022, 16, 3145–3151. [Google Scholar] [CrossRef]
- Richards, V. Post-synthetic pillaring enhances metal–organic framework capacitance. Commun. Chem. 2022, 5, 30. [Google Scholar] [CrossRef]
- Chang, Y.; Lou, J.; Yang, L.; Liu, M.; Xia, N.; Liu, L. Design and application of electrochemical sensors with metal–organic frameworks as the electrode materials or signal tags. Nanomaterials 2022, 12, 3248. [Google Scholar] [CrossRef]
- Iqbal, R.; Yasin, G.; Hamza, M.; Ibraheem, S.; Ullah, B.; Saleem, A.; Ali, S.; Hussain, S.; Anh Nguyen, T.; Slimani, Y.; et al. State of the art two-dimensional covalent organic frameworks: Prospects from rational design and reactions to applications for advanced energy storage technologies. Coord. Chem. Rev. 2021, 447, 214152. [Google Scholar] [CrossRef]
- Talekar, S.; Kim, Y.; Wee, Y.; Kim, J. De novo synthesis of enzyme-embedded covalent organic frameworks (COFs) using deep eutectic solvent: Pushing the COF limits. Chem. Eng. J. 2023, 456, 141058. [Google Scholar] [CrossRef]
- Meng, Z.; Stolz, R.M.; Mirica, K.A. Two-dimensional chemiresistive covalent organic framework with high intrinsic conductivity. J. Am. Chem. Soc. 2019, 141, 11929–11937. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, S.; Liu, L.; Yu, L.; Chen, G.; Xu, W.; Zhu, D. Superconductivity in a copper(II)-based coordination polymer with perfect kagome structure. Angew. Chem. Int. Ed. 2018, 57, 146–150. [Google Scholar] [CrossRef]
- Duhović, S.; Dincă, M. Synthesis and electrical properties of covalent organic frameworks with heavy chalcogens. Chem. Mater. 2015, 27, 5487–5490. [Google Scholar] [CrossRef]
- Ding, X.; Guo, J.; Feng, X.; Honsho, Y.; Guo, J.; Seki, S.; Maitarad, P.; Saeki, A.; Nagase, S.; Jiang, D. Synthesis of metallophthalocyanine covalent organic frameworks that exhibit high carrier mobility and photoconductivity. Angew. Chem. Int. Ed. 2011, 50, 1289–1293. [Google Scholar] [CrossRef]
- Ding, X.; Chen, L.; Honsho, Y.; Feng, X.; Saengsawang, O.; Guo, J.; Saeki, A.; Seki, S.; Irle, S.; Nagase, S.; et al. An n-channel two-dimensional covalent organic framework. J. Am. Chem. Soc. 2011, 133, 14510–14513. [Google Scholar] [CrossRef]
- Wang, H.; Ding, H.; Meng, X.; Wang, C. Two-dimensional porphyrin- and phthalocyanine-based covalent organic frameworks. Chin. Chem. Lett. 2016, 27, 1376–1382. [Google Scholar] [CrossRef]
- Sorokin, A.B. Phthalocyanine metal complexes in catalysis. Chem. Rev. 2013, 113, 8152–8191. [Google Scholar] [CrossRef]
- Zhao, Y.-Y.; Chen, J.-Y.; Hu, J.-Q.; Zhang, L.; Lin, A.-L.; Wang, R.; Zheng, B.-Y.; Ke, M.-R.; Li, X.; Huang, J.-D. The substituted zinc(II) phthalocyanines using “sulfur bridge” as the linkages. Synthesis, red-shifted spectroscopic properties and structure-inherent targeted photodynamic activities. Dyes Pigm. 2021, 189, 109270. [Google Scholar] [CrossRef]
- Ouedraogo, S.; Meunier-Prest, R.; Kumar, A.; Bayo-Bangoura, M.; Bouvet, M. Modulating the electrical properties of organic heterojunction devices based on phthalocyanines for ambipolar sensors. ACS Sens. 2020, 5, 1849–1857. [Google Scholar] [CrossRef]
- Musial, J.; Belet, A.; Mlynarczyk, D.T.; Kryjewski, M.; Goslinski, T.; Lambert, S.D.; Poelman, D.; Stanisz, B.J. Nanocomposites of titanium dioxide and peripherally substituted phthalocyanines for the photocatalytic degradation of sulfamethoxazole. Nanomaterials 2022, 12, 3279. [Google Scholar] [CrossRef]
- Jiang, W.; Wang, T.; Chen, X.; Li, B.; Zeng, M.; Hu, N.; Su, Y.; Zhou, Z.; Zhang, Y.; Yang, Z. Enhancing room-temperature NO2 detection of cobalt phthalocyanine based gas sensor at an ultralow laser exposure. Phys. Chem. Chem. Phys. 2020, 22, 18499–18506. [Google Scholar] [CrossRef]
- Liu, M.; Li, N.; Cao, S.; Wang, X.; Lu, X.; Kong, L.; Xu, Y.; Bu, X.-H. A “Pre-constrained metal twins” strategy to prepare efficient dual-metal-atom catalysts for cooperative oxygen electrocatalysis. Adv. Mater. 2022, 34, 2107421. [Google Scholar] [CrossRef]
- Campos, J. Bimetallic cooperation across the periodic table. Nat. Rev. Chem. 2020, 4, 696–702. [Google Scholar] [CrossRef]
- Ma, Y.; Li, J.; Liao, X.; Luo, W.; Huang, W.; Meng, J.; Chen, Q.; Xi, S.; Yu, R.; Zhao, Y.; et al. Heterostructure design in bimetallic phthalocyanine boosts oxygen reduction reaction activity and durability. Adv. Funct. Mater. 2020, 30, 2005000. [Google Scholar] [CrossRef]
- Yang, C.; Gao, Z.; Wang, D.; Li, S.; Li, J.; Zhu, Y.; Wang, H.; Yang, W.; Gao, X.J.; Zhang, Z.; et al. Bimetallic phthalocyanine heterostructure used for highly selective electrocatalytic CO2 reduction. Sci. China Mater. 2022, 65, 155–162. [Google Scholar] [CrossRef]
- Li, Y.; Shan, W.; Zachman, M.J.; Wang, M.; Hwang, S.; Tabassum, H.; Yang, J.; Yang, X.; Karakalos, S.; Feng, Z.; et al. Atomically dispersed dual-metal site catalysts for enhanced CO2 reduction: Mechanistic insight into active site structures. Angew. Chem. Int. Ed. 2022, 61, e202205632. [Google Scholar]
- Wang, Z.; Jin, X.; Zhu, C.; Liu, Y.; Tan, H.; Ku, R.; Zhang, Y.; Zhou, L.; Liu, Z.; Hwang, S.-J.; et al. Atomically dispersed CO2–N6 and Fe–N4 costructures boost oxygen reduction reaction in both alkaline and acidic media. Adv. Mater. 2021, 33, 2104718. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Ly, K.H.; Wang, M.; Krupskaya, Y.; Han, X.; Zhang, J.; Zhang, J.; Kataev, V.; Buechner, B.; Weidinger, I.M.; et al. A phthalocyanine-based layered two-dimensional conjugated metal-organic framework as a highly efficient electrocatalyst for the oxygen reduction reaction. Angew. Chem. Int. Ed. 2019, 131, 10787–10792. [Google Scholar] [CrossRef]
- Meng, Z.; Aykanat, A.; Mirica, K.A. Welding metallophthalocyanines into bimetallic molecular meshes for ultrasensitive, low-power chemiresistive detection of gases. J. Am. Chem. Soc. 2019, 141, 2046–2053. [Google Scholar] [CrossRef]
- Yue, Y.; Cai, P.; Xu, K.; Li, H.; Chen, H.; Zhou, H.-C.; Huang, N. Stable bimetallic polyphthalocyanine covalent organic frameworks as superior electrocatalysts. J. Am. Chem. Soc. 2021, 143, 18052–18060. [Google Scholar] [CrossRef]
- Han, Y.; Ma, Y.; Liu, Y.; Xu, S.; Chen, X.; Zeng, M.; Hu, N.; Su, Y.; Zhou, Z.; Yang, Z. Construction of MoS2/SnO2 heterostructures for sensitive NO2 detection at room temperature. Appl. Surf. Sci. 2019, 493, 613–619. [Google Scholar] [CrossRef]
- Chen, X.; Wang, S.; Su, C.; Han, Y.; Zou, C.; Zeng, M.; Hu, N.; Su, Y.; Zhou, Z.; Yang, Z. Two-dimensional Cd-doped porous CO3O4 nanosheets for enhanced room-temperature NO2 sensing performance. Sens. Actuators B Chem. 2020, 305, 127393. [Google Scholar] [CrossRef]
- Shi, G.; Xie, Y.; Du, L.; Fu, X.; Chen, X.; Xie, W.; Lu, T.-B.; Yuan, M.; Wang, M. Constructing Cu−C bonds in a graphdiyne-regulated Cu single-atom electrocatalyst for CO2 reduction to CH4. Angew. Chem. Int. Ed. 2022, 61, e202203569. [Google Scholar]
- Yue, Y.; Li, H.; Chen, H.; Huang, N. Piperazine-linked covalent organic frameworks with high electrical conductivity. J. Am. Chem. Soc. 2022, 144, 2873–2878. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, M.; Lin, H.-H.; Ballabio, M.; Zhong, H.; Bonn, M.; Zhou, S.; Heine, T.; Cánovas, E.; Dong, R.; et al. High-mobility semiconducting two-dimensional conjugated covalent organic frameworks with p-type doping. J. Am. Chem. Soc. 2020, 142, 21622–21627. [Google Scholar] [CrossRef]
- Yue, Y.; Cai, P.; Xu, X.; Li, H.; Chen, H.; Zhou, H.; Huang, N. Conductive metallophthalocyanine framework films with high carrier mobility as efficient chemiresistors. Angew. Chem. Int. Ed. 2021, 60, 10806–10813. [Google Scholar] [CrossRef] [PubMed]
- Bohrer, F.I.; Sharoni, A.; Colesniuc, C.; Park, J.; Schuller, I.K.; Kummel, A.C.; Trogler, W.C. Gas sensing mechanism in chemiresistive cobalt and metal-free phthalocyanine thin films. J. Am. Chem. Soc. 2007, 129, 5640–5646. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, K.; Zhu, L.; Xu, M.; Song, Y.; Zhang, Z.; Du, M. Direct growth of two-dimensional phthalocyanine-based COF on Cu-MOF to construct a photoelectrochemical-electrochemical dual-mode biosensing platform for high-efficiency determination of Cr(iii). Dalton Trans. 2021, 50, 14285–14295. [Google Scholar] [CrossRef]
- Kurc, T.; Videnova-Adrabinska, V.; Turowska-Tyrk, I.; Duczmal, M.; Jerzykiewicz, M. Synthesis, crystal structure and magnetic properties of a novel copper(II) complex with sulfoisophthalic acid. J. Mol. Struct. 2013, 1054–1055, 134–142. [Google Scholar] [CrossRef]
- Zhao, J.; Zhou, M.; Chen, J.; Tao, L.; Zhang, Q.; Li, Z.; Zhong, S.; Fu, H.; Wang, H.; Wu, L. Phthalocyanine-based covalent organic frameworks as novel anode materials for high-performance lithium-ion/sodium-ion batteries. Chem. Eng. J. 2021, 425, 131630. [Google Scholar] [CrossRef]
- Singh, S.; Tripathi, S.K.; Saini, G.S.S. Effect of pyridine on infrared absorption spectra of copper phthalocyanine. Spectrochim. Acta Part A 2008, 69, 619–623. [Google Scholar] [CrossRef]
- Szybowicz, M.; Runka, T.; Drozdowski, M.; Bała, W.; Grodzicki, A.; Piszczek, P.; Bratkowski, A. High temperature study of FT-IR and Raman scattering spectra of vacuum deposited CuPc thin films. J. Mol. Struct. 2004, 704, 107–113. [Google Scholar] [CrossRef]
- Li, B.; Chen, X.; Su, C.; Han, Y.; Wang, H.; Zeng, M.; Wang, Y.; Liang, T.; Yang, Z.; Xu, L. Enhanced dimethyl methylphosphonate detection based on two-dimensional WSe2 nanosheets at room temperature. Analyst 2020, 145, 8059–8067. [Google Scholar] [CrossRef] [PubMed]
- Afshari, M.; Dinari, M.; Farrokhpour, H.; Zamora, F. Imine-linked covalent organic framework with a naphthalene moiety as a sensitive phosphate ion sensing. ACS Appl. Mater. Interfaces 2022, 14, 22398–22406. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zheng, B.-D.; Peng, X.-H.; Li, S.-Z.; Ying, J.-W.; Zhao, Y.; Huang, J.-D.; Yoon, J. Phthalocyanines as medicinal photosensitizers: Developments in the last five years. Coord. Chem. Rev. 2019, 379, 147–160. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, S.; Du, K. Chemiresistive gas sensors based on hollow heterojunction: A review. Adv. Mater. Interfaces 2021, 8, 2002122. [Google Scholar] [CrossRef]
- Klyamer, D.; Bonegardt, D.; Krasnov, P.; Sukhikh, A.; Popovetskiy, P.; Basova, T. Tetrafluorosubstituted metal phthalocyanines: Study of the effect of the position of fluorine substituents on the chemiresistive sensor response to ammonia. Chemosensors 2022, 10, 515. [Google Scholar] [CrossRef]
- Kumar, R.; Goel, N.; Kumar, M. UV-activated MoS2 based fast and reversible NO2 sensor at room temperature. ACS Sens. 2017, 2, 1744–1752. [Google Scholar] [CrossRef]
- Wu, E.; Xie, Y.; Yuan, B.; Zhang, H.; Hu, X.; Liu, J.; Zhang, D. Ultrasensitive and fully reversible NO2 gas sensing based on p-type MoTe2 under ultraviolet illumination. ACS Sens. 2018, 3, 1719–1726. [Google Scholar] [CrossRef]
- Yan, X.; Wu, Y.; Li, R.; Shi, C.; Moro, R.; Ma, Y.; Ma, L. High-performance UV-assisted NO2 sensor based on chemical vapor deposition graphene at room temperature. ACS Omega 2019, 4, 14179–14187. [Google Scholar] [CrossRef]
- Reddeppa, M.; Park, B.-G.; Murali, G.; Choi, S.H.; Chinh, N.D.; Kim, D.; Yang, W.; Kim, M.-D. NOx gas sensors based on layer-transferred n-MoS2/p-GaN heterojunction at room temperature: Study of UV light illuminations and humidity. Sens. Actuators B Chem. 2020, 308, 127700. [Google Scholar] [CrossRef]
- Wang, J.; Deng, J.; Li, Y.; Yuan, H.; Xu, M. ZnO nanocrystal-coated MoS2 nanosheets with enhanced ultraviolet light gas sensitive activity studied by surface photovoltage technique. Ceram. Int. 2020, 46, 11427–11431. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Z.; Liu, J.; Peng, Z.; Zhou, J.; Zhang, H.; Li, Y. Optoelectronic gas sensor based on few-layered InSe nanosheets for NO2 detection with ultrahigh antihumidity ability. Anal. Chem. 2020, 92, 11277–11287. [Google Scholar] [CrossRef]
- Fan, C.; Shi, J.; Zhang, Y.; Quan, W.; Chen, X.; Yang, J.; Zeng, M.; Zhou, Z.; Su, Y.; Wei, H.; et al. Fast and recoverable NO2 detection achieved by assembling ZnO on Ti3C2Tx MXene nanosheets under UV illumination at room temperature. Nanoscale 2022, 14, 3441–3451. [Google Scholar] [CrossRef]
- DMello, M.E.; Sundaram, N.G.; Singh, A.; Singh, A.K.; Kalidindi, S.B. An amine functionalized zirconium metal–organic framework as an effective chemiresistive sensor for acidic gases. Chem. Commun. 2019, 55, 349–352. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Wang, Y.; Wang, R.; Zhang, T. Metal-organic-framework-derived In2O3 microcolumnar structures embedded with Pt nanoparticles for NO2 detection near room temperature. Ceram. Int. 2019, 45, 9820–9828. [Google Scholar] [CrossRef]
- Yang, C.; Jiang, K.; Zheng, Q.; Li, X.; Mao, H.; Zhong, W.; Chen, C.; Sun, B.; Zheng, H.; Zhuang, X.; et al. Chemically stable polyarylether-based metallophthalocyanine frameworks with high carrier mobilities for capacitive energy storage. J. Am. Chem. Soc. 2021, 143, 17701–17707. [Google Scholar] [CrossRef]
- Qiu, X.-F.; Huang, J.-R.; Yu, C.; Zhao, Z.-H.; Zhu, H.-L.; Ke, Z.; Liao, P.-Q.; Chen, X.-M. A stable and conductive covalent organic framework with isolated active sites for highly selective electroreduction of carbon dioxide to acetate. Angew. Chem. Int. Ed. 2022, 61, e202206470. [Google Scholar]
- Wang, X.-Z.; Jiang, Y.; Wang, Y.; Ye, C.; Du, C.-F. Probing the tribocorrosion behaviors of three nickel-based superalloys in sodium chloride solution. Tribol. Int. 2022, 172, 107581. [Google Scholar] [CrossRef]
- Kang, T.; Sun, C.; Li, Y.; Song, T.; Guan, Z.; Tong, Z.; Nan, J.; Lee, C.-S. Dendrite-free sodium metal anodes via solid electrolyte interphase engineering with a covalent organic framework separator. Adv. Energy Mater. 2023, 13, 2204083. [Google Scholar] [CrossRef]
- Yuan, J.; Chen, S.; Zhang, Y.; Li, R.; Zhang, J.; Peng, T. Structural regulation of coupled phthalocyanine–porphyrin covalent organic frameworks to highly active and selective electrocatalytic CO2 reduction. Adv. Mater. 2022, 34, 2203139. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, N.; Yang, T.; Liu, D.; Jiang, X.; Wang, D.; Yang, Z.; Xie, Y.; Meng, L. Bimetallic metal–organic frameworks: Enhanced peroxidase-like activities for the self-activated cascade reaction. ACS Appl. Mater. Interfaces 2021, 13, 36106–36116. [Google Scholar] [CrossRef]
- Tran, L.D.; Presley, K.F.; Streit, J.K.; Carpena-Núñez, J.; Beagle, L.K.; Grusenmeyer, T.A.; Dalton, M.J.; Vaia, R.A.; Drummy, L.F.; Glavin, N.R.; et al. Divergent properties in structural isomers of triphenylamine-based covalent organic frameworks. Chem. Mater. 2022, 34, 529–536. [Google Scholar] [CrossRef]
- Wen, Y.; Huang, Z.; Zhao, M.; Zhao, L. Enhanced visible-light-driven photocatalytic activity of bi-phase titanium dioxide @ covalent organic framework Z-scheme system for photocatalytic removal of Cr (VI). Appl. Surf. Sci. 2022, 596, 153485. [Google Scholar] [CrossRef]
- Tomeček, D.; Piliai, L.; Hruška, M.; Fitl, P.; Gadenne, V.; Vorokhta, M.; Matolínová, I.; Vrňata, M. Study of photoregeneration of zinc phthalocyanine chemiresistor after exposure to nitrogen dioxide. Chemosensors 2021, 9, 237. [Google Scholar] [CrossRef]
- Huang, S.; Chen, K.; Li, T.-T. Porphyrin and phthalocyanine based covalent organic frameworks for electrocatalysis. Coord. Chem. Rev. 2022, 464, 214563. [Google Scholar] [CrossRef]
- Zheng, Q.; Li, X.; Zhang, Q.; Lee, D.; Mao, H.; Yang, C.; Bustillo, K.C.; Reimer, J.A.; Liu, Y.; Jiang, J.; et al. A covalent organic framework onion structure. Mater. Today 2022, 60, 98–105. [Google Scholar] [CrossRef]
- Gao, Z.; Yu, Z.; Huang, Y.; He, X.; Su, X.; Xiao, L.; Yu, Y.; Huang, X.; Luo, F. Flexible and robust bimetallic covalent organic frameworks for the reversible switching of electrocatalytic oxygen evolution activity. J. Mater. Chem. A 2020, 8, 5907–5912. [Google Scholar] [CrossRef]
- Mack, J.; Kobayashi, N. Low symmetry phthalocyanines and their analogues. Chem. Rev. 2011, 111, 281–321. [Google Scholar] [CrossRef]
- Archana, V.; Xia, Y.; Fang, R.; Gnana Kumar, G. Hierarchical CuO/NiO-carbon nanocomposite derived from metal organic framework on cello tape for the flexible and high performance nonenzymatic electrochemical glucose sensors. ACS Sustain. Chem. Eng. 2019, 7, 6707–6719. [Google Scholar] [CrossRef]
- Liu, F.; Meng, J.; Jiang, G.; Li, J.; Wang, H.; Xiao, Z.; Yu, R.; Mai, L.; Wu, J. Coordination engineering of metal single atom on carbon for enhanced and robust potassium storage. Matter 2021, 4, 4006–4021. [Google Scholar] [CrossRef]









| Sensing Materials | C (ppm) | T (°C) | Response/Recovery Time (s) | Response (%) | LOD (ppb) | Ref. |
|---|---|---|---|---|---|---|
| MoS2 | 100 | RT | 29/350 (Y) | 35.16 (Y) 1 | — | [46] |
| MoTe2 | 1 | RT | 300/120 (Y) | 18 (Y) 2 | 0.252 | [47] |
| Graphene | 100 | RT | 200/1000 (Y) | 26 (Y) 1 | 42.18 | [48] |
| MoS2/GaN | 50 | RT | 184/369 (Y) | 98.3 (Y) 1 | — | [49] |
| ZnO/MoS2 | 10 | RT | ~258/~72 (Y) | 293 (Y) 1 | 200 | [50] |
| InSe | 1 | RT | 233/350 (Y) | 190 (Y) 1 | 0.98 | [51] |
| Ti3C2Tx-ZnO | 20 | RT | 22/10 (Y) | 78.6 (N) 1 | 50 | [52] |
| MOF (NH2-UiO-66) | 10 | 150 | ~50/~50 (N) | 7.6 ± 0.4 (N) 1 | — | [53] |
| MOF-Pt/In2O3 | 1 | 40 | ~900/420 (N) | 44.9 (N) 1 | 0.1 | [54] |
| COF-CuNiPc | 0.1 | RT | 30/13 (Y) | 226.3 (N) 3 | 5.4 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Zeng, M.; Yang, J.; Hu, N.; Duan, X.; Cai, W.; Su, Y.; Yang, Z. Two-Dimensional Bimetallic Phthalocyanine Covalent-Organic-Framework-Based Chemiresistive Gas Sensor for ppb-Level NO2 Detection. Nanomaterials 2023, 13, 1660. https://doi.org/10.3390/nano13101660
Chen X, Zeng M, Yang J, Hu N, Duan X, Cai W, Su Y, Yang Z. Two-Dimensional Bimetallic Phthalocyanine Covalent-Organic-Framework-Based Chemiresistive Gas Sensor for ppb-Level NO2 Detection. Nanomaterials. 2023; 13(10):1660. https://doi.org/10.3390/nano13101660
Chicago/Turabian StyleChen, Xiyu, Min Zeng, Jianhua Yang, Nantao Hu, Xiaoyong Duan, Wei Cai, Yanjie Su, and Zhi Yang. 2023. "Two-Dimensional Bimetallic Phthalocyanine Covalent-Organic-Framework-Based Chemiresistive Gas Sensor for ppb-Level NO2 Detection" Nanomaterials 13, no. 10: 1660. https://doi.org/10.3390/nano13101660