Biogenic Synthesis of Copper-Based Nanomaterials Using Plant Extracts and Their Applications: Current and Future Directions
Abstract
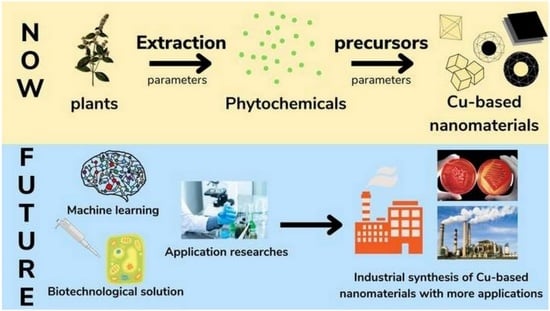
:1. Introduction
2. Synthesis of Nanomaterials: Conventional and Green Approaches
2.1. Disadvantages of Conventional Nanomateiral Synthesis Method
2.2. Green Synthesis Method of Nanomaterials
3. Plant-Mediated Nanomaterial Synthesis
3.1. Plant Extraction Method
3.1.1. Drying
3.1.2. Downsizing
3.1.3. Plant Extraction Methods
Solvents in Plant Extraction
Temperature in Plant Extraction
Extraction Time in Plant Extraction
Filtration and Preservation

3.2. Cu-NMs Synthesis Method
3.2.1. Production of High Tunable Cu-NMs
3.2.2. Precursor
3.2.3. Plant Extract
3.2.4. Temperature
3.2.5. pH
3.2.6. Reaction Time
3.2.7. Indication of Cu-NMs Production
4. Applications of Cu-NMs from Plant-Mediated Synthesis
4.1. Biomedical
4.1.1. Antimicrobial
4.1.2. Nano-Sensor
4.1.3. Anticancer
4.2. Environmental Remediation
5. Future Research Directions
5.1. Limitations and Solutions
5.2. Potential New Applications
5.3. New Research Directions for Synthesis
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coffin, J.D.; Rao, R.; Lurie, D.I. Translational Potential of Ayurveda Prakriti: Concepts in the Area of Personalized Medicine. In Translational Ayurveda; Springer: Singapore, 2019; pp. 21–32. [Google Scholar]
- Liu, W.; Lu, L.; Ma, C.; Yan, C.; Zhao, Z.; Mohammadtursun, N.; Hu, L.; Tulake, W.; Jiang, S.; Gao, Z.; et al. The Evolution of Traditional Chinese Medicine as a Disciplinary Concept and Its Essence throughout History. Tradit. Med. Mod. Med. 2018, 01, 171–180. [Google Scholar] [CrossRef]
- Wang, J.; Wong, Y.-K.; Liao, F. What Has Traditional Chinese Medicine Delivered for Modern Medicine? Expert Rev. Mol. Med. 2018, 20, e4. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for Extraction and Isolation of Natural Products: A Comprehensive Review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Wang, M.; Jiang, L.; Xie, Q.; Yuan, H.; Yang, Y.; Zafar, S.; Liu, Y.; Jian, Y.; Li, B.; et al. The Medicinal Uses of the Genus Bletilla in Traditional Chinese Medicine: A Phytochemical and Pharmacological Review. J. Ethnopharmacol. 2021, 280, 114263. [Google Scholar] [CrossRef]
- Nille, G.C.; Chaudhary, A.K. Potential Implications of Ayurveda in Psoriasis: A Clinical Case Study. J. Ayurveda Integr. Med. 2021, 12, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhai, W.; Chen, H.; Li, L.; Gao, W.; Wei, Y.; Wu, J. Current Understanding of Phytochemicals from Chinese Herbal Medicines for Ferroptosis-Mediated Cancer Prevention and Treatment. Pharmacol. Res. Mod. Chin. Med. 2022, 3, 100100. [Google Scholar] [CrossRef]
- Ekiert, H.; Pajor, J.; Klin, P.; Rzepiela, A.; Ślesak, H.; Szopa, A. Significance of Artemisia vulgaris L. (Common Mugwort) in the History of Medicine and Its Possible Contemporary Applications Substantiated by Phytochemical and Pharmacological Studies. Molecules 2020, 25, 4415. [Google Scholar] [CrossRef]
- Dubey, S.P.; Lahtinen, M.; Sillanpää, M. Green Synthesis and Characterizations of Silver and Gold Nanoparticles Using Leaf Extract of Rosa rugosa. Colloids Surf. A Physicochem. Eng. Asp. 2010, 364, 34–41. [Google Scholar] [CrossRef]
- Dubey, S.P.; Lahtinen, M.; Sillanpää, M. Tansy Fruit Mediated Greener Synthesis of Silver and Gold Nanoparticles. Process Biochem. 2010, 45, 1065–1071. [Google Scholar] [CrossRef]
- Jadoun, S.; Arif, R.; Jangid, N.K.; Meena, R.K. Green Synthesis of Nanoparticles Using Plant Extracts: A Review. Environ. Chem. Lett. 2021, 19, 355–374. [Google Scholar] [CrossRef]
- Azri, F.A.; Selamat, J.; Sukor, R.; Yusof, N.A.; Raston, N.H.A.; Nordin, N.; Jambari, N.N. Etlingera Elatior-Mediated Synthesis of Gold Nanoparticles and Their Application as Electrochemical Current Enhancer. Molecules 2019, 24, 3141. [Google Scholar] [CrossRef]
- Pal, G.; Rai, P.; Pandey, A. Green Synthesis of Nanoparticles: A Greener Approach for a Cleaner Future. In Green Synthesis, Characterization and Applications of Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–26. [Google Scholar]
- Waris, A.; Din, M.; Ali, A.; Ali, M.; Afridi, S.; Baset, A.; Ullah Khan, A. A Comprehensive Review of Green Synthesis of Copper Oxide Nanoparticles and Their Diverse Biomedical Applications. Inorg. Chem. Commun. 2021, 123, 108369. [Google Scholar] [CrossRef]
- Jusoh, R.; Kamarudin, N.H.N.; Kamarudin, N.S.; Sukor, N.F. Green Synthesis of Spherical Shaped Silver Nanoparticles Using Allium cepa Leaves Extract and Its Photocatalytic Activity. Mater. Sci. Forum 2019, 962, 57–62. [Google Scholar] [CrossRef]
- Md Ishak, N.A.I.; Kamarudin, S.K.; Timmiati, S.N. Green Synthesis of Metal and Metal Oxide Nanoparticles via Plant Extracts: An Overview. Mater. Res. Express 2019, 6, 112004. [Google Scholar] [CrossRef]
- Chin, P.P.; Ding, J.; Yi, J.B.; Liu, B.H. Synthesis of FeS2 and FeS Nanoparticles by High-Energy Mechanical Milling and Mechanochemical Processing. J. Alloys Compd. 2005, 390, 255–260. [Google Scholar] [CrossRef]
- De Carvalho, J.F.; De Medeiros, S.N.; Morales, M.A.; Dantas, A.L.; Carriço, A.S. Synthesis of Magnetite Nanoparticles by High Energy Ball Milling. Appl. Surf. Sci. 2013, 275, 84–87. [Google Scholar] [CrossRef]
- Prabhu, S.; Poulose, E.K. Silver Nanoparticles: Mechanism of Antimicrobial Action, Synthesis, Medical Applications, and Toxicity Effects. Int. Nano-Lett. 2012, 2, 32. [Google Scholar] [CrossRef]
- Seino, S.; Kinoshita, T.; Nakagawa, T.; Kojima, T.; Taniguci, R.; Okuda, S.; Yamamoto, T.A. Radiation Induced Synthesis of Gold/Iron-Oxide Composite Nanoparticles Using High-Energy Electron Beam. J. Nanopart. Res. 2008, 10, 1071–1076. [Google Scholar] [CrossRef]
- Agarwal, H.; Nakara, A.; Shanmugam, V.K. Anti-Inflammatory Mechanism of Various Metal and Metal Oxide Nanoparticles Synthesized Using Plant Extracts: A Review. Biomed. Pharmacother. 2019, 109, 2561–2572. [Google Scholar] [CrossRef]
- Iravani, S. Green Synthesis of Metal Nanoparticles Using Plants. Green Chem. 2011, 13, 2638–2650. [Google Scholar] [CrossRef]
- Kumaran, A.; Joel Karunakaran, R. In Vitro Antioxidant Activities of Methanol Extracts of Five Phyllanthus Species from India. LWT Food Sci. Technol. 2007, 40, 344–352. [Google Scholar] [CrossRef]
- Lu, F.; Sun, D.; Huang, J.; Du, M.; Yang, F.; Chen, H.; Hong, Y.; Li, Q. Plant-Mediated Synthesis of Ag-Pd Alloy Nanoparticles and Their Application as Catalyst toward Selective Hydrogenation. ACS Sustain. Chem. Eng. 2014, 2, 1212–1218. [Google Scholar] [CrossRef]
- Madhubala, V.; Kalaivani, T. Phyto and Hydrothermal Synthesis of Fe3O4 @ZnO Core-Shell Nanoparticles Using Azadirachta indica and Its Cytotoxicity Studies. Appl. Surf. Sci. 2018, 449, 584–590. [Google Scholar] [CrossRef]
- Phang, Y.K.; Aminuzzaman, M.; Akhtaruzzaman, M.; Muhammad, G.; Ogawa, S.; Watanabe, A.; Tey, L.H. Green Synthesis and Characterization of CuO Nanoparticles Derived from Papaya Peel Extract for the Photocatalytic Degradation of Palm Oil Mill Effluent (POME). Sustainability 2021, 13, 796. [Google Scholar] [CrossRef]
- Selvanathan, V.; Aminuzzaman, M.; Tey, L.H.; Razali, S.A.; Althubeiti, K.; Alkhammash, H.I.; Guha, S.K.; Ogawa, S.; Watanabe, A.; Shahiduzzaman, M.; et al. Muntingia Calabura Leaves Mediated Green Synthesis of Cuo Nanorods: Exploiting Phytochemicals for Unique Morphology. Materials 2021, 14, 6379. [Google Scholar] [CrossRef]
- Karim, N.A.; Rubinsin, N.J.; Burukan, M.A.A.; Kamarudin, S.K. Sustainable Route of Synthesis Platinum Nanoparticles Using Orange Peel Extract. Int. J. Green Energy 2019, 16, 1518–1526. [Google Scholar] [CrossRef]
- Ali, A.; Sattar, M.; Hussain, F.; Tareen, M.H.K.; Militky, J.; Noman, M.T. Single-step Green Synthesis of Highly Concentrated and Stable Colloidal Dispersion of Core-shell Silver Nanoparticles and Their Antimicrobial and Ultra-high Catalytic Properties. Nanomaterials 2021, 11, 1007. [Google Scholar] [CrossRef]
- Villalobos-Noriega, J.M.A.; Rodríguez-León, E.; Rodríguez-Beas, C.; Larios-Rodríguez, E.; Plascencia-Jatomea, M.; Martínez-Higuera, A.; Acuña-Campa, H.; García-Galaz, A.; Mora-Monroy, R.; Alvarez-Cirerol, F.J.; et al. Au@Ag Core@Shell Nanoparticles Synthesized with Rumex Hymenosepalus as Antimicrobial Agent. Nanoscale Res. Lett. 2021, 16, 118. [Google Scholar] [CrossRef]
- Dauthal, P.; Mukhopadhyay, M. Noble Metal Nanoparticles: Plant-Mediated Synthesis, Mechanistic Aspects of Synthesis, and Applications. Ind. Eng. Chem. Res. 2016, 55, 9557–9577. [Google Scholar] [CrossRef]
- Andra, S.; Balu, S.K.; Jeevanandham, J.; Muthalagu, M.; Vidyavathy, M.; Chan, Y.S.; Danquah, M.K. Phytosynthesized Metal Oxide Nanoparticles for Pharmaceutical Applications. Naunyn Schmiedebergs Arch. Pharmacol. 2019, 392, 755–771. [Google Scholar] [CrossRef]
- Annu, A.A.; Ahmed, S. Green Synthesis of Metal, Metal Oxide Nanoparticles, and Their Various Applications. In Handbook of Ecomaterials; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–45. [Google Scholar]
- Song, X.R.; Yu, S.X.; Jin, G.X.; Wang, X.; Chen, J.; Li, J.; Liu, G.; Yang, H.H. Plant Polyphenol-Assisted Green Synthesis of Hollow CoPt Alloy Nanoparticles for Dual-Modality Imaging Guided Photothermal Therapy. Small 2016, 12, 1506–1513. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Mollick, M.M.R.; Biswas, Y.; Chattopadhyay, D.; Rashid, M.H. Biogenic Synthesis of Shape-Tunable Au-Pd Alloy Nanoparticles with Enhanced Catalytic Activities. J. Alloys Compd. 2018, 763, 399–408. [Google Scholar] [CrossRef]
- Ying, S.; Guan, Z.; Ofoegbu, P.C.; Clubb, P.; Rico, C.; He, F.; Hong, J. Green Synthesis of Nanoparticles: Current Developments and Limitations. Environ. Technol. Innov. 2022, 26, 102336. [Google Scholar] [CrossRef]
- Mutalik, C.; Okoro, G.; Krisnawati, D.I.; Jazidie, A.; Rahmawati, E.Q.; Rahayu, D.; Hsu, W.-T.; Kuo, T.-R. Copper Sulfide with Morphology-Dependent Photodynamic and Photothermal Antibacterial Activities. J. Colloid Interface Sci. 2022, 607, 1825–1835. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Lin, N.; Chen, Y.; Wang, Z.; Xie, Q.; Zheng, T.; Gao, N.; Li, S.; Kang, J.; Cai, D.; et al. Copper Nanowires as Fully Transparent Conductive Electrodes. Sci. Rep. 2013, 3, 2323. [Google Scholar] [CrossRef]
- Hashimi, A.S.; Ginting, R.T.; Chin, S.X.; Lau, K.S.; Nazhif Mohd Nohan, M.A.; Zakaria, S.; Yap, C.C.; Chia, C.H. Fast Microwave-Assisted Synthesis of Copper Nanowires as Reusable High-Performance Transparent Conductive Electrode. Curr. Appl. Phys. 2020, 20, 205–211. [Google Scholar] [CrossRef]
- Jin, M.; He, G.; Zhang, H.; Zeng, J.; Xie, Z.; Xia, Y. Shape-Controlled Synthesis of Copper Nanocrystals in an Aqueous Solution with Glucose as a Reducing Agent and Hexadecylamine as a Capping Agent. Angew. Chem. Int. Ed. 2011, 50, 10560–10564. [Google Scholar] [CrossRef]
- Rathmell, A.R.; Wiley, B.J. The Synthesis and Coating of Long, Thin Copper Nanowires to Make Flexible, Transparent Conducting Films on Plastic Substrates. Adv. Mater. 2011, 23, 4798–4803. [Google Scholar] [CrossRef]
- Ingle, A.P.; Duran, N.; Rai, M. Bioactivity, Mechanism of Action, and Cytotoxicity of Copper-Based Nanoparticles: A Review. Appl. Microbiol. Biotechnol. 2014, 98, 1001–1009. [Google Scholar] [CrossRef]
- Jeong, S.; Woo, K.; Kim, D.; Lim, S.; Kim, J.S.; Shin, H.; Xia, Y.; Moon, J. Controlling the Thickness of the Surface Oxide Layer on Cu Nanoparticles for the Fabrication of Conductive Structures by Ink-Jet Printing. Adv. Funct. Mater. 2008, 18, 679–686. [Google Scholar] [CrossRef]
- Christian, P.; Von Der Kammer, F.; Baalousha, M.; Hofmann, T. Nanoparticles: Structure, Properties, Preparation and Behaviour in Environmental Media. Ecotoxicology 2008, 17, 326–343. [Google Scholar] [CrossRef] [PubMed]
- Abid, N.; Khan, A.M.; Shujait, S.; Chaudhary, K.; Ikram, M.; Imran, M.; Haider, J.; Khan, M.; Khan, Q.; Maqbool, M. Synthesis of Nanomaterials Using Various Top-down and Bottom-up Approaches, Influencing Factors, Advantages, and Disadvantages: A Review. Adv. Colloid Interface Sci. 2022, 300, 102597. [Google Scholar] [CrossRef] [PubMed]
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal Nanoparticles Synthesis: An Overview on Methods of Preparation, Advantages and Disadvantages, and Applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Sopicka-Lizer, M. (Ed.) High Energy Ball Milling; Woodhead Publishing Ltd.: Cambridge, UK, 2010; ISBN 9781845692704. [Google Scholar]
- Rane, A.V.; Kanny, K.; Abitha, V.K.; Thomas, S. Methods for Synthesis of Nanoparticles and Fabrication of Nanocomposites. In Synthesis of Inorganic Nanomaterials; Woodhead Publishing Ltd.: Cambridge, UK, 2018; pp. 121–139. [Google Scholar] [CrossRef]
- Hasanpoor, M.; Aliofkhazraei, M.; Delavari, H. Microwave-Assisted Synthesis of Zinc Oxide Nanoparticles. Procedia Mater. Sci. 2015, 11, 320–325. [Google Scholar] [CrossRef]
- Horwat, D.; Zakharov, D.I.; Endrino, J.L.; Soldera, F.; Anders, A.; Migot, S.; Karoum, R.; Vernoux, P.; Pierson, J.F. Chemistry, Phase Formation, and Catalytic Activity of Thin Palladium-Containing Oxide Films Synthesized by Plasma-Assisted Physical Vapor Deposition. Surf. Coat. Technol. 2011, 205, S171–S177. [Google Scholar] [CrossRef]
- Shende, S.; Ingle, A.P.; Gade, A.; Rai, M. Green Synthesis of Copper Nanoparticles by Citrus medica Linn. (Idilimbu) Juice and Its Antimicrobial Activity. World J. Microbiol. Biotechnol. 2015, 31, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Machado, S.; Pacheco, J.G.; Nouws, H.P.A.; Albergaria, J.T.; Delerue-Matos, C. Characterization of Green Zero-Valent Iron Nanoparticles Produced with Tree Leaf Extracts. Sci. Total Environ. 2015, 533, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Kataria, N.; Garg, V.K. Green Synthesis of Fe3O4 Nanoparticles Loaded Sawdust Carbon for Cadmium (II) Removal from Water: Regeneration and Mechanism; Elsevier: Amsterdam, The Netherlands, 2018; Volume 208, ISBN 9198120581. [Google Scholar]
- Pantidos, N. Biological Synthesis of Metallic Nanoparticles by Bacteria, Fungi and Plants. J. Nanomed. Nanotechnol. 2014, 5, 233. [Google Scholar] [CrossRef]
- Nies, D.H. Microbial Heavy-Metal Resistance. Appl. Microbiol. Biotechnol. 1999, 51, 730–750. [Google Scholar] [CrossRef]
- Silver, S. Bacterial Heavy Metal Resistance Systems and Possibility of Bioremediation. In Biotechnology: Bridging Research and Applications; Springer: Dordrecht, The Netherlands, 1991; pp. 265–287. [Google Scholar]
- Guilger-Casagrande, M.; Lima, R. de Synthesis of Silver Nanoparticles Mediated by Fungi: A Review. Front. Bioeng. Biotechnol. 2019, 7, 287. [Google Scholar] [CrossRef]
- El-Sayed, E.S.R.; Mousa, S.A.; Abdou, D.A.M.; Abo El-Seoud, M.A.; Elmehlawy, A.A.; Mohamed, S.S. Exploiting the Exceptional Biosynthetic Potency of the Endophytic Aspergillus terreus in Enhancing Production of Co3O4, CuO, Fe3O4, NiO, and ZnO Nanoparticles Using Bioprocess Optimization and Gamma Irradiation. Saudi J. Biol. Sci. 2021, 29, 2463–2474. [Google Scholar] [CrossRef] [PubMed]
- Alghuthaymi, M.A.; Almoammar, H.; Rai, M.; Said-Galiev, E.; Abd-Elsalam, K.A. Myconanoparticles: Synthesis and Their Role in Phytopathogens Management. Biotechnol. Biotechnol. Equip. 2015, 29, 221–236. [Google Scholar] [CrossRef] [PubMed]
- Khanna, P.; Kaur, A.; Goyal, D. Algae-Based Metallic Nanoparticles: Synthesis, Characterization and Applications. J. Microbiol. Methods 2019, 163, 105656. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, Q.; Mirza, N.; Shaheen, S. Phytoremediation Using Algae and Macrophytes: I. In Phytoremediation: Management of Environmental Contaminants, Volume 2; Springer International Publishing: Cham, Switzerland, 2015; pp. 265–289. ISBN 9783319109695. [Google Scholar]
- Khan, F.; Shahid, A.; Zhu, H.; Wang, N.; Javed, M.R.; Ahmad, N.; Xu, J.; Alam, M.A.; Mehmood, M.A. Prospects of Algae-Based Green Synthesis of Nanoparticles for Environmental Applications. Chemosphere 2022, 293, 133571. [Google Scholar] [CrossRef]
- Sharma, D.; Kanchi, S.; Bisetty, K. Biogenic Synthesis of Nanoparticles: A Review. Arab. J. Chem. 2019, 12, 3576–3600. [Google Scholar] [CrossRef]
- Verma, N.; Kumar, N. Synthesis and Biomedical Applications of Copper Oxide Nanoparticles: An Expanding Horizon. ACS Biomater. Sci. Eng. 2019, 5, 1170–1188. [Google Scholar] [CrossRef]
- Nagar, N.; Devra, V. Green Synthesis and Characterization of Copper Nanoparticles Using Azadirachta indica Leaves. Mater. Chem. Phys. 2018, 213, 44–51. [Google Scholar] [CrossRef]
- Mani, M.; Pavithra, S.; Mohanraj, K.; Kumaresan, S.; Alotaibi, S.S.; Eraqi, M.M.; Gandhi, A.D.; Babujanarthanam, R.; Maaza, M.; Kaviyarasu, K. Studies on the Spectrometric Analysis of Metallic Silver Nanoparticles (Ag NPs) Using Basella alba Leaf for the Antibacterial Activities. Environ. Res. 2021, 199, 111274. [Google Scholar] [CrossRef]
- Zhang, G.; Du, M.; Li, Q.; Li, X.; Huang, J.; Jiang, X.; Sun, D. Green Synthesis of Au-Ag Alloy Nanoparticles Using Cacumen platycladi Extract. RSC Adv. 2013, 3, 1878–1884. [Google Scholar] [CrossRef]
- Dulta, K.; Ağçeli, G.K.; Chauhan, P.; Chauhan, P.K. Biogenic Production and Characterization of CuO Nanoparticles by Carica Papaya Leaves and Its Biocompatibility Applications. J. Inorg. Organomet. Polym. Mater. 2021, 31, 1846–1857. [Google Scholar] [CrossRef]
- Irum, S.; Jabeen, N.; Ahmad, K.S.; Shafique, S.; Khan, T.F.; Gul, H.; Anwaar, S.; Shah, N.I.; Mehmood, A.; Hussain, S.Z. Biogenic Iron Oxide Nanoparticles Enhance Callogenesis and Regeneration Pattern of Recalcitrant Cicer arietinum L. PLoS ONE 2020, 15, e0242829. [Google Scholar] [CrossRef] [PubMed]
- Beheshtkhoo, N.; Kouhbanani, M.A.J.; Savardashtaki, A.; Amani, A.M.; Taghizadeh, S. Green Synthesis of Iron Oxide Nanoparticles by Aqueous Leaf Extract of Daphne Mezereum as a Novel Dye Removing Material. Appl. Phys. A Mater. Sci. Process. 2018, 124, 363. [Google Scholar] [CrossRef]
- Chung, I.; Rahuman, A.A.; Marimuthu, S.; Kirthi, A.V.; Anbarasan, K.; Padmini, P.; Rajakumar, G. Green Synthesis of Copper Nanoparticles Using Eclipta prostrata Leaves Extract and Their Antioxidant and Cytotoxic Activities. Exp. Ther. Med. 2017, 14, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.; Chakraborty, B.; Kumar, R.S.; Almansour, A.I.; Arumugam, N.; Kotresha, D.; Pallavi, S.S.; Dhanyakumara, S.B.; Shashiraj, K.N.; Nayaka, S. Biogenic Synthesis, Characterization and Antimicrobial Activity of Ixora Brachypoda (DC) Leaf Extract Mediated Silver Nanoparticles. J. King Saud Univ. Sci. 2021, 33, 101296. [Google Scholar] [CrossRef]
- Dipankar, C.; Murugan, S. The Green Synthesis, Characterization and Evaluation of the Biological Activities of Silver Nanoparticles Synthesized from Iresine Herbstii Leaf Aqueous Extracts. Colloids Surf. B Biointerfaces 2012, 98, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Yallappa, S.; Manjanna, J.; Dhananjaya, B.L. Phytosynthesis of Stable Au, Ag and Au-Ag Alloy Nanoparticles Using J. Sambac Leaves Extract, and Their Enhanced Antimicrobial Activity in Presence of Organic Antimicrobials. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 137, 236–243. [Google Scholar] [CrossRef]
- Lee, H.J.; Song, J.Y.; Kim, B.S. Biological Synthesis of Copper Nanoparticles Using Magnolia kobus Leaf Extract and Their Antibacterial Activity. J. Chem. Technol. Biotechnol. 2013, 88, 1971–1977. [Google Scholar] [CrossRef]
- Nouri, A.; Tavakkoli Yaraki, M.; Lajevardi, A.; Rezaei, Z.; Ghorbanpour, M.; Tanzifi, M. Ultrasonic-Assisted Green Synthesis of Silver Nanoparticles Using Mentha aquatica Leaf Extract for Enhanced Antibacterial Properties and Catalytic Activity. Colloids Interface Sci. Commun. 2020, 35, 100252. [Google Scholar] [CrossRef]
- Boruah, J.S.; Devi, C.; Hazarika, U.; Bhaskar Reddy, P.V.; Chowdhury, D.; Barthakur, M.; Kalita, P. Green Synthesis of Gold Nanoparticles Using an Antiepileptic Plant Extract: In Vitrobiological and Photo-Catalytic Activities. RSC Adv. 2021, 11, 28029–28041. [Google Scholar] [CrossRef]
- Usha Rani, P.; Rajasekharreddy, P. Green Synthesis of Silver-Protein (Core-Shell) Nanoparticles Using Piper betle L. Leaf Extract and Its Ecotoxicological Studies on Daphnia Magna. Colloids Surf. A Physicochem. Eng. Asp. 2011, 389, 188–194. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Momeni, S.S.; Sajadi, S.M. Green Synthesis of Copper Nanoparticles Using Plantago asiatica Leaf Extract and Their Application for the Cyanation of Aldehydes Using K 4 Fe(CN) 6. J. Colloid Interface Sci. 2017, 506, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Kocadag Kocazorbaz, E.; Moulahoum, H.; Tut, E.; Sarac, A.; Tok, K.; Yalcin, H.T.; Zihnioglu, F. Kermes Oak (Quercus coccifera L.) Extract for a Biogenic and Eco-Benign Synthesis of Silver Nanoparticles with Efficient Biological Activities. Environ. Technol. Innov. 2021, 24, 102067. [Google Scholar] [CrossRef]
- Vasantharaj, S.; Sathiyavimal, S.; Senthilkumar, P.; LewisOscar, F.; Pugazhendhi, A. Biosynthesis of Iron Oxide Nanoparticles Using Leaf Extract of Ruellia tuberosa: Antimicrobial Properties and Their Applications in Photocatalytic Degradation. J. Photochem. Photobiol. B Biol. 2019, 192, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Elemike, E.E.; Onwudiwe, D.C.; Fayemi, O.E.; Botha, T.L. Green Synthesis and Electrochemistry of Ag, Au, and Ag–Au Bimetallic Nanoparticles Using Golden Rod (Solidago canadensis) Leaf Extract. Appl. Phys. A Mater. Sci. Process. 2019, 125, 42. [Google Scholar] [CrossRef]
- Sadiq, H.; Sher, F.; Sehar, S.; Lima, E.C.; Zhang, S.; Iqbal, H.M.N.; Zafar, F.; Nuhanović, M. Green Synthesis of ZnO Nanoparticles from Syzygium Cumini Leaves Extract with Robust Photocatalysis Applications. J. Mol. Liq. 2021, 335, 116567. [Google Scholar] [CrossRef]
- Sivaraj, R.; Rahman, P.K.S.M.; Rajiv, P.; Salam, H.A.; Venckatesh, R. Biogenic Copper Oxide Nanoparticles Synthesis Using Tabernaemontana divaricate Leaf Extract and Its Antibacterial Activity against Urinary Tract Pathogen. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 133, 178–181. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajadi, S.M.; Rostami-Vartooni, A.; Hussin, S.M. Green Synthesis of CuO Nanoparticles Using Aqueous Extract of Thymus vulgaris L. Leaves and Their Catalytic Performance for N-Arylation of Indoles and Amines. J. Colloid Interface Sci. 2016, 466, 113–119. [Google Scholar] [CrossRef]
- Le, N.T.T.; Thi, T.T.H.; Ching, Y.C.; Nguyen, N.H.; Nguyen, D.Y.P.; Truong, Q.M.; Nguyen, D.H. Garcinia Mangostana Shell and Tradescantia Spathacea Leaf Extract- Mediated One-Pot Synthesis of Silver Nanoparticles with Effective Antifungal Properties. Curr. Nanosci. 2020, 17, 762–771. [Google Scholar] [CrossRef]
- Martínez-cabanas, M.; López-garcía, M.; Rodríguez-barro, P.; Vilariño, T.; Lodeiro, P.; Herrero, R.; Barriada, J.L.; de Vicente, M.E.S. Antioxidant Capacity Assessment of Plant Extracts for Green Synthesis of Nanoparticles. Nanomaterials 2021, 11, 1679. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Maham, M.; Rostami-Vartooni, A.; Bagherzadeh, M.; Sajadi, S.M. Barberry Fruit Extract Assisted in Situ Green Synthesis of Cu Nanoparticles Supported on a Reduced Graphene Oxide-Fe3O4 Nanocomposite as a Magnetically Separable and Reusable Catalyst for the O-Arylation of Phenols with Aryl Halides under Ligand-Free Cond. RSC Adv. 2015, 5, 64769–64780. [Google Scholar] [CrossRef]
- Zarei, M.; Seyedi, N.; Maghsoudi, S.; Nejad, M.S.; Sheibani, H. Green Synthesis of Ag Nanoparticles on the Modified Graphene Oxide Using Capparis spinosa Fruit Extract for Catalytic Reduction of Organic Dyes. Inorg. Chem. Commun. 2021, 123, 108327. [Google Scholar] [CrossRef]
- Jahan, I.; Erci, F.; Isildak, I. Facile Microwave-Mediated Green Synthesis of Non-Toxic Copper Nanoparticles Using Citrus sinensis Aqueous Fruit Extract and Their Antibacterial Potentials. J. Drug Deliv. Sci. Technol. 2021, 61, 102172. [Google Scholar] [CrossRef]
- Lakshmanan, G.; Sathiyaseelan, A.; Kalaichelvan, P.T.; Murugesan, K. Plant-Mediated Synthesis of Silver Nanoparticles Using Fruit Extract of Cleome viscosa L.: Assessment of Their Antibacterial and Anticancer Activity. Karbala Int. J. Mod. Sci. 2018, 4, 61–68. [Google Scholar] [CrossRef]
- Sathishkumar, G.; Logeshwaran, V.; Sarathbabu, S.; Jha, P.K.; Jeyaraj, M.; Rajkuberan, C.; Senthilkumar, N.; Sivaramakrishnan, S. Green Synthesis of Magnetic Fe3O4 Nanoparticles Using Couroupita guianensis Aubl. Fruit Extract for Their Antibacterial and Cytotoxicity Activities. Artif. Cells Nanomed. Biotechnol. 2018, 46, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimzadeh, M.A.; Mortazavi-Derazkola, S.; Zazouli, M.A. Eco-Friendly Green Synthesis and Characterization of Novel Fe3O4/SiO2/Cu2O–Ag Nanocomposites Using Crataegus pentagyna Fruit Extract for Photocatalytic Degradation of Organic Contaminants. J. Mater. Sci. Mater. Electron. 2019, 30, 10994–11004. [Google Scholar] [CrossRef]
- Ramesh, P.S.; Kokila, T.; Geetha, D. Plant Mediated Green Synthesis and Antibacterial Activity of Silver Nanoparticles Using Emblica officinalis Fruit Extract. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 142, 339–343. [Google Scholar] [CrossRef]
- Aksu Demirezen, D.; Yıldız, Y.Ş.; Yılmaz, Ş.; Demirezen Yılmaz, D. Green Synthesis and Characterization of Iron Oxide Nanoparticles Using Ficus carica (Common Fig) Dried Fruit Extract. J. Biosci. Bioeng. 2019, 127, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Yin, Y.; Lv, P.; Su, W.; Zhang, L. Green Controllable Synthesis of Au-Ag Alloy Nanoparticles Using Chinese Wolfberry Fruit Extract and Their Tunable Photocatalytic Activity. RSC Adv. 2018, 8, 3964–3973. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Mohammad Sajadi, S.; Maham, M.; Ehsani, A. Facile and Surfactant-Free Synthesis of Pd Nanoparticles by the Extract of the Fruits of Piper Longum and Their Catalytic Performance for the Sonogashira Coupling Reaction in Water under Ligand- and Copper-Free Conditions. RSC Adv. 2015, 5, 2562–2567. [Google Scholar] [CrossRef]
- Veeramani, C.; Newehy, A.S.E.; Alsaif, M.A.; Al-Numair, K.S. Pouteria Caimito Nutritional Fruit Derived Silver Nanoparticles and Core-Shell Nanospheres Synthesis, Characterization, and Their Oral Cancer Preventive Efficiency. J. Mol. Struct. 2021, 1245, 131227. [Google Scholar] [CrossRef]
- Chelli, V.R.; Golder, A.K. One Pot Green Synthesis of Pt, Co and Pt@Co Core-Shell Nanoparticles Using Sechium edule. J. Chem. Technol. Biotechnol. 2019, 94, 911–918. [Google Scholar] [CrossRef]
- Pilaquinga, F.; Morejón, B.; Ganchala, D.; Morey, J.; Piña, N.; Debut, A.; Neira, M. Green Synthesis of Silver Nanoparticles Using Solanum mammosum L. (Solanaceae) Fruit Extract and Their Larvicidal Activity against Aedes aegypti L. (Diptera: Culicidae). PLoS ONE 2019, 14, e0224109. [Google Scholar] [CrossRef] [PubMed]
- Yugandhar, P.; Vasavi, T.; Jayavardhana Rao, Y.; Uma Maheswari Devi, P.; Narasimha, G.; Savithramma, N. Cost Effective, Green Synthesis of Copper Oxide Nanoparticles Using Fruit Extract of Syzygium alternifolium (Wt.) Walp., Characterization and Evaluation of Antiviral Activity. J. Clust. Sci. 2018, 29, 743–755. [Google Scholar] [CrossRef]
- Khodadadi, B.; Bordbar, M.; Yeganeh-Faal, A.; Nasrollahzadeh, M. Green Synthesis of Ag Nanoparticles/Clinoptilolite Using Vaccinium macrocarpon Fruit Extract and Its Excellent Catalytic Activity for Reduction of Organic Dyes. J. Alloys Compd. 2017, 719, 82–88. [Google Scholar] [CrossRef]
- Yap, Y.H.; Azmi, A.A.; Mohd, N.K.; Yong, F.S.J.; Kan, S.Y.; Thirmizir, M.Z.A.; Chia, P.W. Green Synthesis of Silver Nanoparticle Using Water Extract of Onion Peel and Application in the Acetylation Reaction. Arab. J. Sci. Eng. 2020, 45, 4797–4807. [Google Scholar] [CrossRef]
- Gangapuram, B.R.; Bandi, R.; Alle, M.; Dadigala, R.; Kotu, G.M.; Guttena, V. Microwave Assisted Rapid Green Synthesis of Gold Nanoparticles Using Annona squamosa L. Peel Extract for the Efficient Catalytic Reduction of Organic Pollutants. J. Mol. Struct. 2018, 1167, 305–315. [Google Scholar] [CrossRef]
- Pan, Z.; Lin, Y.; Sarkar, B.; Owens, G.; Chen, Z. Green Synthesis of Iron Nanoparticles Using Red Peanut Skin Extract: Synthesis Mechanism, Characterization and Effect of Conditions on Chromium Removal. J. Colloid Interface Sci. 2019, 558, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Babita Devi, T.; Ahmaruzzaman, M. Bio-Inspired Facile and Green Synthesis of Au@Ag@AgCl Nanoparticles Using Benincasa hispida Peel Extract and Their Photocatalytic Activity for the Removal of Toxic Dye Under Solar Irradiation. In Advances in Waste Management; Kalamdhad, A.S., Singh, J., Dhamodharan, K., Eds.; Springer: Singapore, 2019; pp. 525–534. ISBN 978-981-13-0214-5. [Google Scholar]
- Wicaksono, W.P.; Kadja, G.T.M.; Amalia, D.; Uyun, L.; Rini, W.P.; Hidayat, A.; Fahmi, R.L.; Nasriyanti, D.; Leun, S.G.V.; Ariyanta, H.A.; et al. A Green Synthesis of Gold–Palladium Core–Shell Nanoparticles Using Orange Peel Extract through Two-Step Reduction Method and Its Formaldehyde Colorimetric Sensing Performance. Nano-Struct. Nano-Objects 2020, 24, 100535. [Google Scholar] [CrossRef]
- Yusefi, M.; Shameli, K.; Yee, O.S.; Teow, S.Y.; Hedayatnasab, Z.; Jahangirian, H.; Webster, T.J.; Kuča, K. Green Synthesis of Fe3O4 Nanoparticles Stabilized by a Garcinia Mangostana Fruit Peel Extract for Hyperthermia and Anticancer Activities. Int. J. Nanomed. 2021, 16, 2515–2532. [Google Scholar] [CrossRef]
- Xin Lee, K.; Shameli, K.; Miyake, M.; Kuwano, N.; Bt Ahmad Khairudin, N.B.; Bt Mohamad, S.E.; Yew, Y.P. Green Synthesis of Gold Nanoparticles Using Aqueous Extract of Garcinia mangostana Fruit Peels. J. Nanomater. 2016, 2016, 8489094. [Google Scholar] [CrossRef]
- Sasidharan, D.; Namitha, T.R.; Johnson, S.P.; Jose, V.; Mathew, P. Synthesis of Silver and Copper Oxide Nanoparticles Using Myristica fragrans Fruit Extract: Antimicrobial and Catalytic Applications. Sustain. Chem. Pharm. 2020, 16, 100255. [Google Scholar] [CrossRef]
- Doan Thi, T.U.; Nguyen, T.T.; Thi, Y.D.; Ta Thi, K.H.; Phan, B.T.; Pham, K.N. Green Synthesis of ZnO Nanoparticles Using Orange Fruit Peel Extract for Antibacterial Activities. RSC Adv. 2020, 10, 23899–23907. [Google Scholar] [CrossRef]
- Adebayo, A.E.; Oke, A.M.; Lateef, A.; Oyatokun, A.A.; Abisoye, O.D.; Adiji, I.P.; Fagbenro, D.O.; Amusan, T.V.; Badmus, J.A.; Asafa, T.B.; et al. Biosynthesis of Silver, Gold and Silver–Gold Alloy Nanoparticles Using Persea americana Fruit Peel Aqueous Extract for Their Biomedical Properties. Nanotechnol. Environ. Eng. 2019, 4, 13. [Google Scholar] [CrossRef]
- Siddiqui, V.U.; Ansari, A.; Chauhan, R.; Siddiqi, W.A. Green Synthesis of Copper Oxide (CuO) Nanoparticles by Punica Granatum Peel Extract. Mater. Today Proc. 2019, 36, 751–755. [Google Scholar] [CrossRef]
- Ghidan, A.Y.; Al-Antary, T.M.; Awwad, A.M. Green Synthesis of Copper Oxide Nanoparticles Using Punica granatum Peels Extract: Effect on Green Peach Aphid. Environ. Nanotechnol. Monit. Manag. 2016, 6, 95–98. [Google Scholar] [CrossRef]
- Ravikumar, K.V.G.; Sudakaran, S.V.; Ravichandran, K.; Pulimi, M.; Natarajan, C.; Mukherjee, A. Green Synthesis of NiFe Nano Particles Using Punica granatum Peel Extract for Tetracycline Removal. J. Clean. Prod. 2019, 210, 767–776. [Google Scholar] [CrossRef]
- Ehrampoush, M.H.; Miria, M.; Salmani, M.H.; Mahvi, A.H. Cadmium Removal from Aqueous Solution by Green Synthesis Iron Oxide Nanoparticles with Tangerine Peel Extract. J. Environ. Health Sci. Eng. 2015, 13, 84. [Google Scholar] [CrossRef]
- Nava, O.J.; Soto-Robles, C.A.; Gómez-Gutiérrez, C.M.; Vilchis-Nestor, A.R.; Castro-Beltrán, A.; Olivas, A.; Luque, P.A. Fruit Peel Extract Mediated Green Synthesis of Zinc Oxide Nanoparticles. J. Mol. Struct. 2017, 1147, 43–54. [Google Scholar] [CrossRef]
- Manjari, G.; Saran, S.; Arun, T.; Vijaya Bhaskara Rao, A.; Devipriya, S.P. Catalytic and Recyclability Properties of Phytogenic Copper Oxide Nanoparticles Derived from Aglaia Elaeagnoidea Flower Extract. J. Saudi Chem. Soc. 2017, 21, 610–618. [Google Scholar] [CrossRef]
- Karimi Andeani, J.; Mohsenzadeh, S. Phytosynthesis of Cadmium Oxide Nanoparticles from Achillea Wilhelmsii Flowers. J. Chem. 2013, 2013, 147613. [Google Scholar] [CrossRef]
- Karimi, J.; Mohsenzadeh, S. Rapid, Green, and Eco-Friendly Biosynthesis of Copper Nanoparticles Using Flower Extract of Aloe vera. Synth. React. Inorg. Met. Nano-Met. Chem. 2015, 45, 895–898. [Google Scholar] [CrossRef]
- Karpagavinayagam, P.; Vedhi, C. Green Synthesis of Iron Oxide Nanoparticles Using Avicennia marina Flower Extract. Vacuum 2019, 160, 286–292. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Muniraj, S. Neem Flower Extract Assisted Green Synthesis of Copper Nanoparticles—Optimisation, Characterisation and Anti-Bacterial Study. Mater. Today Proc. 2019, 36, 832–836. [Google Scholar] [CrossRef]
- Elgorban, A.M.; Marraiki, N.; Ansari, S.A.; Syed, A. Green Synthesis of Cu/Fe3O4 nanocomposite Using Calendula Extract and Evaluation of Its Catalytic Activity for Chemoselective Oxidation of Sulfides to Sulfoxides with Aqueous Hydrogen Peroxide. J. Organomet. Chem. 2021, 954–955, 122077. [Google Scholar] [CrossRef]
- Younas, U.; Gulzar, A.; Ali, F.; Pervaiz, M.; Ali, Z.; Khan, S.; Saeed, Z.; Ahmed, M.; Alothman, A.A. Antioxidant and Organic Dye Removal Potential of Cu-Ni Bimetallic Nanoparticles Synthesized Using Gazania rigens Extract. Water 2021, 13, 2653. [Google Scholar] [CrossRef]
- Ghosh, S.; Patil, S.; Ahire, M.; Kitture, R.; Gurav, D.D.; Jabgunde, A.M.; Kale, S.; Pardesi, K.; Shinde, V.; Bellare, J.; et al. Gnidia Glauca Flower Extract Mediated Synthesis of Gold Nanoparticles and Evaluation of Its Chemocatalytic Potential. J. Nanobiotechnol. 2012, 10, 17. [Google Scholar] [CrossRef]
- Thovhogi, N.; Diallo, A.; Gurib-Fakim, A.; Maaza, M. Nanoparticles Green Synthesis by Hibiscus Sabdariffa Flower Extract: Main Physical Properties. J. Alloys Compd. 2015, 647, 392–396. [Google Scholar] [CrossRef]
- Patra, N.; Sahoo, A.; Behera, A. Synthesis and Differential Antibacterial Activity of Bioconjugated Bimetallic Nanoparticles. Pharm. Chem. J. 2020, 54, 865–869. [Google Scholar] [CrossRef]
- Padalia, H.; Moteriya, P.; Chanda, S. Green Synthesis of Silver Nanoparticles from Marigold Flower and Its Synergistic Antimicrobial Potential. Arab. J. Chem. 2015, 8, 732–741. [Google Scholar] [CrossRef] [Green Version]
- Dobrucka, R.; Długaszewska, J. Biosynthesis and Antibacterial Activity of ZnO Nanoparticles Using Trifolium pratense Flower Extract. Saudi J. Biol. Sci. 2016, 23, 517–523. [Google Scholar] [CrossRef]
- Behravan, M.; Hossein Panahi, A.; Naghizadeh, A.; Ziaee, M.; Mahdavi, R.; Mirzapour, A. Facile Green Synthesis of Silver Nanoparticles Using Berberis vulgaris Leaf and Root Aqueous Extract and Its Antibacterial Activity. Int. J. Biol. Macromol. 2019, 124, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Dulta, K.; Koşarsoy Ağçeli, G.; Chauhan, P.; Jasrotia, R.; Chauhan, P.K. A Novel Approach of Synthesis Zinc Oxide Nanoparticles by Bergenia Ciliata Rhizome Extract: Antibacterial and Anticancer Potential. J. Inorg. Organomet. Polym. Mater. 2021, 31, 180–190. [Google Scholar] [CrossRef]
- Nnadozie, E.C.; Ajibade, P.A. Green Synthesis and Characterization of Magnetite (Fe3O4) Nanoparticles Using Chromolaena odorata Root Extract for Smart Nanocomposite. Mater. Lett. 2020, 263, 127145. [Google Scholar] [CrossRef]
- Wang, D.; Markus, J.; Wang, C.; Kim, Y.J.; Mathiyalagan, R.; Aceituno, V.C.; Ahn, S.; Yang, D.C. Green Synthesis of Gold and Silver Nanoparticles Using Aqueous Extract of Cibotium barometz Root. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1548–1555. [Google Scholar] [CrossRef]
- Rao, N.H.; Lakshmidevi, N.; Pammi, S.V.N.; Kollu, P.; Ganapaty, S.; Lakshmi, P. Green Synthesis of Silver Nanoparticles Using Methanolic Root Extracts of Diospyros paniculata and Their Antimicrobial Activities. Mater. Sci. Eng. C 2016, 62, 553–557. [Google Scholar] [CrossRef]
- Al-Radadi, N.S. Facile One-Step Green Synthesis of Gold Nanoparticles (AuNp) Using Licorice Root Extract: Antimicrobial and Anticancer Study against HepG2 Cell Line. Arab. J. Chem. 2021, 14, 102956. [Google Scholar] [CrossRef]
- Suman, T.Y.; Radhika Rajasree, S.R.; Ramkumar, R.; Rajthilak, C.; Perumal, P. The Green Synthesis of Gold Nanoparticles Using an Aqueous Root Extract of Morinda citrifolia L. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 118, 11–16. [Google Scholar] [CrossRef]
- Singh, J.; Dhaliwal, A.S. Novel Green Synthesis and Characterization of the Antioxidant Activity of Silver Nanoparticles Prepared from Nepeta leucophylla Root Extract. Anal. Lett. 2019, 52, 213–230. [Google Scholar] [CrossRef]
- Singh, P.; Kim, Y.J.; Wang, C.; Mathiyalagan, R.; Yang, D.C. The Development of a Green Approach for the Biosynthesis of Silver and Gold Nanoparticles by Using Panax ginseng Root Extract, and Their Biological Applications. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1150–1157. [Google Scholar] [CrossRef] [Green Version]
- Bordbar, M.; Sharifi-Zarchi, Z.; Khodadadi, B. Green Synthesis of Copper Oxide Nanoparticles/Clinoptilolite Using Rheum palmatum L. Root Extract: High Catalytic Activity for Reduction of 4-Nitro Phenol, Rhodamine B, and Methylene Blue. J. Sol-Gel Sci. Technol. 2017, 81, 724–733. [Google Scholar] [CrossRef]
- Arokiyaraj, S.; Vincent, S.; Saravanan, M.; Lee, Y.; Oh, Y.K.; Kim, K.H. Green Synthesis of Silver Nanoparticles Using Rheum palmatum Root Extract and Their Antibacterial Activity against Staphylococcus Aureus and Pseudomonas Aeruginosa. Artif. Cells Nanomed. Biotechnol. 2017, 45, 372–379. [Google Scholar] [CrossRef]
- Hu, D.; Yang, X.; Chen, W.; Feng, Z.; Hu, C.; Yan, F.; Chen, X.; Qu, D.; Chen, Z. Rhodiola RoseaRhizome Extract-Mediated Green Synthesis of Silver Nanoparticles and Evaluation of Their Potential Antioxidant and Catalytic Reduction Activities. ACS Omega 2021, 6, 24450–24461. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Batjikh, I.; Hurh, J.; Han, Y.; Huo, Y.; Ali, H.; Li, J.F.; Rupa, E.J.; Ahn, J.C.; Mathiyalagan, R.; et al. Green Synthesis of Zinc Oxide Nanoparticles from Root Extract of Scutellaria baicalensis and Its Photocatalytic Degradation Activity Using Methylene Blue. Optik 2019, 184, 324–329. [Google Scholar] [CrossRef]
- Judith Vijaya, J.; Jayaprakash, N.; Kombaiah, K.; Kaviyarasu, K.; John Kennedy, L.; Jothi Ramalingam, R.; Al-Lohedan, H.A.; Mansoor-Ali, V.M.; Maaza, M. Bioreduction Potentials of Dried Root of Zingiber Officinale for a Simple Green Synthesis of Silver Nanoparticles: Antibacterial Studies. J. Photochem. Photobiol. B Biol. 2017, 177, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, P.; Anbalagan, K.; Manosathyadevan, M.; Lee, K.J.; Cho, M.; Lee, S.M.; Park, J.H.; Oh, S.G.; Bang, K.S.; Oh, B.T. Green Synthesis of Silver and Gold Nanoparticles Using Zingiber officinale Root Extract and Antibacterial Activity of Silver Nanoparticles against Food Pathogens. Bioprocess Biosyst. Eng. 2014, 37, 1935–1943. [Google Scholar] [CrossRef]
- Maurya, I.C.; Singh, S.; Senapati, S.; Srivastava, P.; Bahadur, L. Green Synthesis of TiO2 Nanoparticles Using Bixa orellana Seed Extract and Its Application for Solar Cells. Sol. Energy 2019, 194, 952–958. [Google Scholar] [CrossRef]
- Sukumar, S.; Rudrasenan, A.; Padmanabhan Nambiar, D. Green-Synthesized Rice-Shaped Copper Oxide Nanoparticles Using Caesalpinia bonducella Seed Extract and Their Applications. ACS Omega 2020, 5, 1040–1051. [Google Scholar] [CrossRef]
- Bogireddy, N.K.R.; Pal, U.; Gomez, L.M.; Agarwal, V. Size Controlled Green Synthesis of Gold Nanoparticles Using Coffea arabica Seed Extract and Their Catalytic Performance in 4-Nitrophenol Reduction. RSC Adv. 2018, 8, 24819–24826. [Google Scholar] [CrossRef]
- Abisharani, J.M.; Devikala, S.; Dinesh Kumar, R.; Arthanareeswari, M.; Kamaraj, P. Green Synthesis of TiO2 Nanoparticles Using Cucurbita pepo Seeds Extract. Mater. Today Proc. 2019, 14, 302–307. [Google Scholar] [CrossRef]
- Shabaani, M.; Rahaiee, S.; Zare, M.; Jafari, S.M. Green Synthesis of ZnO Nanoparticles Using Loquat Seed Extract; Biological Functions and Photocatalytic Degradation Properties. LWT 2020, 134, 110133. [Google Scholar] [CrossRef]
- Girón-Vázquez, N.G.; Gómez-Gutiérrez, C.M.; Soto-Robles, C.A.; Nava, O.; Lugo-Medina, E.; Castrejón-Sánchez, V.H.; Vilchis-Nestor, A.R.; Luque, P.A. Study of the Effect of Persea Americana Seed in the Green Synthesis of Silver Nanoparticles and Their Antimicrobial Properties. Results Phys. 2019, 13, 102142. [Google Scholar] [CrossRef]
- Ansari, M.A.; Alzohairy, M.A. One-Pot Facile Green Synthesis of Silver Nanoparticles Using Seed Extract of Phoenix dactylifera and Their Bactericidal Potential against MRSA. Evid. Based Complement. Altern. Med. 2018, 2018, 4923062. [Google Scholar] [CrossRef] [PubMed]
- Qidwai, A.; Kumar, R.; Dikshit, A. Green Synthesis of Silver Nanoparticles by Seed of Phoenix sylvestris L. and Their Role in the Management of Cosmetics Embarrassment. Green Chem. Lett. Rev. 2018, 11, 176–188. [Google Scholar] [CrossRef]
- Meena Kumari, M.; Jacob, J.; Philip, D. Green Synthesis and Applications of Au-Ag Bimetallic Nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 137, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Nazar, N.; Bibi, I.; Kamal, S.; Iqbal, M.; Nouren, S.; Jilani, K.; Umair, M.; Ata, S. Cu Nanoparticles Synthesis Using Biological Molecule of P. granatum Seeds Extract as Reducing and Capping Agent: Growth Mechanism and Photo-Catalytic Activity. Int. J. Biol. Macromol. 2018, 106, 1203–1210. [Google Scholar] [CrossRef]
- Bibi, I.; Nazar, N.; Ata, S.; Sultan, M.; Ali, A.; Abbas, A.; Jilani, K.; Kamal, S.; Sarim, F.M.; Khan, M.I.; et al. Green Synthesis of Iron Oxide Nanoparticles Using Pomegranate Seeds Extract and Photocatalytic Activity Evaluation for the Degradation of Textile Dye. J. Mater. Res. Technol. 2019, 8, 6115–6124. [Google Scholar] [CrossRef]
- Tabrizi Hafez Moghaddas, S.M.; Elahi, B.; Javanbakht, V. Biosynthesis of Pure Zinc Oxide Nanoparticles Using Quince Seed Mucilage for Photocatalytic Dye Degradation. J. Alloys Compd. 2020, 821, 153519. [Google Scholar] [CrossRef]
- Sabouri, Z.; Rangrazi, A.; Amiri, M.S.; Khatami, M.; Darroudi, M. Green Synthesis of Nickel Oxide Nanoparticles Using Salvia hispanica L. (Chia) Seeds Extract and Studies of Their Photocatalytic Activity and Cytotoxicity Effects. Bioprocess Biosyst. Eng. 2021, 44, 2407–2415. [Google Scholar] [CrossRef]
- Rautela, A.; Rani, J.; Debnath (Das), M. Green Synthesis of Silver Nanoparticles from Tectona Grandis Seeds Extract: Characterization and Mechanism of Antimicrobial Action on Different Microorganisms. J. Anal. Sci. Technol. 2019, 10, 5. [Google Scholar] [CrossRef] [Green Version]
- Yulizar, Y.; Bakri, R.; Apriandanu, D.O.B.; Hidayat, T. ZnO/CuO Nanocomposite Prepared in One-Pot Green Synthesis Using Seed Bark Extract of Theobroma cacao. Nano-Struct. Nano-Objects 2018, 16, 300–305. [Google Scholar] [CrossRef]
- Babu, A.K.; Kumaresan, G.; Raj, V.A.A.; Velraj, R. Review of Leaf Drying: Mechanism and Influencing Parameters, Drying Methods, Nutrient Preservation, and Mathematical Models. Renew. Sustain. Energy Rev. 2018, 90, 536–556. [Google Scholar] [CrossRef]
- Sridhar, A.; Ponnuchamy, M.; Kumar, P.S.; Kapoor, A.; Vo, D.-V.N.; Prabhakar, S. Techniques and Modeling of Polyphenol Extraction from Food: A Review. Environ. Chem. Lett. 2021, 19, 3409–3443. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Weller, C.L. Recent Advances in Extraction of Nutraceuticals from Plants. Trends Food Sci. Technol. 2006, 17, 300–312. [Google Scholar] [CrossRef]
- Bozinou, E.; Karageorgou, I.; Batra, G.; Dourtoglou, V.G.; Lalas, S.I. Pulsed Electric Field Extraction and Antioxidant Activity Determination of Moringa oleifera Dry Leaves: A Comparative Study with Other Extraction Techniques. Beverages 2019, 5, 8. [Google Scholar] [CrossRef]
- Che Sulaiman, I.S.; Basri, M.; Fard Masoumi, H.R.; Chee, W.J.; Ashari, S.E.; Ismail, M. Effects of Temperature, Time, and Solvent Ratio on the Extraction of Phenolic Compounds and the Anti-Radical Activity of Clinacanthus Nutans Lindau Leaves by Response Surface Methodology. Chem. Cent. J. 2017, 11, 54. [Google Scholar] [CrossRef] [PubMed]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.-H. Effect of Extraction Solvent on Total Phenol Content, Total Flavonoid Content, and Antioxidant Activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef]
- Lapornik, B.; Prošek, M.; Golc Wondra, A. Comparison of Extracts Prepared from Plant By-Products Using Different Solvents and Extraction Time. J. Food Eng. 2005, 71, 214–222. [Google Scholar] [CrossRef]
- Vanlalveni, C.; Lallianrawna, S.; Biswas, A.; Selvaraj, M.; Changmai, B.; Rokhum, S.L. Green Synthesis of Silver Nanoparticles Using Plant Extracts and Their Antimicrobial Activities: A Review of Recent Literature. RSC Adv. 2021, 11, 2804–2837. [Google Scholar] [CrossRef]
- Laorko, A.; Tongchitpakdee, S.; Youravong, W. Storage Quality of Pineapple Juice Non-Thermally Pasteurized and Clarified by Microfiltration. J. Food Eng. 2013, 116, 554–561. [Google Scholar] [CrossRef]
- Issaabadi, Z.; Nasrollahzadeh, M.; Sajadi, S.M. Green Synthesis of the Copper Nanoparticles Supported on Bentonite and Investigation of Its Catalytic Activity. J. Clean. Prod. 2017, 142, 3584–3591. [Google Scholar] [CrossRef]
- Dobrucka, R.; Dlugaszewska, J. Antimicrobial Activity of the Biogenically Synthesized Core-Shell Cu@Pt Nanoparticles. Saudi Pharm. J. 2018, 26, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Rajendaran, K.; Muthuramalingam, R.; Ayyadurai, S. Green Synthesis of Ag-Mo/CuO Nanoparticles Using Azadirachta indica Leaf Extracts to Study Its Solar Photocatalytic and Antimicrobial Activities. Mater. Sci. Semicond. Process. 2019, 91, 230–238. [Google Scholar] [CrossRef]
- Rosbero, T.M.S.; Camacho, D.H. Green Preparation and Characterization of Tentacle-like Silver/Copper Nanoparticles for Catalytic Degradation of Toxic Chlorpyrifos in Water. J. Environ. Chem. Eng. 2017, 5, 2524–2532. [Google Scholar] [CrossRef]
- Suvarna, A.R.; Shetty, A.; Anchan, S.; Kabeer, N.; Nayak, S. Cyclea Peltata Leaf Mediated Green Synthesized Bimetallic Nanoparticles Exhibits Methyl Green Dye Degradation Capability. Bionanoscience 2020, 10, 606–617. [Google Scholar] [CrossRef]
- Dayakar, T.; Venkateswara Rao, K.; Park, J.; Krishna, P.; Swaroopa, P.; Ji, Y. Biosynthesis of Ag@CuO Core–Shell Nanostructures for Non-Enzymatic Glucose Sensing Using Screen-Printed Electrode. J. Mater. Sci. Mater. Electron. 2019, 30, 9725–9734. [Google Scholar] [CrossRef]
- Rocha-Rocha, O.; Cortez-Valadez, M.; Hernández-Martínez, A.R.; Gámez-Corrales, R.; Alvarez, R.A.B.; Britto-Hurtado, R.; Delgado-Beleño, Y.; Martinez-Nuñez, C.E.; Pérez-Rodríguez, A.; Arizpe-Chávez, H.; et al. Green Synthesis of Ag-Cu Nanoalloys Using Opuntia ficus-indica. J. Electron. Mater. 2017, 46, 802–807. [Google Scholar] [CrossRef]
- Alshehri, A.A.; Malik, M.A. Facile One-Pot Biogenic Synthesis of Cu-Co-Ni Trimetallic Nanoparticles for Enhanced Photocatalytic Dye Degradation. Catalysts 2020, 10, 1138. [Google Scholar] [CrossRef]
- Suresh, J.; Ragunath, L.; Hong, S.I. Biosynthesis of Mixed Nanocrystalline Zn-Mg-Cu Oxide Nanocomposites and Their Antimicrobial Behavior. Adv. Nat. Sci. Nanosci. Nanotechnol. 2019, 10, 045014. [Google Scholar] [CrossRef]
- Mamatha, G.; Sowmya, P.; Madhuri, D.; Mohan Babu, N.; Suresh Kumar, D.; Vijaya Charan, G.; Varaprasad, K.; Madhukar, K. Antimicrobial Cellulose Nanocomposite Films with In Situ Generations of Bimetallic (Ag and Cu) Nanoparticles Using Vitex negundo Leaves Extract. J. Inorg. Organomet. Polym. Mater. 2021, 31, 802–815. [Google Scholar] [CrossRef]
- Amaliyah, S.; Pangesti, D.P.; Masruri, M.; Sabarudin, A.; Sumitro, S.B. Green Synthesis and Characterization of Copper Nanoparticles Using Piper retrofractum Vahl Extract as Bioreductor and Capping Agent. Heliyon 2020, 6, e04636. [Google Scholar] [CrossRef]
- Biresaw, S.S.; Taneja, P. Copper Nanoparticles Green Synthesis and Characterization as Anticancer Potential in Breast Cancer Cells (MCF7) Derived from Prunus Nepalensis Phytochemicals. Mater. Today Proc. 2022, 49, 3501–3509. [Google Scholar] [CrossRef]
- Hemmati, S.; Mehrazin, L.; Hekmati, M.; Izadi, M.; Veisi, H. Biosynthesis of CuO Nanoparticles Using Rosa canina Fruit Extract as a Recyclable and Heterogeneous Nanocatalyst for C-N Ullmann Coupling Reactions. Mater. Chem. Phys. 2018, 214, 527–532. [Google Scholar] [CrossRef]
- Kumar, B.; Smita, K.; Cumbal, L.; Debut, A.; Angulo, Y. Biofabrication of Copper Oxide Nanoparticles Using Andean Blackberry (Rubus glaucus Benth.) Fruit and Leaf. J. Saudi Chem. Soc. 2017, 21, S475–S480. [Google Scholar] [CrossRef]
- Khani, R.; Roostaei, B.; Bagherzade, G.; Moudi, M. Green Synthesis of Copper Nanoparticles by Fruit Extract of Ziziphus spina-christi (L.) Willd.: Application for Adsorption of Triphenylmethane Dye and Antibacterial Assay. J. Mol. Liq. 2018, 255, 541–549. [Google Scholar] [CrossRef]
- Aminuzzaman, M.; Kei, L.M.; Liang, W.H. Green Synthesis of Copper Oxide (CuO) Nanoparticles Using Banana Peel Extract and Their Photocatalytic Activities. In AIP Conference Proceedings; AIP Publishing LLC: New York, NY, USA, 2017; Volume 1828. [Google Scholar] [CrossRef]
- Ghaffar, A.; Kiran, S.; Rafique, M.A.; Iqbal, S.; Nosheen, S.; Hou, Y.; Afzal, G.; Bashir, M.; Aimun, U. Citrus Paradisi Fruit Peel Extract Mediated Green Synthesis of Copper Nanoparticles for Remediation of Disperse Yellow 125 Dye. Desalin. Water Treat. 2021, 212, 368–375. [Google Scholar] [CrossRef]
- Ituen, E.; Ekemini, E.; Yuanhua, L.; Li, R.; Singh, A. Mitigation of Microbial Biodeterioration and Acid Corrosion of Pipework Steel Using Citrus reticulata Peels Extract Mediated Copper Nanoparticles Composite. Int. Biodeterior. Biodegrad. 2020, 149, 104935. [Google Scholar] [CrossRef]
- Kaur, P.; Thakur, R.; Chaudhury, A. Biogenesis of Copper Nanoparticles Using Peel Extract of Punica granatum and Their Antimicrobial Activity against Opportunistic Pathogens. Green Chem. Lett. Rev. 2016, 9, 33–38. [Google Scholar] [CrossRef]
- Manjari, G.; Saran, S.; Radhakrishanan, S.; Rameshkumar, P.; Pandikumar, A.; Devipriya, S.P. Facile Green Synthesis of Ag–Cu Decorated ZnO Nanocomposite for Effective Removal of Toxic Organic Compounds and an Efficient Detection of Nitrite Ions. J. Environ. Manag. 2020, 262, 110282. [Google Scholar] [CrossRef]
- Roy, K.; Ghosh, C.K.; Sarkar, C.K. Rapid Detection of Hazardous H2O2 by Biogenic Copper Nanoparticles Synthesized Using Eichhornia crassipes Extract. Microsyst. Technol. 2019, 25, 1699–1703. [Google Scholar] [CrossRef]
- Chowdhury, R.; Khan, A.; Rashid, M.H. Green Synthesis of CuO Nanoparticles Using Lantana camara Flower Extract and Their Potential Catalytic Activity towards the Aza-Michael Reaction. RSC Adv. 2020, 10, 14374–14385. [Google Scholar] [CrossRef]
- Thakur, S.; Sharma, S.; Thakur, S.; Rai, R. Green Synthesis of Copper Nano-Particles Using Asparagus adscendens Roxb. Root and Leaf Extract and Their Antimicrobial Activities. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 683–694. [Google Scholar] [CrossRef]
- Pallela, P.N.V.K.; Ummey, S.; Ruddaraju, L.K.; Kollu, P.; Khan, S.; Pammi, S.V.N. Antibacterial Activity Assessment and Characterization of Green Synthesized CuO Nano Rods Using Asparagus racemosus Roots Extract. SN Appl. Sci. 2019, 1, 421. [Google Scholar] [CrossRef]
- Selvam, K.; Sudhakar, C.; Selvankumar, T.; Senthilkumar, B.; Selva Kumar, R.; Kannan, N. Biomimetic Synthesis of Copper Nanoparticles Using Rhizome Extract of Corallocarbus epigaeus and Their Bactericidal with Photocatalytic Activity. SN Appl. Sci. 2020, 2, 1028. [Google Scholar] [CrossRef]
- Maulana, I.; Fasya, D.; Ginting, B. Biosynthesis of Cu Nanoparticles Using Polyalthia longifolia Roots Extracts for Antibacterial, Antioxidant and Cytotoxicity Applications. Mater. Technol. 2022, 1–5. [Google Scholar] [CrossRef]
- Sharma, D.; Ledwani, L.; Kumar, N.; Mehrotra, T.; Pervaiz, N.; Kumar, R. An Investigation of Physicochemical and Biological Properties of Rheum Emodi-Mediated Bimetallic Ag–Cu Nanoparticles. Arab. J. Sci. Eng. 2021, 46, 275–285. [Google Scholar] [CrossRef]
- Sadia, B.O.; Cherutoi, J.K.; Achisa, C.M. Optimization, Characterization, and Antibacterial Activity of Copper Nanoparticles Synthesized Using Senna didymobotrya Root Extract. J. Nanotechnol. 2021, 2021, 1–15. [Google Scholar] [CrossRef]
- Varghese, B.; Kurian, M.; Krishna, S.; Athira, T.S. Biochemical Synthesis of Copper Nanoparticles Using Zingiber officinalis and Curcuma Longa: Characterization and Antibacterial Activity Study. Mater. Today Proc. 2019, 25, 302–306. [Google Scholar] [CrossRef]
- Heydari, R.; Koudehi, M.F.; Pourmortazavi, S.M. Antibacterial Activity of Fe 3 O 4 /Cu Nanocomposite: Green Synthesis Using Carum carvi L. Seeds Aqueous Extract. ChemistrySelect 2019, 4, 531–535. [Google Scholar] [CrossRef]
- Jasrotia, T.; Chaudhary, S.; Kaushik, A.; Kumar, R.; Chaudhary, G.R. Green Chemistry-Assisted Synthesis of Biocompatible Ag, Cu, and Fe2O3 Nanoparticles. Mater. Today Chem. 2020, 15, 100214. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Rinitha, G. Nanostructural Characterization of Antimicrobial and Antioxidant Copper Nanoparticles Synthesized Using Novel persea Americana Seeds. OpenNano 2018, 3, 18–27. [Google Scholar] [CrossRef]
- Sajadi, S.M.; Nasrollahzadeh, M.; Maham, M. Aqueous Extract from Seeds of Silybum marianum L. as a Green Material for Preparation of the Cu/Fe3O4 Nanoparticles: A Magnetically Recoverable and Reusable Catalyst for the Reduction of Nitroarenes. J. Colloid Interface Sci. 2016, 469, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahzadeh, M.; Sajadi, S.M.; Rostami-Vartooni, A.; Bagherzadeh, M. Green Synthesis of Pd/CuO Nanoparticles by Theobroma cacao L. Seeds Extract and Their Catalytic Performance for the Reduction of 4-Nitrophenol and Phosphine-Free Heck Coupling Reaction under Aerobic Conditions. J. Colloid Interface Sci. 2015, 448, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Buazar, F.; Sweidi, S.; Badri, M.; Kroushawi, F. Biofabrication of Highly Pure Copper Oxide Nanoparticles Using Wheat Seed Extract and Their Catalytic Activity: A Mechanistic Approach. Green Process. Synth. 2019, 8, 691–702. [Google Scholar] [CrossRef]
- Singh, P.; Singh, K.R.; Singh, J.; Das, S.N.; Singh, R.P. Tunable Electrochemistry and Efficient Antibacterial Activity of Plant-Mediated Copper Oxide Nanoparticles Synthesized by Annona squamosa Seed Extract for Agricultural Utility. RSC Adv. 2021, 11, 18050–18060. [Google Scholar] [CrossRef] [PubMed]
- Letchumanan, D.; Sok, S.P.M.; Ibrahim, S.; Nagoor, N.H.; Arshad, N.M. Plant-Based Biosynthesis of Copper/Copper Oxide Nanoparticles: An Update on Their Applications in Biomedicine, Mechanisms, and Toxicity. Biomolecules 2021, 11, 564. [Google Scholar] [CrossRef]
- Bhavyasree, P.G.; Xavier, T.S. Green Synthesised Copper and Copper Oxide Based Nanomaterials Using Plant Extracts and Their Application in Antimicrobial Activity: Review. Curr. Res. Green Sustain. Chem. 2022, 5, 100249. [Google Scholar] [CrossRef]
- Akintelu, S.A.; Folorunso, A.S.; Folorunso, F.A.; Oyebamiji, A.K. Green Synthesis of Copper Oxide Nanoparticles for Biomedical Application and Environmental Remediation. Heliyon 2020, 6, e04508. [Google Scholar] [CrossRef]
- Klinger, M.; Theiler, M.; Bosshard, P.P. Epidemiological and Clinical Aspects of Trichophyton Mentagrophytes/Trichophyton Interdigitale Infections in the Zurich Area: A Retrospective Study Using Genotyping. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1017–1025. [Google Scholar] [CrossRef]
- D’Enfert, C.; Kaune, A.-K.; Alaban, L.-R.; Chakraborty, S.; Cole, N.; Delavy, M.; Kosmala, D.; Marsaux, B.; Fróis-Martins, R.; Morelli, M.; et al. The Impact of the Fungus-Host-Microbiota Interplay upon Candida albicans Infections: Current Knowledge and New Perspectives. FEMS Microbiol. Rev. 2021, 45, fuaa060. [Google Scholar] [CrossRef]
- Mali, S.C.; Dhaka, A.; Githala, C.K.; Trivedi, R. Green Synthesis of Copper Nanoparticles Using Celastrus paniculatus Willd. Leaf Extract and Their Photocatalytic and Antifungal Properties. Biotechnol. Rep. 2020, 27, e00518. [Google Scholar] [CrossRef] [PubMed]
- Rehana, D.; Mahendiran, D.; Kumar, R.S.; Rahiman, A.K. Evaluation of Antioxidant and Anticancer Activity of Copper Oxide Nanoparticles Synthesized Using Medicinally Important Plant Extracts. Biomed. Pharmacother. 2017, 89, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Gawande, M.B.; Goswami, A.; Felpin, F.-X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-Based Nanoparticles: Synthesis and Applications in Catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Ni, Z.; Wang, R.; Zhao, B.; Han, Y.; Zheng, Y.; Liu, F.; Gong, Y.; Tang, F.; Liu, Y. The Effects of Cultivar and Climate Zone on Phytochemical Components of Walnut (Juglans regia L.). Food Energy Secur. 2020, 9, e196. [Google Scholar] [CrossRef]
- Szakiel, A.; Pączkowski, C.; Henry, M. Influence of Environmental Abiotic Factors on the Content of Saponins in Plants. Phytochem. Rev. 2011, 10, 471–491. [Google Scholar] [CrossRef]
- Park, Y.D.; Lee, Y.M.; Kang, M.A.; Lee, H.J.; Jin, C.H.; Choi, D.S.; Kim, D.S.; Kang, S.Y.; Kim, W.G.; Jeong, I.Y. Phytochemical Profiles and in Vitro Anti-Inflammatory Properties of Perilla Frutescens Cv. Chookyoupjaso Mutants Induced by Mutagenesis with γ-Ray. Food Sci. Biotechnol. 2010, 19, 305–311. [Google Scholar] [CrossRef]
- Nielsen, E.; Temporiti, M.E.E.; Cella, R. Improvement of Phytochemical Production by Plant Cells and Organ Culture and by Genetic Engineering. Plant Cell Rep. 2019, 38, 1199–1215. [Google Scholar] [CrossRef]
- Nagajyothi, P.C.; Muthuraman, P.; Sreekanth, T.V.M.; Kim, D.H.; Shim, J. Green Synthesis: In-Vitro Anticancer Activity of Copper Oxide Nanoparticles against Human Cervical Carcinoma Cells. Arab. J. Chem. 2017, 10, 215–225. [Google Scholar] [CrossRef]
- Panneerselvam, C.; Murugan, K.; Roni, M.; Aziz, A.T.; Suresh, U.; Rajaganesh, R.; Madhiyazhagan, P.; Subramaniam, J.; Dinesh, D.; Nicoletti, M.; et al. Fern-Synthesized Nanoparticles in the Fight against Malaria: LC/MS Analysis of Pteridium aquilinum Leaf Extract and Biosynthesis of Silver Nanoparticles with High Mosquitocidal and Antiplasmodial Activity. Parasitol. Res. 2016, 115, 997–1013. [Google Scholar] [CrossRef]
- De Araujo, A.R.; Ramos-Jesus, J.; de Oliveira, T.M.; de Carvalho, A.M.A.; Nunes, P.H.M.; Daboit, T.C.; Carvalho, A.P.; Barroso, M.F.; de Almeida, M.P.; Plácido, A.; et al. Identification of Eschweilenol C in Derivative of Terminalia fagifolia Mart. and Green Synthesis of Bioactive and Biocompatible Silver Nanoparticles. Ind. Crops Prod. 2019, 137, 52–65. [Google Scholar] [CrossRef]
- Tao, H.; Wu, T.; Aldeghi, M.; Wu, T.C.; Aspuru-Guzik, A.; Kumacheva, E. Nanoparticle Synthesis Assisted by Machine Learning. Nat. Rev. Mater. 2021, 6, 701–716. [Google Scholar] [CrossRef]
- Lv, H.; Chen, X. Intelligent Control of Nanoparticle Synthesis through Machine Learning. Nanoscale 2022, 14, 6688–6708. [Google Scholar] [CrossRef] [PubMed]
- Mekki-Berrada, F.; Ren, Z.; Huang, T.; Wong, W.K.; Zheng, F.; Xie, J.; Tian, I.P.S.; Jayavelu, S.; Mahfoud, Z.; Bash, D.; et al. Two-Step Machine Learning Enables Optimized Nanoparticle Synthesis. NPJ Comput. Mater. 2021, 7, 55. [Google Scholar] [CrossRef]
- Devaraj, T.; Aathika, S.; Mani, Y.; Jagadiswary, D.; Evangeline, S.J.; Dhanasekaran, A.; Palaniyandi, S.; Subramanian, S. Application of Artificial Neural Network as a Nonhazardous Alternative on Kinetic Analysis and Modeling for Green Synthesis of Cobalt Nanocatalyst from Ocimum tenuiflorum. J. Hazard. Mater. 2021, 416, 125720. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, F.; Isopescu, R.; Pellutiè, L.; Sordello, F.; Rossi, A.M.; Ortel, E.; Martra, G.; Hodoroaba, V.-D.; Maurino, V. Machine Learning Approach for Elucidating and Predicting the Role of Synthesis Parameters on the Shape and Size of TiO2 Nanoparticles. Sci. Rep. 2020, 10, 18910. [Google Scholar] [CrossRef]






| Species | Drying | Downsizing Method | Extraction Method | Temperature (°C)/Power | Time | Solvent | Reference |
|---|---|---|---|---|---|---|---|
| Leaves | |||||||
| Azadirachta indica | Oven drying at 50 °C | - | Heating | 60 | 20 min | DI-H2O | [65] |
| Basella alba | Shade drying at room temperature | Grinding and pulverizing | Boiling | 60 | 20 min | DI-H2O | [66] |
| Cacumen platycladi | Acquired in dried form | Milling | Heating | 30 | 4 h | DI-H2O | [67] |
| Carica papaya | Shade drying | Grinding | Boiling | 60 | 30 min | DI-H2O | [68] |
| Cymbopogon jwarancusa | Shade drying at room temperature | Grinding | Boiling | Step 1: 100 Step 2: 37 | Step 1: 30 min Step 2: overnight | Double DS-H2O | [69] |
| Daphne mezereum | Acquired in dried form | Acquired in cut form | Reflux extraction | - | 15 min | DI-H2O | [70] |
| Eclipta prostrata | - | - | Boiling | 80 | 30 min | Double DS-H2O | [71] |
| Ixora brachypoda | Air drying at room temperature | Cutting | Boiling | 60 | 1 h | DI-H2O | [72] |
| Iresine herbstii | Drying | Pulverizing | Soxhlet extraction | - | 12–16 h | Ethanol | [73] |
| Jasminum sambac | - | Cutting | Microwave irradiation | - | 200 s | DS-H2O | [74] |
| Magnolia kobus | Drying at room temperature | Cutting | Boiling | - | 5 min | DS-H2O | [75] |
| Menthaaquatica | Drying | Grinding | Ultrasonication | 400 W | 10 min (On/Off = 7 s/3 s) | DI-H2O | [76] |
| Moringa oleifera | Drying at room temperature | Grinding | Soxhlet extraction | 35–45 | 10 h | Methanol | [77] |
| Piper betle | Shade drying at room temperature | Cutting | Boiling | - | 5 min | Double-distilled deionised water | [78] |
| Plantago asiatica | Acquired in dried form | Acquired in powder form | Reflux extraction | 80 | 30 min | Double DS-H2O | [79] |
| Quercus coccifera | Drying at room temperature | Grinding | Boiling | 90 | 30 min | DS-H2O | [80] |
| Ruellia tuberosa | - | Chopping | Boiling | 50 | 10 min | DI-H2O | [81] |
| Solidago canadensis | Drying at room temperature | Grinding | Heating | 80 | - | DS-H2O | [82] |
| Syzygium cumini | Oven drying at 60 °C | Crumpling | Boiling | 100 | 35 min | DI-H2O | [83] |
| Tabernaemontana divaricate | - | Grinding | Boiling | - | 10 min | DI-H2O | [84] |
| Thymus vulgaris | Acquired in dried form | Grinding | Reflux extraction | 70 | 2 h | DS-H2O | [85] |
| Tradescantia spathacea | - | Chopping | Boiling | 60 | 60 min | DI-H2O | [86] |
| Camellia sinensis | Acquired in dried form | - | Reflux extraction | - | 40 min | DI-H2O | [87] |
| Citrus limon | |||||||
| Eucalyptus globulus | |||||||
| Laurus nobilis | |||||||
| Mentha sp. | |||||||
| Quercus robur | |||||||
| Rosmarinus officinalis | |||||||
| Thimus mastichina | |||||||
| Thimus vulgaris | |||||||
| Thuja occidentalis | |||||||
| Fruits | |||||||
| Berberis vulgaris | Acquired in dry form | Acquired in powder form | Heating | 80 | 30 min | Double DS-H2O | [88] |
| Capparis spinosa | Oven drying (12 h) (383 K) | - | Boiling | - | 30 min | Ethanol/ H2O (ratio-1:1) | [89] |
| Citrus medica | - | - | Squeezing to get juice | - | - | - | [51] |
| Citrus sinensis | - | Cutting | Squeezing to get juice | - | - | - | [90] |
| Cleome viscosa | - | - | Boiling | 60 | 30 min | DS-H2O | [91] |
| Couroupita guianensis | Shade drying for 8–10 days | Chopping, grinding | Decoction | 60 | 20 min | DS-H2O | [92] |
| Crataegus pentagyna | - | - | Maceration | - | - | Methanol | [93] |
| Emblica officinalis | - | Crushing | Boiling | - | 10 min | Double DS-H2O | [94] |
| Ficus carica | Acquired in dry form | Chopping | Heating | 100 | 1 h | Double DS-H2O | [95] |
| Lycium barbarum | - | - | Boiling | - | 8 min | DI-H2O | [96] |
| Piper longum | Acquired in dry form | Acquired in powder form | Heating | 70 | 30 min | 30% methanolic solution | [97] |
| Pouteria caimito | Shade drying at room temperature | Cutting | Steeping | - | - | DS-H2O | [98] |
| Sechium edule | - | - | Heating | 90 ± 2 | 12 h | DS-H2O | [99] |
| Solanum mammosum | Oven drying (25 °C) | Grinding | Mixing with solvent/maceration | - | 1 h | DI-H2O | [100] |
| Syzygium alternifolium | Acquired in dry form | Acquired in powder form | Boiling | 80 | 30 min | Milli-Q water | [101] |
| Vaccinium macrocarpon | Acquired in dry form | Grinding | Reflux extraction | 90 | 45 min | DS-H2O | [102] |
| Peelings | |||||||
| Allium cepa | Acquired in dry form | Cutting | Heating | 90 | 30 min | DS-H2O | [103] |
| Annona squamosa | Air-drying | Grinding | Heating | 60 | 30 min | Double DS-H2O | [104] |
| Arachis hypogaea | Oven drying method for 70 °C for 30 min | Peeling via oven drying method | Heating | 70 | 30 min | Water | [105] |
| Benincasa hispida | - | - | Boiling | - | 30 min | DS-H2O | [106] |
| Carica papaya | - | Acquired in small pieces | Heating | 70–80 | 20 min | DI-H2O | [26] |
| Citrus sinensis | - | Smashing and grinding | Mixing | - | 4 h | DI-H2O | [107] |
| Garcinia mangostana | Drying at ambient conditions; later with crude extract via oven drying | Grinding | Heating | 80 | 1 h | Double DI-H2O | [108] |
| Garcinia mangostana | Oven drying at 40 °C | Grinding | Boiling | 60 | 30 min | DS-H2O | [109] |
| Myristica fragrans | Acquired in dry form | Acquired in ground form | Boiling | 100 | 1 h | DI-H2O | [110] |
| Orange peel | Drying by food drier for 12 h | Peeling and grinding | Stage 1: Maceration Stage 2: Heating | Stage 1: none Stage 2: 60 | Stage 1: 3 h Stage 2: 60 min | DI-H2O | [111] |
| Persea americana | - | Milling | Maceration | - | 24 h | DS-H2O | [112] |
| Punica granatum | Air-drying under shade | Chopping and grinding | Soxhlet extraction | 55 | 30 min | DI-H2O | [113] |
| Punica granatum | Shade drying | - | Boiling | - | 10 min | DS-H2O | [114] |
| Punica granatum | Oven drying 60 °C for 40 h | Acquired in powder form | Mixing | - | 24 h | 100% Ethanol | [115] |
| Tangerine | Shade drying (27 ± 2 °C) | Milling by electric mill and sieving | Heating | 80 | 15 min | DS-H2O | [116] |
| Citrusaurantifolia | Drying via food dryer | Grinding | Stage 1: Maceration with solvent Stage 2: Heating | Stage 1: none Stage 2: 60 | Stage 1: 3 h Stage 2: 60 min | DI-H2O | [117] |
| Citrus paradisi | |||||||
| Citrus sinensis | |||||||
| Lycopersiconesculentum | |||||||
| Flowers | |||||||
| Aglaia elaeagnoidea | Shade drying for 3 days | Grinding | Reflux extrication | - | 10 min | DI-H2O | [118] |
| Achillea wilhelmsii | Air drying | Cutting | Boiling | - | 10 min | Sterile DS-H2O | [119] |
| Aloe vera | Oven drying at 50 °C for 72 h | Cutting | Boiling | - | 5 min | Double DS-H2O | [120] |
| Avicennia marina | - | Grinding | Boiling | - | 5 min | DS-H2O | [121] |
| Azadirachta indica | Shade drying for a week | Crushing | Heating | 80 | 1 h | DI-H2O | [122] |
| Calendula | Drying at room temperature | - | Heating | 80 | 30 min | DI-H2O | [123] |
| Gazania rigens | Shade drying with oven drying | Cutting and grinding | - | - | 3 h | Methanol | [124] |
| Gnidia glauca | Shade drying for 2 days at room temperature | Grinding | Boiling | - | 5 min | DS-H2O | [125] |
| Hibiscus sabdariffa | Air drying under shade at room temperature | Soaking | Room temperature | 2 h | DS-H2O | [126] | |
| Muntingia calabura | - | - | Boiling via microwave oven | - | Boiling: 1 min * Process repeated at 1 h intervals for up to 6 h | DS-H2O | [127] |
| Tagetes erecta | - | Cutting | Boiling | - | 10 min | Ultra-pure water | [128] |
| Trifolium pratense | Air drying for 5 days at room temperature | - | Heating | 80 | 45 min | Double DS-H2O | [129] |
| Roots and Rhizomes | |||||||
| Berberis vulgaris | Drying at ambient temperature for 2 days | Grinding | - | Room temperature | 2 days | Sterile DS-H2O | [130] |
| Bergenia ciliata | Air drying at 25 °C | Acquired in powder form | Boiling | 60 | 30 min | Milli-Q water | [131] |
| Chromolaena odorata | Sun drying At 22 °C ± 2 °C for 14 days | Crushing | Heating | 85 | 2 h | DI-H2O | [132] |
| Cibotium barometz | Drying | Cutting and pulverizing | Boiling | 100 | 30 min | DS-H2O | [133] |
| Diospyros paniculata | Air drying | Grinding | Soxhlet extraction | - | - | Methanol | [134] |
| Licorice | - | - | Heating | - | - | Ethanol and double-ionised water | [135] |
| Morinda citrifolia | Shade drying at room temperature | Grinding | Boiling | - | 15 min | DS-H2O | [136] |
| Nepeta leucophylla | Shade drying for 30 days at room temperature (24–32 °C) | Grinding | Soxhlet extraction | Boiling point of methanol | 8 h | Methanol | [137] |
| Panax ginseng | - | Cutting and grinding | Boiling | - | 30 min | Sterile water | [138] |
| Rheum palmatum | Acquired in dry form | Acquired in powder form | Reflux extraction | 80 | 45 min | Ethanol | [139] |
| Rheum palmatum | - | Acquired in powder form | Incubating/heating | 40 | 24 h | Milli-Q DI-H2O | [140] |
| Rhodiola rosea | - | Grinding and screening via sieve | Boiling | 100 | 30 min | DI-H2O | [141] |
| Scutellaria baicalensis | Acquired in dry form | Grinding | Autoclave heating | 100 | 30 min | DS-H2O | [142] |
| Zingiber officinale | - | Grinding | Microwave | 1 min | DI-H2O | [143] | |
| Zingiber officinale | - | Cutting and pulverizing | Squeezing | - | - | - | [144] |
| Seeds | |||||||
| Bixa orellana | Vacuum drying at 60 °C | Crushing | Steeping | In dark environment | 24 h | Ethanol | [145] |
| Caesalpinia bonducella | - | Grinding | Sonication | - | 30 min | DI-H2O | [146] |
| Coffea arabica | - | Grinding | Heating | 85 | 25 min | DS-H2O | [147] |
| Cucurbita pepo | Shade air drying for 2 days | - | Heating | 90 | 2 h | DS-H2O | [148] |
| Eriobotrya japonica | Oven drying at 50 °C for 24 h | Grinding | Heating | 40 | 60 min | DI-H2O | [149] |
| Persea americana | Drying in dryer for 12 h | Grinding | - | Stage 1—room temperature Stage 2—65 ± 1 | Stage 1: 60 min Stage 2: 60 min | DI-H2O | [150] |
| Phoenix dactylifera | - | Milling | Boiling | 80 | 20 min | Sterile DS-H2O | [151] |
| Phoenix sylvestris | - | - | Steeping | 45 | 12 h | Sterile double DI-H2O | [152] |
| Pomegranate | - | - | Crushing to get juice | - | - | DI-H2O | [153] |
| Punica granatum | Drying by pressing in filter paper | Grinding | Heating | 80–85 | 10 min | Ultra-pure water | [154] |
| Punica granatum | - | Grinding | Mixing | - | 2 h | Water | [155] |
| Quince | - | - | Heating | 60 | 4 h | DS-H2O | [156] |
| Salvia hispanica | Drying | - | Heating | 60 | 120 min | DS-H2O | [157] |
| Tectona grandis | Drying at room temperature for 3–4 days | Crushing | Boiling | 80 | 15–20 min | Double DS-H2O | [158] |
| Theobroma cacao | Drying at room temperature for a week | Grinding | Maceration | - | A week | Methanol | [159] |
| Extraction Methods | ||
|---|---|---|
| Pros | Cons | |
| Boiling/heating/decoction |
|
|
| Maceration |
|
|
| Microwave extraction |
|
|
| Reflux extraction |
|
|
| Soxhlet extraction |
|
|
| Ultrasonication |
|
|
| Plant | Cu Precursor | Synthesis Time | Synthesis Temperature (°C) | Key Compounds | Colour of the Product | Nanomaterials | Size (nm) | Geometry | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Leaves | |||||||||
| Agrimoniae herba |
|
| 65 |
| - | Core-shell Cu-core Pt-shell | 30 | Spherical | [170] |
| Azadirachta indica |
| Stage 1—26 h Stage 2—1 h | Stage 1—none Stage 2—500 (calcination) | - | - | CuO nanoparticles | - | Nanoflake | [171] |
| Ag-CuO nanoparticles | - | ||||||||
| Mo-CuO nanoparticles | - | ||||||||
| Ag-Mo-CuO nanoparticles | 12 | ||||||||
| Carica papaya |
| 24 h | 50–60 |
| Green to blackish brown | CuO nanoparticles | <50 | Spherical | [68] |
| Carica papaya |
| 2 h | 90 | - | Light yellow green to olive green precipitate | Bimetallic Ag-Cu alloy | TEM-90-150 DLS-420.7 | Tentacle-like | [172] |
| Cyclea peltata |
| 4 h | Room temperature |
| Light yellow to green | Core-shell Cu-core Fe-shell | 45–50 | Spherical | [173] |
| Eclipta prostrata |
| 24 h | Room temperature | - | - | Cu nanoparticles | 28–45 | Spherical, hexagonal, cubical | [71] |
| Magnolia kobus |
| - | 95 |
| - | Cu nanoparticles | 37–91 | Spherical | [75] |
| 25 | 110 | ||||||||
| 60 | 90 | ||||||||
| Ocimum tenuiflorum |
| 6 h | 80 | - | Brownish blue | Core-shell CuO-shell Ag-core | Ag core: 28–30 CuO shells: 6–10 | Spherical | [174] |
| Opuntiaficus-indica |
| 1 h | 55 |
| Slight green shade | Core-shell Ag-coreCu-shell | 10–20 | Ellipsoidal | [175] |
| Slight blue shade | Bimetallic Ag-Cu alloy | - | |||||||
| Origanum vulgare |
| Until alteration of colour | 40 |
| Dark greenish-brown | Trimetallic Cu-Co-Nialloy | 28.25 | Nanoflake | [176] |
| Pisonia grandis |
| Stage 1—4 h | Stage 1—80 Stage 2—450 (calcination) |
| Green to brownish black | Zn-Mg-Cu oxide nanocomposites | 50 | Cubic | [177] |
| Plantago asiatica |
| 5 min | 80 |
| Dark | Cu nanoparticles | 7–35 | Spherical | [79] |
| Tabernaemontanadivaricate |
| 7–8 h | 100 |
| Brownish black | CuO nanoparticles | 46 ± 4 | Spherical | [84] |
| Thymus vulgaris |
| 5 min | 60 |
| Change from yellow to dark brown | CuO nanoparticles | <30 | - | [85] |
| Vitex negundo |
| 24 h | - | - | Green to Brown | Bimetallic Ag-Cu nanoparticles | 60 | Spherical | [178] |
| Fruits | |||||||||
| Crataegus pentagyna |
| - | Room temperature | - | - | Fe3O-SiO2- Cu2O-Ag nanocomposites | 55–75 | Spherical | [93] |
| Piper retrofractum |
| 60 min | 60 |
| Dark green | Cu nanoparticles | 2–10 | Spherical | [179] |
| Prunus nepalensis |
| Overnight | Room temperature | - | Light green to brown and then to pink | Cu nanoparticles | 35–50 | Centred cubic | [180] |
| Rosa canina |
| 1 h | 100 | - | Dark brown | CuO nanoparticles | 15–25 | Spherical | [181] |
| Rubus glaucus |
| 6 h | 75–80 |
| - | CuO nanoparticles | 45 | Spherical | [182] |
| Syzygium alternifolium |
| 2 h | 50 | - | - | CuO nanoparticles | 2–21 | Spherical | [101] |
| Ziziphus spina-christi |
| - | 80 |
| Green to reddish brown | Cu nanoparticles | 5–20 | Elongated spherical | [183] |
| Peelings | |||||||||
| Carica papaya |
| Stage 1— none Stage 2—2 h | Stage 1—70–80 Stage 2—450 (calcination) |
| Greenish-blue to green to dark green to black powder | CuO nanoparticles | 85–140 | Agglomerated spherical | [26] |
| Cavendish banana |
| Stage 1— none Stage 2—2 h | Stage 1—Boiling Stage 2—400 | - | Brown paste to black powder | CuO nanoparticles | 50–85 | Agglomerated spherical | [184] |
| Citrus paradisi(grapefruit) |
| Stage 1—20 min Stage 2—72 h | Stage 1—70 Stage 2—room temperature | - | Brown precipitate | Cu nanoparticles | 56–59 | Spherical | [185] |
| Citrus reticulata |
| 10 min | 25 ± 2 | - | Brown | Cu nanoparticles | 54–72 | Spherical | [186] |
| 30 | |||||||||
| 40 | |||||||||
| 50 | |||||||||
| Punica granatum |
| Stage 1—10 min Stage 2—4 h | Stage 1—80 Stage 2—40 | - | - | Cu nanoparticles | 15–20 | Spherical | [187] |
| Flowers | |||||||||
| Acacia caesia |
| - | Stage 1—none Stage 2—400 (calcination) | - | - | Ag-Cu-ZnO nanocomposite | Ag -7 Cu- 12 ZnO-none | Spherical | [188] |
| Cu-ZnO nanocomposite | 14 | |||||||
| Aglaia elaeagnoidea |
| 5 min | Room temperature |
| Light brownish red to brick red | CuO nanoparticles | 3–54 | Spherical | [118] |
| Aloe vera |
| Stage 1—30 min Stage 2— overnight | Stage 1—50 Stage 2—room temperature | - | Light green to dark green | Cu nanoparticles | 40 | Spherical | [120] |
| Azadirachta indica |
| 1 h | 80 |
| Light blue to light green to dark yellow to brown precipitate | Cu nanoparticles | 5 | Spherical | [122] |
| Bougainvillea sp. |
| - | - | - | Blue to black-blue colour | CuO nanoparticles | 12–20 | Spherical | [188] |
| Calendula sp. |
| Stage 1—1 hStage 2—6 h | Room temperature | - | - | Cu-Fe3O4 nanocomposite | 20–40 | Globular | [123] |
| Eichhornia crassipes |
| 48 h | Room temperature |
| Colourless to light red | Cu nanoparticles | 12–15 | Spherical | [189] |
| Lantana camara |
| Stage 1—10 min Stage 2—2 h | 65 | - | - | CuO nanoparticles | 13–28 | Spherical | [190] |
| Roots and Rhizomes | |||||||||
| Asparagus adscendens |
| 1 h | Room temperature | - | Pale yellow to sky blue | Cu nanoparticles | 10–15 | Spherical | [191] |
| Asparagus racemosus |
| 8 h | 60 |
| - | CuO nanoparticles | Diameter: 50–100 Length: 400–500 | Rod-like | [192] |
| Corallocarbus epigaeus |
| 12 h | 80–100 | - | Deep blue to colourless and then to brick red and dark red | Cu nanoparticles | 65–80 | Spherical | [193] |
| Polyalthia longifolia |
| 30 min with stirring and 24 h storage | - |
| Dark green colour | Cu, CuO2, Cu2O, and CuO nanoparticles | 30 | Spherical | [194] |
| Rheum emodi |
| 3 h | 90 |
| Light brown to black | Bimetallic Ag-Cu nanoparticles | 40–50 | Pseudo-spherical | [195] |
| 4 h | 90 | Blue to brown | Cu nanoparticles | - | - | |||
| Senna didymobotrya |
| - | 40 |
| - | Cu nanoparticles | 5.55–63.60 | Spherical | [196] |
| 60 | |||||||||
| 80 | |||||||||
| Zingiber officinalis |
| - | Room temperature | - | Straw yellow to sea green | Cu nanoparticles | Around 20–100 | Spherical | [197] |
| Curcuma longa | |||||||||
| Seeds | |||||||||
| Caesalpinia bonducella |
| Stage 1—5 hStage 2—2 h | - | - | Stage 1—blue-coloured solution turned green Stage 2—dark brown precipitate | CuO nanoparticles | - | Rice-grain-shaped | [146] |
| Carum carvi | - | - | - | - | - | Cu nanoparticles | 37 | Spherical | [198] |
| Fe3O4-Cu nanocomposite | 62 | Spherical | |||||||
| Koelreuteria apiculata |
| 24 h | - | - | Precipitate formation | Cu nanoparticles | 20 | Spherical | [199] |
| Persea americana |
| 6–7 h | 45–50 |
| Brownish black | Cu nanoparticles | 42–90 | Spherical | [200] |
| Punica granatum |
| Stage 1—10 min Stage 2—1–2 h Stage 3—4–6 h | Stage 1—60–70 Stage 2—60 Stage 3—room temperature |
| Stage 2—dull bluish brown colour Changed to dark green | Cu nanoparticles | 40–80 | Spherical | [154] |
| Silybum marianum |
| 5 h | 60 |
| Dark solution and forming of precipitate | Cu-Fe3O4 nanoparticles | 8.5–60 | Spherical | [201] |
| Theobroma cacao |
| 2 h | 50 |
| - | Pd-CuO nanoparticles | 40 | - | [202] |
| Triticum aestivum |
| Stage 1—1 h Stage 2—10 min Stage 3—20 min | Stage 1—room temperature (25) Stage 2—sonication Stage 3—70 |
| Dark blue to dark brown | CuO nanoparticles | 21–42 | Spherical | [203] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vincent, J.; Lau, K.S.; Evyan, Y.C.-Y.; Chin, S.X.; Sillanpää, M.; Chia, C.H. Biogenic Synthesis of Copper-Based Nanomaterials Using Plant Extracts and Their Applications: Current and Future Directions. Nanomaterials 2022, 12, 3312. https://doi.org/10.3390/nano12193312
Vincent J, Lau KS, Evyan YC-Y, Chin SX, Sillanpää M, Chia CH. Biogenic Synthesis of Copper-Based Nanomaterials Using Plant Extracts and Their Applications: Current and Future Directions. Nanomaterials. 2022; 12(19):3312. https://doi.org/10.3390/nano12193312
Chicago/Turabian StyleVincent, Jei, Kam Sheng Lau, Yang Chia-Yan Evyan, Siew Xian Chin, Mika Sillanpää, and Chin Hua Chia. 2022. "Biogenic Synthesis of Copper-Based Nanomaterials Using Plant Extracts and Their Applications: Current and Future Directions" Nanomaterials 12, no. 19: 3312. https://doi.org/10.3390/nano12193312