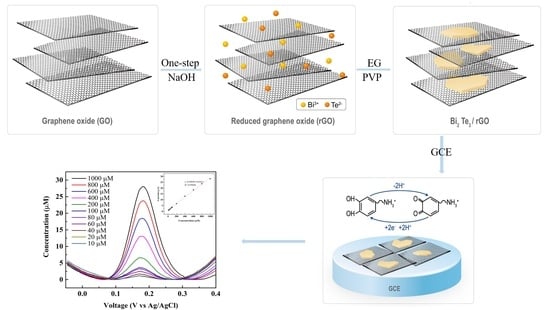
In Situ Synthesis of a Bi2Te3-Nanosheet/Reduced-Graphene-Oxide Nanocomposite for Non-Enzymatic Electrochemical Dopamine Sensing
Abstract
:1. Introduction
2. Experimental
2.1. Synthesis of BT/rGO Nanocomposite
2.2. Preparation of Modified GCEs
2.3. Characterization
2.4. Electrochemical Measurements
3. Results and Discussion
3.1. Characterization of BT/rGO
3.2. EIS Analysis
3.3. Electrochemical Behavior of Dopamine with Various Modified GCEs
3.4. Optimization of pH
3.5. Analytical Application of Sensor
3.6. Interference and Repeatability Analyses
3.7. Real Sample Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schultz, S.K. Principles of neural science, fourth edition. Am. J. Psychiatry 2001, 158, 662. [Google Scholar] [CrossRef]
- Liu, X.X.; Liu, J.W. Biosensors and sensors for dopamine detection. View 2021, 2, 20200102. [Google Scholar] [CrossRef]
- Fabregat, G.; Estrany, F.; Casas, M.T.; Aleman, C.; Armelin, E. Detection of Dopamine Using Chemically Synthesized Multilayered Hollow Microspheres. J. Phys. Chem. B 2014, 118, 4702–4709. [Google Scholar] [CrossRef] [PubMed]
- Britto, J.; Antunes, N.J.; Campos, R.; Sucupira, M.; Mendes, G.D.; Fernandes, F.; Moraes, M.O.; Moraes, M.E.A.; De Nucci, G. Determination of dopamine, noradrenaline, and adrenaline in Krebs-Henseleit solution by liquid chromatography coupled with tandem mass spectrometry and measurement of their basal release fromChelonoidis carbonariaaortaein vitro. Biomed. Chromatogr. 2021, 35, e4978. [Google Scholar] [CrossRef]
- Wang, Y.F.; Li, L.; Jiang, M.; Yang, X.; Yu, X.; Xu, L. One-pot synthesis of boron and nitrogen co-doped silicon-carbon dots for fluorescence enhancement and on-site colorimetric detection of dopamine with high selectivity. Appl. Surf. Sci. 2022, 573, 151457. [Google Scholar] [CrossRef]
- Lan, Y.X.; Yuan, F.; Fereja, T.H.; Wang, C.; Lou, B.H.; Li, J.P.; Xu, G.B. Chemiluminescence of Lucigenin/Riboflavin and Its Application for Selective and Sensitive Dopamine Detection. Anal. Chem. 2019, 91, 2135–2139. [Google Scholar] [CrossRef]
- Xing, Y.Y.; Chen, M.L.; Zhao, Y.K.; Xu, J.B.; Hou, X.H. Triple-enzyme mimetic activity of Fe3O4@C@MnO2 composites derived from metal-organic frameworks and their application to colorimetric biosensing of dopamine. Microchim. Acta 2022, 189, 12. [Google Scholar] [CrossRef]
- Algov, I.; Feiertag, A.; Shikler, R.; Alfonta, L. Sensitive enzymatic determination of neurotransmitters in artificial sweat. Biosens. Bioelectron. 2022, 210, 114264. [Google Scholar] [CrossRef]
- Khan, M.I.; Muhammad, N.; Tariq, M.; Nishan, U.; Razaq, A.; Saleh, T.A.; Abu Haija, M.; Ismail, I.; Rahim, A. Non-enzymatic electrochemical dopamine sensing probe based on hexagonal shape zinc-doped cobalt oxide (Zn-Co2O4) nanostructure. Microchim. Acta 2022, 189, 37. [Google Scholar] [CrossRef]
- Sabar, M.; Amara, U.; Riaz, S.; Hayat, A.; Nasir, M.; Nawaz, M.H. Fabrication of MoS2 enwrapped carbon cloth as electrochemical probe for non-enzymatic detection of dopamine. Mater. Lett. 2022, 308, 131233. [Google Scholar] [CrossRef]
- Nayak, S.P.; Ramamurthy, S.S.; Kumar, J.K.K. Green synthesis of silver nanoparticles decorated reduced graphene oxide nanocomposite as an electrocatalytic platform for the simultaneous detection of dopamine and uric acid. Mater. Chem. Phys. 2020, 252, 123302. [Google Scholar] [CrossRef]
- Minta, D.; Gonzalez, Z.; Wiench, P.; Gryglewicz, S.; Gryglewicz, G. N-Doped Reduced Graphene Oxide/Gold Nanoparticles Composite as an Improved Sensing Platform for Simultaneous Detection of Dopamine, Ascorbic Acid, and Uric Acid. Sensors 2020, 20, 4427. [Google Scholar] [CrossRef] [PubMed]
- Murali, A.; Lan, Y.P.; Sarswat, P.K.; Free, M.L. Synthesis of CeO2/reduced graphene oxide nanocomposite for electrochemical determination of ascorbic acid and dopamine and for photocatalytic applications. Mater. Today Chem. 2019, 12, 222–232. [Google Scholar] [CrossRef]
- Kumar, R.S.; Govindan, K.; Ramakrishnan, S.; Kim, A.R.; Kim, J.S.; Yoo, D.J. Fe3O4 nanorods decorated on polypyrrole/reduced graphene oxide for electrochemical detection of dopamine and photocatalytic degradation of acetaminophen. Appl. Surf. Sci. 2021, 556, 149765. [Google Scholar] [CrossRef]
- Han, Z.Y.; Zhang, H.; Li, H.K.; Zhu, Q.Q.; He, H.M. Ingenious construction of an electrochemical aptasensor based on a Au@COF/GO-NH2 composite with excellent detection performance. J. Mater. Chem. C 2021, 9, 4576–4582. [Google Scholar] [CrossRef]
- Song, Z.; Sheng, G.; Cui, Y.G.; Li, M.R.; Song, Z.L.; Ding, C.F.; Luo, X.L. Low fouling electrochemical sensing in complex biological media by using the ionic liquid-doped conducting polymer PEDOT: Application to voltammetric determination of dopamine. Microchim. Acta 2019, 186, 220. [Google Scholar] [CrossRef]
- Ran, G.; Li, Y.; Xia, Y. Graphene oxide and electropolymerized p-aminobenzenesulfonic acid mixed film used as dopamine and serotonin electrochemical sensor. Monatshefte Fur Chem. 2020, 151, 293–299. [Google Scholar] [CrossRef]
- Zhu, Q.Q.; Zhang, H.W.; Yuan, R.R.; He, H.M. Ingenious fabrication of metal-organic framework/graphene oxide composites as aptasensors with superior electrochemical recognition capability. J. Mater. Chem. C 2020, 8, 15823–15829. [Google Scholar] [CrossRef]
- Li, H.K.; Ye, H.L.; Zhao, X.X.; Sun, X.L.; Zhu, Q.Q.; Han, Z.Y.; Yuan, R.R.; He, H.M. Artful union of a zirconium-porphyrin MOF/GO composite for fabricating an aptamer-based electrochemical sensor with superb detecting performance. Chin. Chem. Lett. 2021, 32, 2851–2855. [Google Scholar] [CrossRef]
- Choo, S.S.; Kang, E.S.; Song, I.; Lee, D.; Choi, J.W.; Kim, T.H. Electrochemical Detection of Dopamine Using 3D Porous Graphene Oxide/Gold Nanoparticle Composites. Sensors 2017, 17, 861. [Google Scholar] [CrossRef]
- Sharma, V.; Kaur, G.A.; Gupta, N.; Shandilya, M. Growth mechanism of rGO/CDs by electrospun calcination process: Structure and application. Flatchem 2020, 24, 100195. [Google Scholar] [CrossRef]
- Anuar, N.S.; Basirun, W.J.; Shalauddin, M.; Akhter, S. A dopamine electrochemical sensor based on a platinum-silver graphene nanocomposite modified electrode. RSC Adv. 2020, 10, 17336–17344. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.Q.; Fang, X.; Zhao, H.; Li, Z.X.; Ren, H.X. An electrochemical sensor for dopamine based on polydopamine modified reduced graphene oxide anchored with tin dioxide and gold nanoparticles. Anal. Methods 2017, 9, 5322–5332. [Google Scholar] [CrossRef]
- Chen, D.; Feng, H.B.; Li, J.H. Graphene Oxide: Preparation, Functionalization, and Electrochemical Applications. Chem. Rev. 2012, 112, 6027–6053. [Google Scholar] [CrossRef]
- Chen, X.; Li, N.; Rong, Y.Q.; Hou, Y.L.; Huang, Y.; Liang, W.T. beta-Cyclodextrin functionalized 3D reduced graphene oxide composite-based electrochemical sensor for the sensitive detection of dopamine. RSC Adv. 2021, 11, 28052–28060. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Deng, P.H.; Tian, Y.L.; Feng, J.X.; Xiao, J.Y.; Li, J.H.; Liu, J.; Li, G.L.; He, Q.G. Simultaneous and sensitive determination of ascorbic acid, dopamine and uric acid via an electrochemical sensor based on PVP-graphene composite. J. Nanobiotechnol. 2020, 18, 112. [Google Scholar] [CrossRef]
- Gu, M.; Xiao, H.L.; Wei, S.S.; Chen, Z.C.; Cao, L.L. A portable and sensitive dopamine sensor based on AuNPs functionalized ZnO-rGO nanocomposites modified screen-printed electrode. J. Electroanal. Chem. 2022, 908, 116117. [Google Scholar] [CrossRef]
- Li, S.K.; Zheng, J.X.; Zuo, S.Y.; Wu, Z.G.; Yan, P.X.; Pan, F. 2D hybrid anode based on SnS nanosheet bonded with graphene to enhance electrochemical performance for lithium-ion batteries. RSC Adv. 2015, 5, 46941–46946. [Google Scholar] [CrossRef]
- Mohan, V.B.; Brown, R.; Jayaraman, K.; Bhattacharyya, D. Characterisation of reduced graphene oxide: Effects of reduction variables on electrical conductivity. Mater. Sci. Eng. B-Adv. Funct. Solid-State Mater. 2015, 193, 49–60. [Google Scholar] [CrossRef]
- Wu, D.; Guo, J.; Ge, Z.H.; Feng, J. Facile Synthesis Bi2Te3 Based Nanocomposites: Strategies for Enhancing Charge Carrier Separation to Improve Photocatalytic Activity. Nanomaterials 2021, 11, 3390. [Google Scholar] [CrossRef]
- Zhu, B.; Liu, X.X.; Wang, Q.; Qiu, Y.; Shu, Z.; Guo, Z.T.; Tong, Y.; Cui, J.; Gu, M.; He, J.Q. Realizing record high performance in n-type Bi2Te3-based thermoelectric materials. Energy Environ. Sci. 2020, 13, 2106–2114. [Google Scholar] [CrossRef]
- Liu, J.J.; Li, Y.Y.; Song, Y.X.; Ma, Y.J.; Chen, Q.M.; Zhu, Z.Y.S.; Lu, P.F.; Wang, S.M. Bi2Te3 photoconductive detectors on Si. Appl. Phys. Lett. 2017, 110, 141109. [Google Scholar] [CrossRef]
- Mousavi, M.; Ghasemian, M.B.; Han, J.L.; Wang, Y.F.; Abbasi, R.; Yang, J.; Tang, J.B.; Idrus-Saidi, S.A.; Guan, X.W.; Christoe, M.J.; et al. Bismuth telluride topological insulator synthesized using liquid metal alloys: Test of NO2 selective sensing. Appl. Mater. Today 2021, 22, 100954. [Google Scholar] [CrossRef]
- Buathet, S.; Simalaotao, K.; Reunchan, P.; Vailikhit, V.; Teesetsopon, P.; Duanghatai, R.; Kitisripanya, N.; Tubtimtae, A. Electrochemical performance of Bi2Te3 heterostructure thin film and Cu7Te4 nanocrystals on undoped and In3+-doped WO3 films for energy storage applications. Electrochim. Acta 2020, 341, 136049. [Google Scholar] [CrossRef]
- Rajaji, U.; Chen, T.W.; Chinnapaiyan, S.; Chen, S.M.; Govindasamy, M. Two-dimensional binary nanosheets (Bi2Te3@g-C3N4): Application toward the electrochemical detection of food toxic chemical. Anal. Chim. Acta 2020, 1125, 220–230. [Google Scholar] [CrossRef]
- Rajaji, U.; Chinnapaiyan, S.; Chen, S.M.; Mani, G.; Alothman, A.A.; Alshgari, R.A. Bismuth telluride decorated on graphitic carbon nitrides based binary nanosheets: Its application in electrochemical determination of salbutamol (feed additive) in meat samples. J. Hazard. Mater. 2021, 413, 125265. [Google Scholar] [CrossRef]
- Zhao, F.J.; Zhou, S.; Zhang, Y.J. Ultrasensitive Detection of Hydrogen Peroxide Using Bi2Te3 Electrochemical Sensors. ACS Appl. Mater. Interfaces 2021, 13, 4761–4767. [Google Scholar] [CrossRef]
- Ludwig, T.; Guo, L.L.; McCrary, P.; Zhang, Z.T.; Gordon, H.; Quan, H.Y.; Stanton, M.; Frazier, R.M.; Rogers, R.D.; Wang, H.T.; et al. Mechanism of Bismuth Telluride Exfoliation in an Ionic Liquid Solvent. Langmuir 2015, 31, 3644–3652. [Google Scholar] [CrossRef]
- Pareek, A.; Sravan, J.S.; Mohan, S.V. Graphene modified electrodes for bioelectricity generation in mediator-less microbial fuel cell. J. Mater. Sci. 2019, 54, 11604–11617. [Google Scholar] [CrossRef]
- Wang, B.; Huang, Z.Y.; Tang, P.H.; Luo, S.W.; Liu, Y.D.; Li, J.; Qi, X. One-pot synthesized Bi2Te3/graphene for a self-powered photoelectrochemical-type photodetector. Nanotechnology 2020, 31, 115201. [Google Scholar] [CrossRef]
- Du, Y.; Li, J.; Xu, J.Y.; Eklund, P. Thermoelectric Properties of Reduced Graphene Oxide/Bi2Te3 Nanocomposites. Energies 2019, 12, 2430. [Google Scholar] [CrossRef]
- Liu, D.M.; He, C.Y.; Chen, L.; Li, W.L.; Zu, Y.L. The nonlinear absorption effects and optical limiting properties of Bi2Te3/rGO thin films. Opt. Mater. 2021, 111, 110634. [Google Scholar] [CrossRef]
- Salavati-Niasari, M.; Bazarganipour, M.; Davar, F. Hydrothermal preparation and characterization of based-alloy Bi2Te3 nanostructure with different morphology. J. Alloy. Compd. 2010, 489, 530–534. [Google Scholar] [CrossRef]
- Moulder, J.F. Photoelectron Spectroscopy: A Reference Book of Standard Spectra for Identification and Interpretation of XPS Data; Physical Electronics Division, Perkin-Elmer Corporation: Waltham, MA, USA, 1992. [Google Scholar]
- Chen, R.Z.; Shen, Y.; Li, T.S.; Huang, J.; Gu, F.; Liang, X.Y.; Cao, M.; Wang, L.J.; Min, J.H. Interface optimization of free-standing CdZnTe films for solar-blind ultraviolet detection: Substrate dependence. Vacuum 2021, 193, 110484. [Google Scholar] [CrossRef]
- Lee, J.G.; Joshi, B.N.; Lee, J.H.; Kim, T.G.; Kim, D.Y.; Al-Deyab, S.S.; Seong, I.W.; Swihart, M.T.; Yoon, W.Y.; Yoon, S.S. Stable High-Capacity Lithium Ion Battery Anodes Produced by Supersonic Spray Deposition of Hematite Nanoparticles and Self-Healing Reduced Graphene Oxide. Electrochim. Acta 2017, 228, 604–610. [Google Scholar] [CrossRef]
- Kang, S.S.; Zhuang, J.Y.; Kang, S.H.; Peng, Y.; Guan, S.Y. Synthesis of high-quality graphene with enhanced electrochemical properties by two-step reduction method. Ceram. Int. 2019, 45, 23954–23965. [Google Scholar] [CrossRef]
- Kim, C.K.; Ji, J.M.; Aftabuzzaman, M.; Kim, H.K. Three-dimensional tellurium and nitrogen Co-doped mesoporous carbons for high performance supercapacitors. RSC Adv. 2021, 11, 8628–8635. [Google Scholar] [CrossRef]
- Jeon, I.Y.; Kim, H.M.; Kweon, D.H.; Jung, S.M.; Seo, J.M.; Shin, S.H.; Choi, I.T.; Eom, Y.K.; Kang, S.H.; Kim, H.K.; et al. Metalloid tellurium-doped graphene nanoplatelets as ultimately stable electrocatalysts for cobalt reduction reaction in dye-sensitized solar cells. Nano Energy 2016, 30, 867–876. [Google Scholar] [CrossRef]
- Namba, T.; Tamura, K.; Hatsuda, K.; Nakamura, T.; Ohata, C.; Katsumoto, S.; Haruyama, J. Spin-orbit interaction in Pt or Bi2Te3 nanoparticle-decorated graphene realized by a nanoneedle method. Appl. Phys. Lett. 2018, 113, 053106. [Google Scholar] [CrossRef]
- Boronin, A.I.; Koscheev, S.V.; Zhidomirov, G.M. XPS and UPS study of oxygen states on silver. J. Electron. Spectrosc. Relat. Phenom. 1998, 96, 43–51. [Google Scholar] [CrossRef]
- Li, S.K.; Fan, T.J.; Liu, X.R.; Liu, F.S.; Meng, H.; Liu, Y.D.; Pan, F. Graphene Quantum Dots Embedded in Bi2Te3 Nanosheets To Enhance Thermoelectric Performance. Acs Appl. Mater. Interfaces 2017, 9, 3677–3685. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Cui, Z.D.; Zheng, X.R.; Yang, X.J.; Zhu, S.L.; Li, Z.Y.; Liang, Y.Q. A Bi2Te3@CoNiMo composite as a high performance bifunctional catalyst for hydrogen and oxygen evolution reactions. J. Mater. Chem. A 2015, 3, 22770–22780. [Google Scholar] [CrossRef]
- Renuka, H.; Joshna, P.; Wani, W.A.; Venkataraman, B.H.; Ramaswamy, K.; Kundu, S. Plasmonic gold nanorods mediated p-BFCrO/n-rGO heterojunction in realizing efficient ferroelectric photovoltaic devices. Mater. Sci. Semicond. Process. 2020, 109, 104937. [Google Scholar] [CrossRef]
- Li, X.C.; She, F.S.; Shen, D.; Liu, C.P.; Chen, L.H.; Li, Y.; Deng, Z.; Chen, Z.H.; Wang, H.E. Coherent nanoscale cobalt/cobalt oxide heterostructures embedded in porous carbon for the oxygen reduction reaction. RSC Adv. 2018, 8, 28625–28631. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.T.; Lu, C.G.; Ding, D.F.; Zhao, M.; Wang, D.W.; Hu, C.; Qiu, J.S.; Xie, G.; Tang, Z.Y. Capping nanoparticles with graphene quantum dots for enhanced thermoelectric performance. Chem. Sci. 2015, 6, 4103–4108. [Google Scholar] [CrossRef] [PubMed]
- Bharath, G.; Anwer, S.; Mangalaraja, R.V.; Alhseinat, E.; Banat, F.; Ponpandian, N. Sunlight-Induced photochemical synthesis of Au nanodots on alpha-Fe2O3@Reduced graphene oxide nanocomposite and their enhanced heterogeneous catalytic properties. Sci. Rep. 2018, 8, 5718. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.N.; Li, D.L.; Fu, Q.; Pan, C.X. Influence of graphene microstructures on electrochemical performance for supercapacitors. Prog. Nat. Sci.-Mater. Int. 2015, 25, 379–385. [Google Scholar] [CrossRef]
- Zhou, L.Y.; Yan, S.C.; Lin, Z.X.; Shi, Y. In situ reduction of WS2 nanosheets for WS2/reduced graphene oxide composite with superior Li-ion storage. Mater. Chem. Phys. 2016, 171, 16–21. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.Y.; Li, N.; Xia, J.H.; Meng, Q.M.; Ding, J.C.; Lu, J. Synthesis and characterization of TiO2/graphene oxide nanocomposites for photoreduction of heavy metal ions in reverse osmosis concentrate. RSC Adv. 2018, 8, 34241–34251. [Google Scholar] [CrossRef]
- Rahman, M.M.; Lopa, N.S.; Ju, M.J.; Lee, J.J. Highly sensitive and simultaneous detection of dopamine and uric acid at graphene nanoplatelet-modified fluorine-doped tin oxide electrode in the presence of ascorbic acid. J. Electroanal. Chem. 2017, 792, 54–60. [Google Scholar] [CrossRef]
- Sheng, Z.H.; Zheng, X.Q.; Xu, J.Y.; Bao, W.J.; Wang, F.B.; Xia, X.H. Electrochemical sensor based on nitrogen doped graphene: Simultaneous determination of ascorbic acid, dopamine and uric acid. Biosens. Bioelectron. 2012, 34, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Walczak, M.M.; Dryer, D.A.; Jacobson, D.D.; Foss, M.G.; Flynn, N.T. pH-dependent redox couple: Illustrating the Nernst equation using cyclic voltammetry. J. Chem. Educ. 1997, 74, 1195–1197. [Google Scholar] [CrossRef]
- Li, S.H.; Ma, Y.S.; Liu, Y.K.; Xin, G.; Wang, M.H.; Zhang, Z.H.; Liu, Z.Y. Electrochemical sensor based on a three dimensional nanostructured MoS2 nanosphere-PANI/reduced graphene oxide composite for simultaneous detection of ascorbic acid, dopamine, and uric acid. RSC Adv. 2019, 9, 2997–3003. [Google Scholar] [CrossRef] [PubMed]
- Arumugasamy, S.K.; Govindaraju, S.; Yun, K. Electrochemical sensor for detecting dopamine using graphene quantum dots incorporated with multiwall carbon nanotubes. Appl. Surf. Sci. 2020, 508, 145294. [Google Scholar] [CrossRef]
- Kim, K.T.; Choi, S.Y.; Shin, E.H.; Moon, K.S.; Koo, H.Y.; Lee, G.G.; Ha, G.H. The influence of CNTs on the thermoelectric properties of a CNT/Bi2Te3 composite. Carbon 2013, 52, 541–549. [Google Scholar] [CrossRef]
- Chen, S.; Fang, Y.M.; Li, J.; Sun, J.J.; Chen, G.N.; Yang, H.H. Study on the electrochemical catalytic properties of the topological insulator Bi2Se3. Biosens. Bioelectron. 2013, 46, 171–174. [Google Scholar] [CrossRef]
- Nagao, J.; Hatta, E.; Mukasa, K. Evaluation of metal-Bi2Te3 contacts by electron tunneling spectroscopy. In Proceedings of the Ict ’96—Fifteenth International Conference on Thermoelectrics, Pasadena, CA, USA, 26–29 March 1996; pp. 404–407. [Google Scholar] [CrossRef]
- Takahashi, T.; Tokailin, H.; Sagawa, T. Angle-Resolved Ultraviolet Photoelectron-Spectroscopy of the Unoccupied Band-Structure of Graphite. Phys. Rev. B 1985, 32, 8317–8324. [Google Scholar] [CrossRef]










| Modified Electrode | Technique | Sensitivity (µA·mM−1cm−2) | Linear Range (µM) | LOD (µM) | Ref. |
|---|---|---|---|---|---|
| 3D porous GO | CV | - | 0.1–30 | 1.28 | [20] |
| rGO/CD 1 | CV | - | 0.5–20 | 1.41 | [21] |
| Ag-rGO | DPV | 110 | 10–70 | 1 | [11] |
| Au-N-rGO 2 | DPV | 190 | 20–1000 | 2.4 | [12] |
| CeO/rGO | DPV | - | 10–150 | 2 | [13] |
| MoS2-PANI/rGO | DPV | - | 5–500 | 0.7 | [64] |
| GQDs 3@MWCNTs 4 | DPV | - | 0.25–250 | 0.095 | [65] |
| BT/rGO | DPV | 222.93 | 10–1000 | 0.06 | Present study |
| Content (µM) | Detected (µM) | Recovered (%) | RSD (%) |
|---|---|---|---|
| 10 | 9.11 | 91.11 | 7.8 |
| 240 | 237.62 | 99.01 | 2.85 |
| 480 | 481.25 | 100.26 | 3.91 |
| 560 | 556.99 | 99.46 | 4.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, H.; Jang, B.; Park, J.; Mun, H.-j.; Cho, H.-B.; Choa, Y.-H. In Situ Synthesis of a Bi2Te3-Nanosheet/Reduced-Graphene-Oxide Nanocomposite for Non-Enzymatic Electrochemical Dopamine Sensing. Nanomaterials 2022, 12, 2009. https://doi.org/10.3390/nano12122009
Shen H, Jang B, Park J, Mun H-j, Cho H-B, Choa Y-H. In Situ Synthesis of a Bi2Te3-Nanosheet/Reduced-Graphene-Oxide Nanocomposite for Non-Enzymatic Electrochemical Dopamine Sensing. Nanomaterials. 2022; 12(12):2009. https://doi.org/10.3390/nano12122009
Chicago/Turabian StyleShen, Haishan, Byungkwon Jang, Jiyoung Park, Hyung-jin Mun, Hong-Baek Cho, and Yong-Ho Choa. 2022. "In Situ Synthesis of a Bi2Te3-Nanosheet/Reduced-Graphene-Oxide Nanocomposite for Non-Enzymatic Electrochemical Dopamine Sensing" Nanomaterials 12, no. 12: 2009. https://doi.org/10.3390/nano12122009