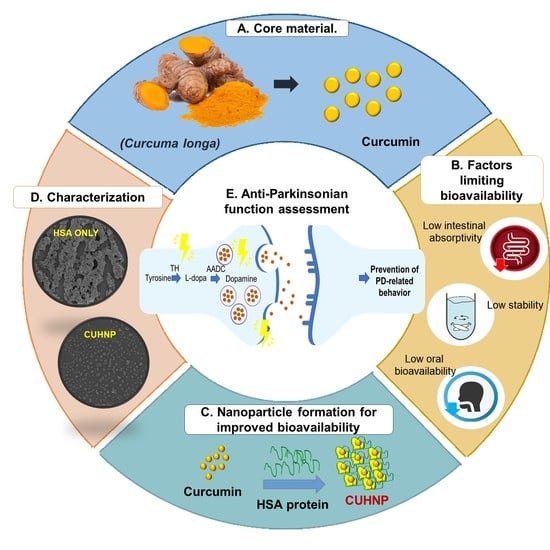
Curcumin-Loaded Human Serum Albumin Nanoparticles Prevent Parkinson’s Disease-like Symptoms in C. elegans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of CU-Loaded HSA Nanoparticles (CUHNP)
2.3. Fabrication/Characterization of CUHNP
2.3.1. Morphology, Particle Size, PDI, and ζ-Potential
2.3.2. Encapsulation Efficiency (EE)
2.4. In Vivo Safety of CUHNP in C. elegans
2.4.1. Nematode Cultivation
2.4.2. Analysis of Lifespan
2.4.3. Locomotion Assay
2.4.4. Assessment of DA Neuron Degeneration and DA-Related Behaviors
2.4.5. Assessment of DA-Related Behaviors
Basal Slowing Response (BSR) Assay
2.4.6. Ethanol Avoidance Test
3. Results
3.1. Fabrication/Characterization of CU-Loaded HSA Nanoparticles (CUHNP)
3.1.1. Effect of CU Concentrations on Particle Size and ζ-Potential
3.1.2. Morphology of CUHNP
3.2. In Vivo Assessment of CUHNP in C. elegans
3.2.1. Enhanced Lifespan after Feeding with CUHNP
3.2.2. Enhanced Body Movement after Feeding with CUHNP
3.3. Improvement of DA-Regulatory Behaviors by CUHNP
3.3.1. Basal Slowing Response (BSR) Assay
3.3.2. Ethanol Avoidance
3.4. Prevention of Dopaminergic Neuron Degeneration by CUHNP
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ishihara, L.S.; Cheesbrough, A.; Brayne, C.; Schrag, A. Estimated life expectancy of Parkinson’s patients compared with the UK population. J. Neurol. Neurosurg. Psychiatry 2007, 78, 1304–1309. [Google Scholar] [CrossRef] [PubMed]
- Gelb, D.J.; Oliver, E.; Gilman, S. Diagnostic criteria for Parkinson disease. Arch. Neurol. 1999, 56, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Dauer, W.; Przedborski, S. Parkinson’s disease: Mechanisms and models. Neuron 2003, 39, 889–909. [Google Scholar] [CrossRef]
- Ramassamy, C.; Clostre, F.; Christen, Y.; Costentin, J. Prevention by a Ginkgo biloba extract (GBE 761) of the dopaminergic neurotoxicity of MPTP. J. Pharm. Pharmacol. 1990, 42, 785–789. [Google Scholar] [CrossRef]
- Wu, W.R.; Zhu, X.Z. Involvement of monoamine oxidase inhibition in neuroprotective and neurorestorative effects of Ginkgo biloba extract against MPTP-induced nigrostriatal dopaminergic toxicity in C57 mice. Life Sci. 1999, 65, 157–164. [Google Scholar] [CrossRef]
- Rudakewich, M.; Ba, F.; Benishin, C.G. Neurotrophic and neuroprotective actions of ginsenosides Rb(1) and Rg(1). Planta Med. 2001, 67, 533–537. [Google Scholar] [CrossRef]
- Van Kampen, J.; Robertson, H.; Hagg, T.; Drobitch, R. Neuroprotective actions of the ginseng extract G115 in two rodent models of Parkinson’s disease. Exp. Neurol. 2003, 184, 521–529. [Google Scholar] [CrossRef]
- Haleagrahara, N.; Siew, C.J.; Mitra, N.K.; Kumari, M. Neuroprotective effect of bioflavonoid quercetin in 6-hydroxydopamine-induced oxidative stress biomarkers in the rat striatum. Neurosci. Lett. 2011, 500, 139–143. [Google Scholar] [CrossRef]
- Lv, C.; Hong, T.; Yang, Z.; Zhang, Y.; Wang, L.; Dong, M.; Zhao, J.; Mu, J.; Meng, Y. Effect of Quercetin in the 1-Methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-Induced Mouse Model of Parkinson’s Disease. Evid.-Based Complement. Altern. Med. 2012, 2012, 928643. [Google Scholar] [CrossRef]
- de Guzman, A.C.V.; Razzak, M.A.; Purevdulam, B.; Choi, S.S. Anti-Parkinson’s Disease Function of Dioscin-Zein-Carboxymethyl Cellulose Nanocomplex in Caenorhabditis elegans. Biotechnol. J. 2020, 15, 2000080. [Google Scholar] [CrossRef]
- Wang, X.S.; Zhang, Z.R.; Zhang, M.M.; Sun, M.X.; Wang, W.W.; Xie, C.L. Neuroprotective properties of curcumin in toxin-base animal models of Parkinson’s disease: A systematic experiment literatures review. BMC Complement. Altern. Med. 2017, 17, 412. [Google Scholar] [CrossRef]
- Bhat, A.; Mahalakshmi, A.M.; Ray, B.; Tuladhar, S.; Hediyal, T.A.; Manthiannem, E.; Padamati, J.; Chandra, R.; Chidambaram, S.B.; Sakharkar, M.K. Benefits of curcumin in brain disorders. Benefits of curcumin in brain disorders. BioFactors 2019, 45, 666–689. [Google Scholar] [CrossRef]
- Mythri, R.B.; Bharath, M.M. Curcumin: A potential neuroprotective agent in Parkinson’s disease. Curr. Pharm. Des. 2012, 18, 91–99. [Google Scholar] [CrossRef]
- Siddique, Y.H.; Naz, F.; Jyoti, S. Effect of curcumin on lifespan, activity pattern, oxidative stress, and apoptosis in the brains of transgenic Drosophila model of Parkinson’s disease. Biomed. Res. Int. 2014, 2014, 606928. [Google Scholar] [CrossRef]
- Ganesan, P.; Ko, H.M.; Kim, I.S.; Choi, D.K. Recent trends in the development of nanophytobioactive compounds and delivery systems for their possible role in reducing oxidative stress in Parkinson’s disease models. Int. J. Nanomed. 2015, 10, 6757–6772, Published 29 October 2015. [Google Scholar] [CrossRef]
- Bollimpelli, V.S.; Kumar, P.; Kumari, S.; Kondapi, A.K. Neuroprotective effect of curcumin-loaded lactoferrin nano particles against rotenone induced neurotoxicity. Neurochem. Int. 2016, 95, 37–45. [Google Scholar] [CrossRef]
- Siddique, Y.H.; Khan, W.; Singh, B.R.; Naqvi, A.H. Synthesis of alginate-curcumin nanocomposite and its protective role in transgenic Drosophila model of Parkinson’s disease. ISRN Pharmacol. 2013, 2013, 794582. [Google Scholar] [CrossRef]
- Zhang, N.; Yan, F.; Liang, X.; Wu, M.; Shen, Y.; Chen, M.; Xu, Y.; Zou, G.; Jiang, P.; Tang, C.; et al. Localized delivery of curcumin into brain with polysorbate 80-modified cerasomes by ultrasound-targeted microbubble destruction for improved Parkinson’s disease therapy. Theranostics 2018, 8, 2264–2277. [Google Scholar] [CrossRef]
- Kundu, P.; Das, M.; Tripathy, K.; Sahoo, S.K. Delivery of Dual Drug Loaded Lipid Based Nanoparticles across the Blood-Brain Barrier Impart Enhanced Neuroprotection in a Rotenone Induced Mouse Model of Parkinson’s Disease. ACS Chem. Neurosci. 2016, 7, 1658–1670. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Harikumar, K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int. J. Biochem. Cell Biol. 2009, 41, 40–59. [Google Scholar] [CrossRef]
- Epstein, J.; Sanderson, I.R.; Macdonald, T.T. Curcumin as a therapeutic agent: The evidence from in vitro, animal and human studies. Br. J. Nutr. 2010, 103, 1545–1557. [Google Scholar] [CrossRef]
- Chainani-Wu, N. Safety and anti-inflammatory activity of curcumin: A component of tumeric (Curcuma longa). J. Altern. Complement. Med. 2003, 9, 161–168. [Google Scholar] [CrossRef]
- Strimpakos, A.S.; Sharma, R.A. Curcumin: Preventive and therapeutic properties in laboratory studies and clinical trials. Antioxid. Redox Signal. 2008, 10, 511–545. [Google Scholar] [CrossRef]
- Funk, J.L.; Frye, J.B.; Oyarzo, J.N.; Zhang, H.; Timmermann, B.N. Anti-Arthritic Effects and Toxicity of the Essential Oils of Turmeric (Curcuma longa L.). J. Agric. Food Chem. 2010, 58, 842–849. [Google Scholar] [CrossRef]
- Roy, G.C.; Chakraborty, K.; Nandy, P.; Moitra, M. Pros and Cons of Curcumin as Bioactive Phyto- Compound for Effective Management of Insect Pests. Am. Sci. Res. J. Eng. Technol. Sci. 2015, 7, 31–43. [Google Scholar]
- Prasad, S.; Tyagi, A.K.; Aggarwal, B.B. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: The golden pigment from golden spice. Cancer Res. Treat. 2014, 46, 2–18. [Google Scholar] [CrossRef]
- Hu, K.; McClements, D.J. Fabrication of surfactant-stabilized zein nanoparticles: A pH modulated antisolvent precipitation method. Food Res. Int. 2014, 64, 329–335. [Google Scholar] [CrossRef]
- Zheng, B.; Peng, S.; Zhang, X.; McClements, D.J. Impact of Delivery System Type on Curcumin Bioaccessibility: Comparison of Curcumin-Loaded Nanoemulsions with Commercial Curcumin Supplements. J. Agric. Food Chem. 2018, 66, 10816–10826. [Google Scholar] [CrossRef]
- Sobh, R.A.; Mohamed, W.S.; Moustafa, A.B.; Nasr, H.E. Encapsulation of Curcumin and Curcumin Derivative in Polymeric Nanospheres. Polymer-Plast. Technol. Eng. 2015, 54, 1457–1467. [Google Scholar] [CrossRef]
- Pignanelli, C.; Ma, D.; Noel, M.; Ropat, J.; Mansour, F.; Curran, C.; Pupulin, S.; Larocque, K.; Wu, J.; Liang, G.; et al. Targeting of Cancer Cells by Oxidative Vulnerabilities with Novel Curcumin Analogs. Sci. Rep. 2017, 7, 1105. [Google Scholar] [CrossRef]
- Sneharani, A.H. Curcumin-sunflower protein nanoparticles-A potential antiinflammatory agent. J. Food Biochem. 2019, 43, e12909. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Diao, J.X.; Sun, Y.; Sun, Y. Bioevaluation of human serum albumin-hesperidin bioconjugate: Insight into protein vector function and conformation. J. Agric. Food Chem. 2012, 60, 7218–7228. [Google Scholar] [CrossRef] [PubMed]
- Patra, D.; Barakat, C.; Tafech, R.M. Study on effect of lipophilic curcumin on sub-domain IIA site of human serum albumin during unfolded and refolded states: A synchronous fluorescence spectroscopic study. Colloids Surf. B Biointerfaces 2012, 94, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xiao, K.; Luo, J.; Lee, J.; Pan, S.; Lam, K.S. A novel size-tunable nanocarrier system for targeted anticancer drug delivery. J. Control. Release 2010, 144, 314–323. [Google Scholar] [CrossRef]
- Elzoghby, A.O.; Samy, W.M.; Elgindy, N.A. Albumin-based nanoparticles as potential controlled release drug delivery systems. J. Control. Release 2012, 157, 168–182. [Google Scholar] [CrossRef]
- Shimanovich, U.; Tkacz, I.; Eliaz, D.; Cavaco-Paulo, A.; Michaeli, S.; Gedanken, A. Encapsulation of RNA molecules in BSA microspheres and internalization into Trypanosoma Brucei parasites and human U2OS cancer cells. Adv. Funct. Mater. 2011, 21, 3659–3666. [Google Scholar] [CrossRef]
- Altintas, I.; Heukers, R.; van der Meel, R.; Lacombe, M.; Amidi, M.; van Bergen En Henegouwen, P.M.; Hennink, W.E.; Schiffelers, R.M.; Kok, R.J. Nanobody-albumin nanoparticles (NANAPs) for the delivery of a multikinase inhibitor 17864 to EGFR overexpressing tumor cells. J. Control. Release 2013, 165, 110–118. [Google Scholar] [CrossRef]
- Bakare, R.A.; Bhan, C.; Raghavan, D. Synthesis and characterization of collagen grafted poly(hydroxybutyrate-valerate) (PHBV) scaffold for loading of bovine serum albumin capped silver (Ag/BSA) nanoparticles in the potential use of tissue engineering application. Biomacromolecules 2014, 15, 423–435. [Google Scholar] [CrossRef]
- Shahpiri, Z.; Bahramsoltani, R.; Hosein Farzaei, M.; Farzaei, F.; Rahimi, R. Phytochemicals as future drugs for Parkinson’s disease: A comprehensive review. Rev. Neurosci. 2016, 27, 651–668. [Google Scholar] [CrossRef]
- Razzak, M.A.; Choi, S.S. Delineating the interaction mechanism of glabridin and ovalbumin by spectroscopic and molecular docking techniques. Food Chem. 2021, 347, 128981. [Google Scholar] [CrossRef] [PubMed]
- Razzak, M.A.; Lee, J.E.; Choi, S.S. Structural insights into the binding behavior of isoflavonoid glabridin with human serum albumin. Food Hydrocoll. 2019, 91, 290–300. [Google Scholar] [CrossRef]
- Lewis, J.A.; Fleming, J.T. Basic culture methods. Methods Cell Biol. 1995, 48, 3–29. [Google Scholar] [PubMed]
- Sutphin, G.L.; Kaeberlein, M. Measuring Caenorhabditis elegans life span on solid media. J. Vis. Exp. 2009, 27, 1152. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.A.; Wu, C.H.; Berg, H.; Levine, J.H. The genetics of levamisole resistance in the nematode Caenorhabditis elegans. Genetics 1980, 95, 905–928. [Google Scholar] [CrossRef] [PubMed]
- Chase, D.L.; Pepper, J.S.; Koelle, M.R. Mechanism of extrasynaptic dopamine signaling in Caenorhabditis elegans. Nat. Neurosci. 2004, 7, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Jahanban-Esfahlan, A.; Dastmalchi, S.; Davaran, S. A simple improved desolvation method for the rapid preparation of albumin nanoparticles. Int. J. Biol. Macromol. 2016, 91, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Huang, X.; Gao, Y.; Huang, X.; Xiao, H.; McClements, D.J. Core–shell biopolymer nanoparticle delivery systems: Synthesis and characterization of curcumin fortified zein–pectin nanoparticles. Food Chem. 2015, 182, 275–281. [Google Scholar] [CrossRef]
- Razzak, M.A.; Lee, J.E.; Park, H.H.; Park, T.H.; Choi, S.S. Exploring Binding Mechanisms between Curcumin and Silkworm 30Kc19 Protein Using Spectroscopic Analyses and Computational Simulations. Biotechnol. Bioprocess Eng. 2018, 23, 605–616. [Google Scholar] [CrossRef]
- Howarth, C.; Gleeson, P.; Attwell, D. Updated energy budgets for neural computation in the neocortex and cerebellum. J. Cereb. Blood Flow Metab. 2012, 32, 1222–1232. [Google Scholar] [CrossRef]
- van Ham, T.J.; Thijssen, K.L.; Breitling, R.; Hofstra, R.M.; Plasterk, R.H.; Nollen, E.A. C. elegans model identifies genetic modifiers of alpha-synuclein inclusion formation during aging. PLoS Genet. 2008, 4, e1000027. [Google Scholar] [CrossRef]
- Davies, A.G.; Pierce-Shimomura, J.T.; Kim, H.; VanHoven, M.K.; Thiele, T.R.; Bonci, A.; Bargmann, C.I.; McIntire, S.L. A central role of the BK potassium channel in behavioral responses to ethanol in C. elegans. Cell 2003, 115, 656–666. [Google Scholar] [CrossRef]
- Geny, B.; Mettauer, B.; Muan, B.; Bischoff, P.; Epailly, E.; Piquard, F.; Eisenmann, B.; Haberey, P. Safety and efficacy of a new transpulmonary echo contrast agent in echocardiographic studies in patients. J. Am. Coll. Cardiol. 1993, 22, 1193–1198. [Google Scholar] [CrossRef]
- Ibrahim, N.K.; Desai, N.; Legha, S.; Soon-Shiong, P.; Theriault, R.L.; Rivera, E.; Esmaeli, B.; Ring, S.E.; Bedikian, A.; Hortobagyi, G.N.; et al. Phase I and pharmacokinetic study of ABI-007, a Cremophor-free, protein-stabilized, nanoparticle formulation of paclitaxel. Clin. Cancer Res. 2002, 8, 1038–1044. [Google Scholar]
- Wang, G.; Siggers, K.; Zhang, S.; Jiang, H.; Xu, Z.; Zernicke, R.F.; Matyas, J.; Uludağ, H. Preparation of BMP-2 containing bovine serum albumin (BSA) nanoparticles stabilized by polymer coating. Pharm. Res. 2008, 25, 2896–2909. [Google Scholar] [CrossRef]
- Langer, K.; Balthasar, S.; Vogel, V.; Dinauer, N.; von Briesen, H.; Schubert, D. Optimization of the preparation process for human serum albumin (HSA) nanoparticles. Int. J. Pharm. 2003, 257, 169–180. [Google Scholar] [CrossRef]
- Lee, T.K.; Sokoloski, T.D.; Royer, G.P. Serum albumin beads: An injectable, biodegradable system for the sustained release of drugs. Science 1981, 213, 233–235. [Google Scholar] [CrossRef]
- Merodio, M.; Arnedo, A.; Renedo, M.J.; Irache, J.M. Ganciclovir-loaded albumin nanoparticles: Characterization and in vitro release properties. Eur. J. Pharm. Sci. 2001, 12, 251–259. [Google Scholar] [CrossRef]
- Migneault, I.; Dartiguenave, C.; Bertrand, M.J.; Waldron, K.C. Glutaraldehyde: Behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. Biotechniques 2004, 37, 790–802. [Google Scholar] [CrossRef]
- Razzak, M.A.; Li, J.; Choi, S.S. Egg-Curry: Insights into the Interaction Between Curcumin and Ovalbumin Using Spectroscopic Analyses and Protein-Ligand Docking Simulations. Food Biophys. 2021, 1–12. [Google Scholar] [CrossRef]
- Speer, D.P.; Chvapil, M.; Eskelson, C.D.; Ulreich, J. Biological effects of residual glutaraldehyde in glutaraldehyde-tanned collagen biomaterials. J. Biomed. Mater. Res. 1980, 14, 753–764. [Google Scholar] [CrossRef]
- Gendler, E.; Gendler, S.; Nimni, M.E. Toxic reactions evoked by glutaraldehyde-fixed pericardium and cardiac valve tissue bioprosthesis. J. Biomed. Mater. Res. 1984, 18, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.K.; Hang, X.; Liu, R.; Hasan, A.; Feng, Z. Levodopa-Induced Motor and Dopamine Receptor Changes in Caenorhabditis elegans Overexpressing Human Alpha-Synuclein. Neurodegener. Dis. 2016, 16, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jee, C.; McIntire, S.L. Ethanol preference in C. elegans. Genes Brain Behav. 2009, 8, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Kikuchi, K.; Tsuruta-Numayama, K.; Ishikawa, T. Particle selectivity of filtering by C. elegans. Theor. Appl. Mech. Lett. 2019, 9, 61–65. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Guzman, A.C.V.; Razzak, M.A.; Cho, J.H.; Kim, J.Y.; Choi, S.S. Curcumin-Loaded Human Serum Albumin Nanoparticles Prevent Parkinson’s Disease-like Symptoms in C. elegans. Nanomaterials 2022, 12, 758. https://doi.org/10.3390/nano12050758
de Guzman ACV, Razzak MA, Cho JH, Kim JY, Choi SS. Curcumin-Loaded Human Serum Albumin Nanoparticles Prevent Parkinson’s Disease-like Symptoms in C. elegans. Nanomaterials. 2022; 12(5):758. https://doi.org/10.3390/nano12050758
Chicago/Turabian Stylede Guzman, Arvie Camille V., Md. Abdur Razzak, Joong Hee Cho, Ji Yi Kim, and Shin Sik Choi. 2022. "Curcumin-Loaded Human Serum Albumin Nanoparticles Prevent Parkinson’s Disease-like Symptoms in C. elegans" Nanomaterials 12, no. 5: 758. https://doi.org/10.3390/nano12050758