Polymer Functionalized Nanoparticles in Blue Phase LC: Effect of Particle Shape
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Functionalization of Gold Nanoparticles
2.3. Preparation of BPLC Mixture
2.4. Preparation of the Blue Phase Nanoparticle Dispersions
2.5. Characterization
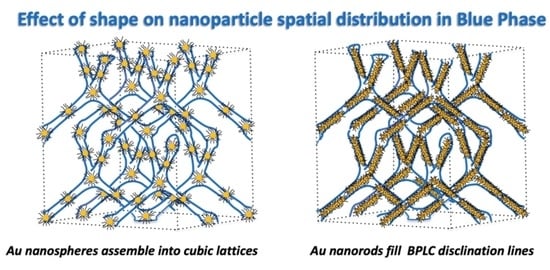
3. Results
3.1. Stabilization of BP Mixture by Short Chain PEO
3.2. Stabilization of BP Mixture by AuNP-PEO
3.3. Stabilization of 6BA:4-BCHA BP Mixture by AuNR-PEO
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johnson, D.; Flack, J.; Crooker, P.P. Structure and properties of the cholesteric blue phases. Phys. Rev. Lett. 1980, 27, 641–644. [Google Scholar] [CrossRef]
- Meiboom, S.; Sethna, J.P.; Anderson, P.W.; Brinkman, W.F. Theory of the blue phase of cholesteric liquid crystals. Phys. Rev. Lett. 1981, 46, 1216–1219. [Google Scholar] [CrossRef]
- Kitzerow, H.S. Blue phases come of age: A review. Emerg. Liq. Cryst. Technol. IV 2009, 7232, 723205. [Google Scholar]
- Rahman, M.A.; Said, S.M.; Balamurugan, S. Blue phase liquid crystal: Strategies for phase stabilization and device development. Sci. Technol. Adv. Mater. 2015, 16, 033501. [Google Scholar] [CrossRef]
- Fukuda, J.I.; Žumer, S. Novel Defect Structures in a Strongly Confined Liquid-Crystalline Blue Phase. Phys. Rev. Lett. 2010, 104, 017801. [Google Scholar] [CrossRef]
- Bahr, C.; Kitzerow, H.S. Chirality in Liquid Crystals; Springer: Berlin/Heidelberg, Germany, 2001. [Google Scholar]
- Kikuchi, H. Liquid Crystalline Blue Phases. In Liquid Crystalline Blue Phases in Liquid Crystalline Functional Assemblies and Their Supramolecular Structures; Springer: Berlin/Heidelberg, Germany, 2007; Volume 128, pp. 99–117. [Google Scholar]
- Yoshizawa, A. Material design for blue phase liquid crystals and their electro-optical effects. RSC Adv. 2013, 3, 25475–25497. [Google Scholar] [CrossRef]
- Ravnik, M.; Alexander, G.P.; Yeomans, J.M.; Zumer, S. Mesoscopic modelling of colloids in chiral nematics. Faraday Discuss. 2010, 144, 159–169. [Google Scholar] [CrossRef]
- Dierking, I.; Blenkhorn, W.; Credland, E.; Drake, W.; Kociuruba, R.; Kayser, B.; Michael, T. Stabilising liquid crystalline blue phases. Soft Matter 2012, 8, 4355–4362. [Google Scholar] [CrossRef]
- Kasch, N.; Dierking, I.; Turner, M. Stabilization of the liquid crystalline blue phase by the addition of short-chain polystyrene. Soft Matter 2013, 9, 4789–4793. [Google Scholar] [CrossRef]
- Jo, S.Y.; Jeon, S.W.; Kim, B.C.; Bae, J.H.; Araoka, F.; Choi, S.W. Polymer stabilization of liquid-crystal blue phase II toward photonic crystals. ACS Appl. Mater. Interfaces 2017, 9, 8941–8947. [Google Scholar] [CrossRef]
- Senyuk, B.; Evans, J.S.; Ackerman, P.; Lee, T.; Manna, P.; Vigderman, L.; Zubarev, E.R.; van de Lagemaat, J.; Smalyukh, I.I. Shape-dependent oriented trapping and scaffolding of plasmonic nanoparticles by topological defects for self-assembly of colloidal dimers in liquid crystals. Nano Lett. 2012, 12, 955–963. [Google Scholar] [CrossRef] [Green Version]
- Karatairi, E.; Rožič, B.; Kutnjak, Z.; Tzitzios, V.; Nounesis, G.; Cordoyiannis, G.; Thoen, J.; Glorieux, C.; Kralj, S. Nanoparticle-induced widening of the temperature range of liquid-crystalline blue phases. Phys. Rev. E 2010, 8, 041703. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, H.; Tanaka, Y.; Kawamoto, K.; Kubo, H.; Tsuda, T.; Fujii, A.; Kuwabata, S.; Kikuchi, H.; Ozaki, M. Nanoparticle-stabilized cholesteric blue phases. Appl. Phys. Exp. 2009, 2, 121501. [Google Scholar] [CrossRef]
- Senyuk, B.; Glugla, D.; Smalyukh, I.I. Rotational and translational diffusion of anisotropic gold nanoparticles in liquid crystals controlled by varying surface anchoring. Phys. Rev. E 2013, 88, 062507. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Tang, J.; Zhang, Y.; Martinez, A.; Wang, S.; He, S.; White, T.J.; Smalyukh, I.I. Shape-dependent dispersion and alignment of nonaggregating plasmonic gold nanoparticles in lyotropic and thermotropic liquid crystals. Phys. Rev. E 2014, 89, 052505. [Google Scholar] [CrossRef]
- Wong, J.M.; Hwang, J.Y.; Chien, L.C. Electrically reconfigurable and thermally sensitive optical properties of gold nanorods dispersed liquid crystal blue phase. Soft Matter 2011, 7, 7956–7959. [Google Scholar] [CrossRef]
- Wang, L.; Gutierrez-Cuevas, K.S.; Krishna Bisoyi, H.; Xiang, J.; Singh, G.; Zola, R.S.; Kumar, S.; Lavrentovich, O.D.; Urbas, A.; Li, Q. NIR Light-directing self-organized 3D photonic superstructures loaded with anisotropic plasmonic hybrid nanorods. Chem. Commun. 2015, 51, 15039–15042. [Google Scholar] [CrossRef]
- Ravnik, M.; Alexander, G.P.; Yeomans, J.M.; Žumer, S. Three-dimensional colloidal crystals in liquid crystalline blue phases. Proc. Natl. Acad. Sci. USA 2011, 108, 5188–5192. [Google Scholar] [CrossRef] [Green Version]
- Stratford, K.; Henrich, O.; Lintuvuori, J.S.; Cates, M.E.; Marenduzzo, D. Self-assembly of colloid-cholesteric composites provides a possible route to switchable optical materials. Nat. Commun. 2014, 5, 3954. [Google Scholar] [CrossRef] [Green Version]
- Gharbi, M.A.; Manet, S.; Lhermitte, J.; Brown, S.; Milette, J.; Toader, V.; Sutton, M.; Reven, L. Reversible nanoparticle cubic lattices in blue phase liquid crystals. ACS Nano 2016, 10, 3410–3415. [Google Scholar] [CrossRef]
- Roohnikan, M.; Cummings Premack, K.; Guzman-Juarez, B.; Toader, V.; Rey, A.; Reven, L. Hydrogen-bonded LC nanocomposites: Characterization of nanoparticle-LC interactions by solid-state NMR and FTIR spectroscopies. Liq. Cryst. 2019, 46, 1067–1078. [Google Scholar] [CrossRef]
- Gvozdovskyy, I. ‘Blue phases’ of highly chiral thermotropic liquid crystals with a wide range of near-room temperature. Liq. Cryst. 2015, 42, 1391–1404. [Google Scholar] [CrossRef]
- Roohnikan, M.; Toader, V.; Rey, A.; Reven, L. Hydrogen-bonded liquid crystal nanocomposites. Langmuir 2016, 32, 8442–8450. [Google Scholar] [CrossRef] [PubMed]
- Rucareanu, S.; Maccarini, M.; Shepherd, J.L.; Lennox, R.B. Polymer-capped gold nanoparticles by ligand-exchange reactions. J. Mater. Chem. 2008, 18, 5830–5834. [Google Scholar] [CrossRef]
- Chang, H.H.; Murphy, C.J. Mini gold nanorods with tunable plasmonic peaks beyond 1000 nm. Chem. Mater. 2018, 30, 1427–1435. [Google Scholar] [CrossRef]
- Corbierre, M.K.; Cameron, N.S.; Sutton, M.; Laaziri, K.; Lennox, R. Gold nanoparticle/polymer nanocomposites: Dispersion of nanoparticles as a function of capping agent molecular weight and grafting density. Langmuir 2005, 21, 6063–6072. [Google Scholar] [CrossRef] [PubMed]
- Ni, S. Gold Nanocomposites: Synthesis and Applications. Ph.D. Dissertation, McGill University, Montreal, QC, Canada, 2021. [Google Scholar]
- Matsuyama, A.; Kato, T. Phase separations and orientational ordering of polymers in liquid crystal solvents. Phys. Rev. E 1999, 59, 763. [Google Scholar] [CrossRef]
- Matsuyama, A.; Kato, T. Induced nematic phase in a polymer/liquid crystal mixture. J. Chem. Phys. 2000, 112, 1046–1049. [Google Scholar] [CrossRef]
- Matsuyama, A. Conformational transitions of a semiflexible polymer in nematic solvents. Phys. Rev. E 2003, 67, 042701. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.M.; Gauza, S.; Xianyu, H.; Wu, S.T. Hysteresis effects in blue-phase liquid crystals. J. Display Technol. 2010, 6, 318–322. [Google Scholar] [CrossRef]
- Cordoyiannis, G.; Losada-Pérez, P.; Tripathi, C.S.P.; Rozic, B.; Tkalec, U.; Tzitzios, V.; Karatairi, E.; Nounesis, G.; Kutnjak, Z.; Musevic, I.; et al. Blue phase III widening in CE6-dispersed surface-functionalised CdSe nanoparticles. Liq. Cryst. 2010, 37, 1419–1426. [Google Scholar] [CrossRef]
- Wang, L.; He, W.; Wang, Q.; Yu, M.; Xiao, X.; Zhang, Y.; Ellahi, M.; Zhao, D.; Yang, H.; Guo, L. Polymer-stabilized nanoparticle-enriched blue phase liquid crystals. J. Mater. Chem. C 2013, 1, 6526–6531. [Google Scholar] [CrossRef]
- Wang, L.; He, W.; Xiao, X.; Meng, F.; Zhang, Y.; Yang, P.; Wang, L.; Xiao, J.; Yang, H.; Lu, Y. Hysteresis-free blue phase liquid-crystal-stabilized by ZnS nanoparticles. Small 2012, 8, 2189–2193. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, A.; Singh, G.; Biradar, A.M. Advances in gold nanoparticle–liquid crystal composites. Nanoscale 2014, 6, 7743–7756. [Google Scholar] [CrossRef] [PubMed]
- Schulz, F.; Friedrich, W.; Hoppe, K.; Vossmeyer, T.; Weller, H.; Lange, H. Effective PEGylation of gold nanorods. Nanoscale 2016, 8, 7296–7308. [Google Scholar] [CrossRef] [Green Version]
- Oton, E.; Netter, E.; Nakano, T.; D-Katayama, Y.; Inoue, F. Monodomain blue phase liquid crystal layers for phase modulation. Sci. Rep. 2017, 7, 44575. [Google Scholar] [CrossRef] [Green Version]








| Chemicals | Structure | Mass Percentage (%) |
|---|---|---|
| 4-hexylbenzoic acid (6BA) |  | 31.2 |
| trans-4-butylcyclohexanecarboxylic acid (4BCHA) |  | 32.5 |
| (S)-octan-2-yl 4-((4-(hexyloxy)benzoyl)oxy)benzoate (chiral dopant) |  | 36.2 |
| BP Mixture | Observed Phases | BP Temperature Ranges, °C | ||
|---|---|---|---|---|
| Cooling (ΔTBP) | Heating (ΔTBP) | |||
| 1:1 6BA:4CA 36 %wt chiral dopant (BPM36) | BP I, II and III, Ch | 54.5–61 (6.5) | 57.5–60 (6.5) | |
| BPM36 | 1 wt% PEO400 | BP I, II and III, Ch | 57.6–44.4 (13.1) | 52.5–58 (5.5) |
| BPM36 | 2 wt% PEO400 | Ch | 53 | |
| BPM36 | 1 wt% PEO700 | Ch | 57.9 | |
| BP Mixture wt% (vol%) AuNP | Observed Phases | BP Temperature Ranges | ||
|---|---|---|---|---|
| Cooling (ΔTBP) | Heating (ΔTBP) | |||
| BMP36 | BP I, II and III, Ch | 54.5–61 (6.5) | 57.5–60.6 (7.1) | |
| BMP36 | 1 wt% (0.39 vol%) Au-PEO300 | BP I, II and III, Ch | 52.5–58.5 (6) | 56.9–60 (3.1) |
| BMP36 | 5 wt% (2.0 vol%) Au-PEO300 | BP I, II and III, Ch | 50.8–57.8 (7) | 51.7–58 (6.3) |
| BMP36 | 10 wt% (4.2 vol%) Au-PEO300 | Ch | 56 | |
| BMP36 | 1 wt% (2.4 vol%) Au-PEO700 | BP I, II and III, Ch | 50.5–57.5 (7) | |
| BMP36 | 2 wt% (4.79 vol%) Au-PEO700 | BP I, II and III, Ch | 51–53.5 (2.5) | |
| BM36 | 5 wt% (11.49 vol%) Au-PEO700 | Ch | 52.8 | |
| Ligands | Pure BP Temperature Range | Mass Percentage of Gold in BP | Temperature Range |
|---|---|---|---|
| mPEG-SH 5K | 54.8–60.7 °C (5.9 °C) | 0.0692% | No blue phase formed |
| 0.0374% | 51.0–57.2 °C (6.2 °C) |
| BP Mixture | wt% AuNR | Observed Phases | BP Temperature Ranges Cooling (ΔTBP) |
|---|---|---|---|
| BMP36 | BP I, II and III, Ch | 48.5–55.8 (7.3) | |
| BMP36 | 0.13 wt% AuNR-PEO300 | BP I, II and III, Ch | <30.5–44.0 (>13.5) |
| BMP36 | 0.03 wt% AuNR-PEO300 | BP I, II and III, Ch | 40.8–49.7 (8.9) |
| BMP36 | 0.03 wt% AuNR-PEO2000 | BP I, II and III, Ch | 47.0–56.3 (9.3) 30.7–42.6 (11.9) 37.3–47.0 (9.7) |
| BMP36 | 0.07 wt% AuNR-PEO2000 | BP I, II and III, Ch | 31.1–40.6 (8.5) |
| BMP36 | 0.04 wt% AuNR-PEO5000 | BP I, II and III, Ch | 42.4–50.0 (7.6) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Lindner-D’Addario, M.; Roohnikan, M.; Toader, V.; Lennox, R.B.; Reven, L. Polymer Functionalized Nanoparticles in Blue Phase LC: Effect of Particle Shape. Nanomaterials 2022, 12, 91. https://doi.org/10.3390/nano12010091
Zhang M, Lindner-D’Addario M, Roohnikan M, Toader V, Lennox RB, Reven L. Polymer Functionalized Nanoparticles in Blue Phase LC: Effect of Particle Shape. Nanomaterials. 2022; 12(1):91. https://doi.org/10.3390/nano12010091
Chicago/Turabian StyleZhang, Manlin, Michael Lindner-D’Addario, Mahdi Roohnikan, Violeta Toader, Robert Bruce Lennox, and Linda Reven. 2022. "Polymer Functionalized Nanoparticles in Blue Phase LC: Effect of Particle Shape" Nanomaterials 12, no. 1: 91. https://doi.org/10.3390/nano12010091