Surface Coating-Modulated Phytotoxic Responses of Silver Nanoparticles in Plants and Freshwater Green Algae
Abstract
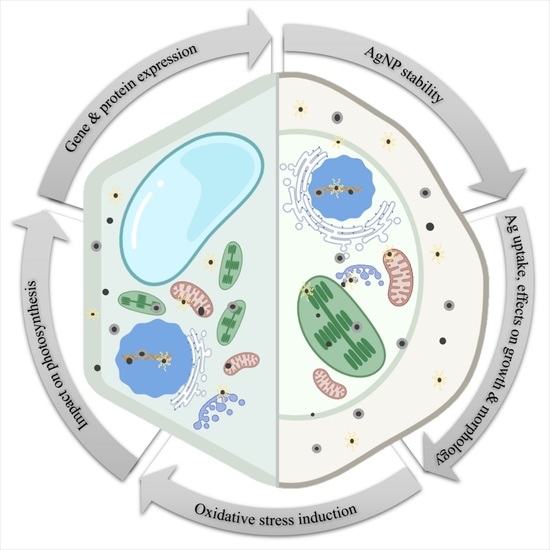
:1. Introduction
2. AgNP Stability in Various Exposure Media
3. Silver Uptake and Effects on Growth and Morphology
4. Oxidative Stress Induction and Mobilization of Antioxidant Machinery
5. Impact on Photosynthesis
6. Changes in Gene and Protein Expression
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Tkalec, M.; Peharec Štefanić, P.; Balen, B. Phytotoxicity of silver nanoparticles and defence mechanisms. Compr. Anal. Chem. 2019, 84, 145–198. [Google Scholar] [CrossRef]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The antibacterial mechanism of silver nanoparticles and its application in dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef] [Green Version]
- Ray, Paresh Chandra; Hongtao, Y.; Fu, P.P. Toxicity and environmental risks of nanomaterials: Challenges and future needs. Env. Sci Heal. C Env. Carcinog. Ecotoxicol. Rev. 2009, 27, 1–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smita, S.; Gupta, S.K.; Bartonova, A.; Dusinska, M.; Gutleb, A.C.; Rahman, Q. Nanoparticles in the environment: Assessment using the causal diagram approach. Environ. Heal. A Glob. Access Sci. Source 2012, 11, S13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bae, S.; Hwang, Y.S.; Lee, Y.-J.; Lee, S.-K. Effects of water chemistry on aggregation and soil adsorption of silver nanoparticles. Environ. Health Toxicol. 2013, 28, e2013006. [Google Scholar] [CrossRef]
- Behra, R.; Sigg, L.; Clift, M.J.D.; Herzog, F.; Minghetti, M.; Johnston, B.; Petri-Fink, A.; Rothen-Rutishauser, B. Bioavailability of silver nanoparticles and ions: From a chemical and biochemical perspective. J. R. Soc. Interface 2013, 10. [Google Scholar] [CrossRef] [PubMed]
- De Leersnyder, I.; De Gelder, L.; Van Driessche, I.; Vermeir, P. Revealing the importance of aging, environment, size and stabilization mechanisms on the stability of metal nanoparticles: A case study for silver nanoparticles in a minimally defined and complex undefined bacterial growth medium. Nanomaterials 2019, 9. [Google Scholar] [CrossRef] [Green Version]
- Levard, C.; Reinsch, B.C.; Michel, F.M.; Oumahi, C.; Lowry, G.V.; Brown, G.E. Sulfidation processes of PVP-coated silver nanoparticles in aqueous solution: Impact on dissolution rate. Environ. Sci. Technol. 2011, 45, 5260–5266. [Google Scholar] [CrossRef] [PubMed]
- Levard, C.; Hotze, E.M.; Lowry, G.V.; Brown, G.E. Environmental transformations of silver nanoparticles: Impact on stability and toxicity. Environ. Sci. Technol. 2012, 46, 6900–6914. [Google Scholar] [CrossRef]
- Yin, L.; Cheng, Y.; Espinasse, B.; Colman, B.P.; Auffan, M.; Wiesner, M.; Rose, J.; Liu, J.; Bernhardt, E.S. More than the ions: The effects of silver nanoparticles on Lolium multiflorum. Environ. Sci. Technol. 2011, 45, 2360–2367. [Google Scholar] [CrossRef]
- Sharma, V.K.; Siskova, K.M.; Zboril, R.; Gardea-Torresdey, J.L. Organic-coated silver nanoparticles in biological and environmental conditions: Fate, stability and toxicity. Adv. Colloid Interface Sci. 2014, 204, 15–34. [Google Scholar] [CrossRef]
- Tolaymat, T.M.; El Badawy, A.M.; Genaidy, A.; Scheckel, K.G.; Luxton, T.P.; Suidan, M. An evidence-based environmental perspective of manufactured silver nanoparticle in syntheses and applications: A systematic review and critical appraisal of peer-reviewed scientific papers. Sci. Total Environ. 2010, 408, 999–1006. [Google Scholar] [CrossRef] [Green Version]
- Biba, R.; Peharec Štefanić, P.; Cvjetko, P.; Tkalec, M.; Balen, B. Silver nanoparticles phytotoxicity mechanisms. In Nanobiotechnology for Plant Protection; Silver Nanomaterials for Agri-Food Applications; Kamel, A.A.-E., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 317–356. ISBN 9780128235287. [Google Scholar]
- Wang, S.; Lv, J.; Ma, J.; Zhang, S. Cellular internalization and intracellular biotransformation of silver nanoparticles in Chlamydomonas reinhardtii. Nanotoxicology 2016, 10, 1129–1135. [Google Scholar] [CrossRef]
- Navarro, E.; Baun, A.; Behra, R.; Hartmann, N.B.; Filser, J.; Miao, A.J.; Quigg, A.; Santschi, P.H.; Sigg, L. Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology 2008, 17, 372–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piccapietra, F.; Allué, C.G.; Sigg, L.; Behra, R. Intracellular silver accumulation in Chlamydomonas reinhardtii upon exposure to carbonate coated silver nanoparticles and silver nitrate. Environ. Sci. Technol. 2012, 46, 7390–7397. [Google Scholar] [CrossRef]
- Dewez, D.; Oukarroum, A. Silver nanoparticles toxicity effect on photosystem II photochemistry of the green alga Chlamydomonas reinhardtii treated in light and dark conditions. Toxicol. Environ. Chem. 2012, 94, 1536–1546. [Google Scholar] [CrossRef]
- Oukarroum, A.; Bras, S.; Perreault, F.; Popovic, R. Inhibitory effects of silver nanoparticles in two green algae, Chlorella vulgaris and Dunaliella tertiolecta. Ecotoxicol. Environ. Saf. 2012, 78, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Matorin, D.N.; Todorenko, D.A.; Seifullina, N.K.; Zayadan, B.K.; Rubin, A.B. Effect of silver nanoparticles on the parameters of chlorophyll fluorescence and P700 reaction in the green alga Chlamydomonas reinhardtii. Microbiology 2013, 82, 809–814. [Google Scholar] [CrossRef]
- Navarro, E.; Wagner, B.; Odzak, N.; Sigg, L.; Behra, R. Effects of differently coated silver nanoparticles on the photosynthesis of Chlamydomonas reinhardtii. Environ. Sci. Technol. 2015, 49, 8041–8047. [Google Scholar] [CrossRef] [Green Version]
- Qian, H.; Zhu, K.; Lu, H.; Lavoie, M.; Chen, S.; Zhou, Z.; Deng, Z.; Chen, J.; Fu, Z. Contrasting silver nanoparticle toxicity and detoxification strategies in Microcystis aeruginosa and Chlorella vulgaris: New insights from proteomic and physiological analyses. Sci. Total Environ. 2016, 572, 1213–1221. [Google Scholar] [CrossRef]
- Romero, N.; Visentini, F.F.; Márquez, V.E.; Santiago, L.G.; Castro, G.R.; Gagneten, A.M. Physiological and morphological responses of green microalgae Chlorella vulgaris to silver nanoparticles. Environ. Res. 2020, 189. [Google Scholar] [CrossRef]
- Wang, Z.; Quik, J.T.K.; Song, L.; Van Den Brandhof, E.J.; Wouterse, M.; Peijnenburg, W.J.G.M. Humic substances alleviate the aquatic toxicity of polyvinylpyrrolidone-coated silver nanoparticles to organisms of different trophic levels. Environ. Toxicol. Chem. 2015, 34, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Kleiven, M.; Macken, A.; Oughton, D.H. Growth inhibition in Raphidocelis subcapita – evidence of nanospecific toxicity of silver nanoparticles. Chemosphere 2019, 221, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Lekamge, S.; Miranda, A.F.; Abraham, A.; Ball, A.S.; Shukla, R.; Nugegoda, D. The toxicity of coated silver nanoparticles to the alga Raphidocelis subcapitata. SN Appl. Sci. 2020, 2, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Pham, T.L. Effect of silver nanoparticles on tropical freshwater and marine microalgae. J. Chem. 2019, 2019. [Google Scholar] [CrossRef]
- Dash, A.; Singh, A.P.; Chaudhary, B.R.; Singh, S.K.; Dash, D. Effect of silver nanoparticles on growth of eukaryotic green algae. Nano-Micro Lett. 2012, 4, 158–165. [Google Scholar] [CrossRef] [Green Version]
- Lindgren, A.L. The effects of silver nitrate and silver nanoparticles on Chlamydomonas reinhardtii: A proteomic approach. Master’s Thesis, University of Gothenburg, Gothenburg, Sweden, 2014. [Google Scholar]
- Zhang, J.; Shen, L.; Xiang, Q.; Ling, J.; Zhou, C.; Hu, J.; Chen, L. Proteomics reveals surface electrical property-dependent toxic mechanisms of silver nanoparticles in Chlorella vulgaris. Environ. Pollut. 2020, 265, 114743. [Google Scholar] [CrossRef]
- Leclerc, S.; Wilkinson, K.J. Bioaccumulation of nanosilver by Chlamydomonas reinhardtii—Nanoparticle or the free ion? Environ. Sci. Technol. 2014, 48, 358–364. [Google Scholar] [CrossRef]
- Zhao, Z.; Xu, L.; Wang, Y.; Li, B.; Zhang, W.; Li, X. Toxicity mechanism of silver nanoparticles to Chlamydomonas reinhardtii: Photosynthesis, oxidative stress, membrane permeability, and ultrastructure analysis. Environ. Sci. Pollut. Res. 2020, 28, 15032–15042. [Google Scholar] [CrossRef]
- Oukarroum, A.; Polchtchikov, S.; Perreault, F.; Popovic, R. Temperature influence on silver nanoparticles inhibitory effect on photosystem II photochemistry in two green algae, Chlorella vulgaris and Dunaliella tertiolecta. Environ. Sci. Pollut. Res. 2012, 19, 1755–1762. [Google Scholar] [CrossRef]
- Kalman, J.; Paul, K.B.; Khan, F.R.; Stone, V.; Fernandes, T.F. Characterisation of bioaccumulation dynamics of three differently coated silver nanoparticles and aqueous silver in a simple freshwater food chain. Environ. Chem. 2015, 12, 662–672. [Google Scholar] [CrossRef] [Green Version]
- Hazeem, L.J.; Kuku, G.; Dewailly, E.; Slomianny, C.; Barras, A.; Hamdi, A.; Boukherroub, R.; Culha, M.; Bououdina, M. Toxicity effect of silver nanoparticles on photosynthetic pigment content, growth, ROS production and ultrastructural changes of microalgae Chlorella vulgaris. Nanomaterials 2019, 9. [Google Scholar] [CrossRef] [Green Version]
- Mariano, S.; Panzarini, E.; Inverno, M.D.; Voulvoulis, N.; Dini, L. Toxicity, bioaccumulation and biotransformation of glucose-capped silver nanoparticles in green microalgae Chlorella vulgaris. Nanomaterials 2020, 10, 1377. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.J.; Hull, M.S.; Bednar, A.J.; Goss, J.D.; Gunter, J.C.; Bouldin, J.L.; Vikesland, P.J.; Steevens, J.A. Fractionating nanosilver: Importance for determining toxicity to aquatic test organisms. Environ. Sci. Technol. 2010, 44, 9571–9577. [Google Scholar] [CrossRef] [PubMed]
- Angel, B.M.; Batley, G.E.; Jarolimek, C.V.; Rogers, N.J. The impact of size on the fate and toxicity of nanoparticulate silver in aquatic systems. Chemosphere 2013, 93, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.J.; Yin, Y.G.; Liu, J.F. Silver nanoparticles in the environment. Environ. Sci. Process. Impacts 2013, 15, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Schirmer, K.; Bernard, L.; Sigg, L.; Pillai, S.; Behra, R. Silver nanoparticle toxicity and association with the alga Euglena gracilis. Environ. Sci. Nano 2015, 2, 594–602. [Google Scholar] [CrossRef]
- Yue, Y.; Li, X.; Sigg, L.; Suter, M.J.F.; Pillai, S.; Behra, R.; Schirmer, K. Interaction of silver nanoparticles with algae and fish cells: A side by side comparison. J. Nanobiotechnol. 2017, 15, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, K.; Hu, Y.; Zhang, L.; Yang, K.; Lin, D. The role of exopolymeric substances in the bioaccumulation and toxicity of Ag nanoparticles to algae. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Koczkur, K.M.; Mourdikoudis, S.; Polavarapu, L.; Skrabalak, S.E. Polyvinylpyrrolidone (PVP) in nanoparticle synthesis. Dalt. Trans. 2015, 44, 17883–17905. [Google Scholar] [CrossRef] [Green Version]
- Yu, W.; Xie, H. A review on nanofluids: Preparation, stability mechanisms, and applications. J. Nanomater. 2012, 2012. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.L.; Jiang, H.S.; Gu, S.P.; Zhou, X.H.; Lu, Z.W.; Kang, X.H.; Yin, L.; Huang, J. Combination analysis of the physiology and transcriptome provides insights into the mechanism of silver nanoparticles phytotoxicity. Environ. Pollut. 2019, 252, 1539–1549. [Google Scholar] [CrossRef]
- Vannini, C.; Domingo, G.; Onelli, E.; De Mattia, F.; Bruni, I.; Marsoni, M.; Bracale, M. Phytotoxic and genotoxic effects of silver nanoparticles exposure on germinating wheat seedlings. J. Plant Physiol. 2014, 171, 1142–1148. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.S.; Yin, L.Y.; Ren, N.N.; Zhao, S.T.; Li, Z.; Zhi, Y.; Shao, H.; Li, W.; Gontero, B. Silver nanoparticles induced reactive oxygen species via photosynthetic energy transport imbalance in an aquatic plant. Nanotoxicology 2017, 11, 157–167. [Google Scholar] [CrossRef]
- Cvjetko, P.; Zovko, M.; Štefanić, P.P.; Biba, R.; Tkalec, M.; Domijan, A.M.; Vrček, I.V.; Letofsky-Papst, I.; Šikić, S.; Balen, B. Phytotoxic effects of silver nanoparticles in tobacco plants. Environ. Sci. Pollut. Res. 2018, 25, 5590–5602. [Google Scholar] [CrossRef]
- Noori, A.; Donnelly, T.; Colbert, J.; Cai, W.; Newman, L.A.; White, J.C. Exposure of tomato (Lycopersicon esculentum) to silver nanoparticles and silver nitrate: Physiological and molecular response. Int. J. Phytoremediation 2019, 22, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Falco, W.F.; Scherer, M.D.; Oliveira, S.L.; Wender, H.; Colbeck, I.; Lawson, T.; Caires, A.R.L. Phytotoxicity of silver nanoparticles on Vicia faba: Evaluation of particle size effects on photosynthetic performance and leaf gas exchange. Sci. Total Environ. 2020, 701, 134816. [Google Scholar] [CrossRef] [PubMed]
- Biba, R.; Matić, D.; Lyons, D.M.; Štefanić, P.P.; Cvjetko, P.; Tkalec, M.; Pavoković, D.; Letofsky-Papst, I.; Balen, B. Coating-dependent effects of silver nanoparticles on tobacco seed germination and early growth. Int. J. Mol. Sci. 2020, 21. [Google Scholar] [CrossRef] [PubMed]
- Peharec Štefanić, P.; Košpić, K.; Lyons, D.M.; Jurković, L.; Balen, B.; Tkalec, M. Phytotoxicity of silver nanoparticles on tobacco plants: Evaluation of coating effects on photosynthetic performance and chloroplast ultrastructure. Nanomaterials 2021, 11, 744. [Google Scholar] [CrossRef]
- Wang, J.; Koo, Y.; Alexander, A.; Yang, Y.; Westerhof, S.; Zhang, Q.; Schnoor, J.L.; Colvin, V.L.; Braam, J.; Alvarez, P.J.J. Phytostimulation of poplars and Arabidopsis exposed to silver nanoparticles and Ag+ at sublethal concentrations. Environ. Sci. Technol. 2013, 47, 5442–5449. [Google Scholar] [CrossRef] [PubMed]
- Abdelsalam, N.R.; Abdel-Megeed, A.; Ali, H.M.; Salem, M.Z.M.; Al-Hayali, M.F.A.; Elshikh, M.S. Genotoxicity effects of silver nanoparticles on wheat (Triticum aestivum L.) root tip cells. Ecotoxicol. Environ. Saf. 2018, 155, 76–85. [Google Scholar] [CrossRef]
- Das, P.; Barua, S.; Sarkar, S.; Chatterjee, S.K.; Mukherjee, S.; Goswami, L.; Das, S.; Bhattacharya, S.; Karak, N.; Bhattacharya, S.S. Mechanism of toxicity and transformation of silver nanoparticles: Inclusive assessment in earthworm-microbe-soil-plant system. Geoderma 2018, 314, 73–84. [Google Scholar] [CrossRef]
- Mirzajani, F.; Askari, H.; Hamzelou, S.; Farzaneh, M.; Ghassempour, A. Effect of silver nanoparticles on Oryza sativa L. and its rhizosphere bacteria. Ecotoxicol. Environ. Saf. 2013, 88, 48–54. [Google Scholar] [CrossRef]
- Mirzajani, F.; Askari, H.; Hamzelou, S.; Schober, Y.; Römpp, A.; Ghassempour, A.; Spengler, B. Proteomics study of silver nanoparticles toxicity on Oryza sativa L. Ecotoxicol. Environ. Saf. 2014, 108, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Lalau, C.M.; Simioni, C.; Vicentini, D.S.; Ouriques, L.C.; Mohedano, R.A.; Puerari, R.C.; Matias, W.G. Toxicological effects of AgNPs on duckweed (Landoltia punctata). Sci. Total Environ. 2020, 710, 136318. [Google Scholar] [CrossRef]
- Jiang, H.S.; Li, M.; Chang, F.Y.; Li, W.; Yin, L.Y. Physiological analysis of silver nanoparticles and AgNO3 toxicity to Spirodela polyrhiza. Environ. Toxicol. Chem. 2012, 31, 1880–1886. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.; Yang, J.; Zhang, Y.; Fang, Y.; Nishinari, K.; Phillips, G.O. Synthesis and antioxidant properties of gum arabic-stabilized selenium nanoparticles. Int. J. Biol. Macromol. 2014, 65, 155–162. [Google Scholar] [CrossRef]
- Biba, R.; Tkalec, M.; Cvjetko, P.; Štefanić, P.P.; Šikić, S.; Pavoković, D.; Balen, B. Silver nanoparticles affect germination and photosynthesis in tobacco seedlings. Acta Bot. Croat. 2021, 80, 1–11. [Google Scholar] [CrossRef]
- Peharec Štefanić, P.; Cvjetko, P.; Biba, R.; Domijan, A.M.; Letofsky-Papst, I.; Tkalec, M.; Šikić, S.; Cindrić, M.; Balen, B. Physiological, ultrastructural and proteomic responses of tobacco seedlings exposed to silver nanoparticles and silver nitrate. Chemosphere 2018, 209, 640–653. [Google Scholar] [CrossRef]
- Gubbins, E.J.; Batty, L.C.; Lead, J.R. Phytotoxicity of silver nanoparticles to Lemna minor L. Environ. Pollut. 2011, 159, 1551–1559. [Google Scholar] [CrossRef]
- Abdel-Aziz, H.M.M.; Rizwan, M. Chemically synthesized silver nanoparticles induced physio-chemical and chloroplast ultrastructural changes in broad bean seedlings. Chemosphere 2019, 235, 1066–1072. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Niu, J.; Chen, Y. Surface-coating-dependent dissolution, aggregation, and reactive oxygen species (ROS) generation of silver nanoparticles under different irradiation conditions. Environ. Sci. Technol. 2013, 47, 10293–10301. [Google Scholar] [CrossRef] [PubMed]
- Cvjetko, P.; Milošić, A.; Domijan, A.M.; Vinković Vrček, I.; Tolić, S.; Peharec Štefanić, P.; Letofsky-Papst, I.; Tkalec, M.; Balen, B. Toxicity of silver ions and differently coated silver nanoparticles in Allium cepa roots. Ecotoxicol. Environ. Saf. 2017, 137, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Barabanov, P.V.; Gerasimov, A.V.; Blinov, A.V.; Kravtsov, A.A.; Kravtsov, V.A. Influence of nanosilver on the efficiency of Pisum sativum crops germination. Ecotoxicol. Environ. Saf. 2018, 147, 715–719. [Google Scholar] [CrossRef]
- Gusev, A.A.; Kudrinsky, A.A.; Zakharova, O.V.; Klimov, A.I.; Zherebin, P.M.; Lisichkin, G.V.; Vasyukova, I.A.; Denisov, A.N.; Krutyakov, Y.A. Versatile synthesis of PHMB-stabilized silver nanoparticles and their significant stimulating effect on fodder beet (Beta vulgaris L.). Mater. Sci. Eng. C 2016, 62, 152–159. [Google Scholar] [CrossRef]
- Zhao, C.M.; Wang, W.X. Importance of surface coatings and soluble silver in silver nanoparticles toxicity to Daphnia magna. Nanotoxicology 2012, 6, 361–370. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, Q.F.; Liu, J.Y.; Fu, J.J.; Liu, S.J.; Jiang, G. Bin Environmental and biological influences on the stability of silver nanoparticles. Chinese Sci. Bull. 2011, 56, 2009–2015. [Google Scholar] [CrossRef] [Green Version]
- Argentiere, S.; Cella, C.; Cesaria, M.; Milani, P.; Lenardi, C. Silver nanoparticles in complex biological media: Assessment of colloidal stability and protein corona formation. J. Nanoparticle Res. 2016, 18. [Google Scholar] [CrossRef]
- Pem, B.; Ćurlin, M.; Jurašin, D.D.; Vrček, V.; Barbir, R.; Micek, V.; Fratila, R.M.; de la Fuente, J.M.; Vrček, I.V. Fate and transformation of silver nanoparticles in different biological conditions. Beilstein J. Nanotechnol. 2021, 12, 665–679. [Google Scholar] [CrossRef] [PubMed]
- MacCuspie, R.I. Colloidal stability of silver nanoparticles in biologically relevant conditions. J. Nanoparticle Res. 2011, 13, 2893–2908. [Google Scholar] [CrossRef]
- Akter, M.; Sikder, M.T.; Rahman, M.M.; Ullah, A.K.M.A.; Hossain, K.F.B.; Banik, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. A systematic review on silver nanoparticles-induced cytotoxicity: Physicochemical properties and perspectives. J. Adv. Res. 2018, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, D.K.; Tripathi, A.; Shweta; Singh, S.; Singh, Y.; Vishwakarma, K.; Yadav, G.; Sharma, S.; Singh, V.K.; Mishra, R.K.; et al. Uptake, accumulation and toxicity of silver nanoparticle in autotrophic plants, and heterotrophic microbes: A concentric review. Front. Microbiol. 2017, 8, 1–16. [Google Scholar] [CrossRef]
- Tejamaya, M.; Römer, I.; Merrifield, R.C.; Lead, J.R. Stability of citrate, PVP, and PEG coated silver nanoparticles in ecotoxicology media. Environ. Sci. Technol. 2012, 46, 7011–7017. [Google Scholar] [CrossRef] [PubMed]
- Azodi, M.; Sultan, Y.; Ghoshal, S. Dissolution behavior of silver nanoparticles and formation of secondary silver nanoparticles in municipal wastewater by single-particle ICP-MS. Environ. Sci. Technol. 2016, 50, 13318–13327. [Google Scholar] [CrossRef] [PubMed]
- Reidy, B.; Haase, A.; Luch, A.; Dawson, K.A.; Lynch, I. Mechanisms of silver nanoparticle release, transformation and toxicity: A critical review of current knowledge and recommendations for future studies and applications. Materials 2013, 6, 2295–2350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muraleetharan, V.; Mantaj, J.; Swedrowska, M.; Vllasaliu, D. Nanoparticle modification in biological media: Implications for oral nanomedicines. RSC Adv. 2019, 9, 40487–40497. [Google Scholar] [CrossRef] [Green Version]
- Thwala, M.; Musee, N.; Sikhwivhilu, L.; Wepener, V. The oxidative toxicity of Ag and ZnO nanoparticles towards the aquatic plant Spirodela punctuta and the role of testing media parameters. Environ. Sci. Process. Impacts 2013, 15, 1830–1843. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.M.G.; Chung, I.M. Assessment of silver nanoparticle-induced physiological and molecular changes in Arabidopsis thaliana. Environ. Sci. Pollut. Res. 2014, 21, 8858–8869. [Google Scholar] [CrossRef] [PubMed]
- Dimkpa, C.O.; McLean, J.E.; Martineau, N.; Britt, D.W.; Haverkamp, R.; Anderson, A.J. Silver nanoparticles disrupt wheat (Triticum aestivum L.) growth in a sand matrix. Environ. Sci. Technol. 2013, 47, 1082–1090. [Google Scholar] [CrossRef]
- Patlolla, A.K.; Berry, A.; May, L.; Tchounwou, P.B. Genotoxicity of silver nanoparticles in Vicia faba: A pilot study on the environmental monitoring of nanoparticles. Int. J. Environ. Res. Public Health 2012, 9, 1649–1662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrena, R.; Casals, E.; Colón, J.; Font, X.; Sánchez, A.; Puntes, V. Evaluation of the ecotoxicity of model nanoparticles. Chemosphere 2009, 75, 850–857. [Google Scholar] [CrossRef] [Green Version]
- Ju-Nam, Y.; Lead, J.R. Manufactured nanoparticles: An overview of their chemistry, interactions and potential environmental implications. Sci. Total Environ. 2008, 400, 396–414. [Google Scholar] [CrossRef] [PubMed]
- Kvítek, L.; Panáček, A.; Soukupová, J.; Kolář, M.; Večeřová, R.; Prucek, R.; Holecová, M.; Zbořil, R. Effect of surfactants and polymers on stability and antibacterial activity of silver nanoparticles (NPs). J. Phys. Chem. C 2008, 112, 5825–5834. [Google Scholar] [CrossRef]
- Cartwright, A.; Jackson, K.; Morgan, C.; Anderson, A.; Britt, D.W. A review of metal and metal-oxide nanoparticle coating technologies to inhibit agglomeration and increase bioactivity for agricultural applications. Agronomy 2020, 10, 1018. [Google Scholar] [CrossRef]
- Schubert, J.; Chanana, M. Coating matters: Review on colloidal stability of nanoparticles with biocompatible coatings in biological media, living cells and organisms. Curr. Med. Chem. 2018, 25, 4553–4586. [Google Scholar] [CrossRef] [PubMed]
- Pokhrel, L.R.; Dubey, B. Evaluation of developmental responses of two crop plants exposed to silver and zinc oxide nanoparticles. Sci. Total Environ. 2013, 452–453, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Peharec Štefanić, P.; Jarnević, M.; Cvjetko, P.; Biba, R.; Šikić, S.; Tkalec, M.; Cindrić, M.; Letofsky-Papst, I.; Balen, B. Comparative proteomic study of phytotoxic effects of silver nanoparticles and silver ions on tobacco plants. Environ. Sci. Pollut. Res. 2019, 26, 22529–22550. [Google Scholar] [CrossRef] [PubMed]
- Ke, M.; Qu, Q.; Peijnenburg, W.J.G.M.; Li, X.; Zhang, M.; Zhang, Z.; Lu, T.; Pan, X.; Qian, H. Phytotoxic effects of silver nanoparticles and silver ions to Arabidopsis thaliana as revealed by analysis of molecular responses and of metabolic pathways. Sci. Total Environ. 2018, 644, 1070–1079. [Google Scholar] [CrossRef] [PubMed]
- Geisler-Lee, J.; Wang, Q.; Yao, Y.; Zhang, W.; Geisler, M.; Li, K.; Huang, Y.; Chen, Y.; Kolmakov, A.; Ma, X. Phytotoxicity, accumulation and transport of silver nanoparticles by Arabidopsis thaliana. Nanotoxicology 2013, 7, 323–337. [Google Scholar] [CrossRef]
- Li, X.; Ke, M.; Zhang, M.; Peijnenburg, W.J.G.M.; Fan, X.; Xu, J.; Zhang, Z.; Lu, T.; Fu, Z.; Qian, H. The interactive effects of diclofop-methyl and silver nanoparticles on Arabidopsis thaliana: Growth, photosynthesis and antioxidant system. Environ. Pollut. 2017, 232, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lenhart, J.J.; Walker, H.W. Dissolution-accompanied aggregation kinetics of silver nanoparticles. Langmuir 2010, 26, 16690–16698. [Google Scholar] [CrossRef] [PubMed]
- Saleeb, N.; Gooneratne, R.; Cavanagh, J.; Bunt, C.; Hossain, A.K.M.M.; Gaw, S.; Robinson, B. The mobility of silver nanoparticles and silver ions in the soil-plant system. J. Environ. Qual. 2019, 48, 1835–1841. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.M.; Kwak, J., II; An, Y.J. Effect of silver nanoparticles in crop plants Phaseolus radiatus and Sorghum bicolor: Media effect on phytotoxicity. Chemosphere 2012, 86, 491–499. [Google Scholar] [CrossRef]
- Fernando, I.; Zhou, Y. Impact of pH on the stability, dissolution and aggregation kinetics of silver nanoparticles. Chemosphere 2019, 216, 297–305. [Google Scholar] [CrossRef]
- Moore, T.L.; Rodriguez-Lorenzo, L.; Hirsch, V.; Balog, S.; Urban, D.; Jud, C.; Rothen-Rutishauser, B.; Lattuada, M.; Petri-Fink, A. Nanoparticle colloidal stability in cell culture media and impact on cellular interactions. Chem. Soc. Rev. 2015, 44, 6287–6305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mogoşanu, G.D.; Grumezescu, A.M.; Bejenaru, C.; Bejenaru, L.E. Polymeric protective agents for nanoparticles in drug delivery and targeting. Int. J. Pharm. 2016, 510, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Stegemeier, J.P.; Colman, B.P.; Schwab, F.; Wiesner, M.R.; Lowry, G.V. Uptake and distribution of silver in the aquatic plant Landoltia punctata (duckweed) exposed to silver and silver sulfide nanoparticles. Environ. Sci. Technol. 2017, 51, 4936–4943. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.S.; Qiu, X.N.; Li, G.B.; Li, W.; Yin, L.Y. Silver nanoparticles induced accumulation of reactive oxygen species and alteration of antioxidant systems in the aquatic plant Spirodela polyrhiza. Environ. Toxicol. Chem. 2014, 33, 1398–1405. [Google Scholar] [CrossRef]
- Yang, Q.; Shan, W.; Hu, L.; Zhao, Y.; Hou, Y.; Yin, Y.; Liang, Y.; Wang, F.; Cai, Y.; Liu, J.; et al. Uptake and transformation of silver nanoparticles and ions by rice plants revealed by dual stable isotope tracing. Environ. Sci. Technol. 2019, 53, 625–633. [Google Scholar] [CrossRef]
- Yang, Q.; Xu, W.; Liu, G.; Song, M.; Tan, Z.; Mao, Y.; Yin, Y.; Cai, Y.; Liu, J.; Jiang, G. Transformation and uptake of silver nanoparticles and silver ions in rice plant (Oryza sativa L.): The effect of iron plaque and dissolved iron. Environ. Sci. Nano 2020, 7, 599–609. [Google Scholar] [CrossRef]
- Lv, J.; Christie, P.; Zhang, S. Uptake, translocation, and transformation of metal-based nanoparticles in plants: Recent advances and methodological challenges. Environ. Sci. Nano 2019, 6, 41–59. [Google Scholar] [CrossRef]
- Wheeler, K.E.; Chetwynd, A.J.; Fahy, K.M.; Hong, B.S.; Tochihuitl, J.A.; Foster, L.A.; Lynch, I. Environmental dimensions of the protein corona. Nat. Nanotechnol. 2021, 16, 617–629. [Google Scholar] [CrossRef]
- Szőllősi, R.; Molnár, Á.; Kondak, S.; Kolbert, Z. Dual effect of nanomaterials on germination and seedling growth: Stimulation vs. phytotoxicity. Plants 2020, 9, 1745. [Google Scholar] [CrossRef] [PubMed]
- Tymoszuk, A. Silver nanoparticles effects on in vitro germination, growth, and biochemical activity of tomato, radish, and kale seedlings. Materials 2021, 14. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, I.; Buzea, C. Nanoparticle interaction with plants. In Nanoscience and Plant-Soil Systems. Soil Biology, Vol. 48; Ghorbanpour, M., Manika, K., Varma, A., Eds.; Springer: Berlin, Germany, 2017; pp. 323–355. ISBN 9783319468358. [Google Scholar]
- Navarro, E.; Piccapietra, F.; Wagner, B.; Marconi, F.; Kaegi, R.; Odzak, N.; Sigg, L.; Behra, R. Toxicity of silver nanoparticles to Chlamydomonas reinhardtii. Environ. Sci. Technol. 2008, 42, 8959–8964. [Google Scholar] [CrossRef]
- Fabrega, J.; Luoma, S.N.; Tyler, C.R.; Galloway, T.S.; Lead, J.R. Silver nanoparticles: Behaviour and effects in the aquatic environment. Environ. Int. 2011, 37, 517–531. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Geiser-Lee, J.; Deng, Y.; Kolmakov, A. Interactions between engineered nanoparticles (ENPs) and plants: Phytotoxicity, uptake and accumulation. Sci. Total Environ. 2010, 408, 3053–3061. [Google Scholar] [CrossRef]
- Yan, A.; Chen, Z. Impacts of silver nanoparticles on plants: A focus on the phytotoxicity and underlying mechanism. Int. J. Mol. Sci. 2019, 20, 1003. [Google Scholar] [CrossRef]
- Prażak, R.; Święciło, A.; Krzepiłko, A.; Michałek, S.; Arczewska, M. Impact of Ag nanoparticles on seed germination and seedling growth of green beans in normal and chill temperatures. Agriculture 2020, 10, 312. [Google Scholar] [CrossRef]
- Song, U.; Jun, H.; Waldman, B.; Roh, J.; Kim, Y.; Yi, J.; Lee, E.J. Functional analyses of nanoparticle toxicity: A comparative study of the effects of TiO2 and Ag on tomatoes (Lycopersicon esculentum). Ecotoxicol. Environ. Saf. 2013, 93, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Geisler-Lee, J.; Brooks, M.; Gerfen, J.; Wang, Q.; Fotis, C.; Sparer, A.; Ma, X.; Berg, R.; Geisler, M. Reproductive toxicity and life history study of silver nanoparticle effect, uptake and transport in Arabidopsis thaliana. Nanomaterials 2014, 4, 301–318. [Google Scholar] [CrossRef] [Green Version]
- Scherer, M.D.; Sposito, J.C.V.; Falco, W.F.; Grisolia, A.B.; Andrade, L.H.C.; Lima, S.M.; Machado, G.; Nascimento, V.A.; Gonçalves, D.A.; Wender, H.; et al. Cytotoxic and genotoxic effects of silver nanoparticles on meristematic cells of Allium cepa roots: A close analysis of particle size dependence. Sci. Total Environ. 2019, 660, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Vannini, C.; Domingo, G.; Onelli, E.; Prinsi, B.; Marsoni, M.; Espen, L.; Bracale, M. Morphological and proteomic responses of Eruca sativa exposed to silver nanoparticles or silver nitrate. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasur, J.; Rani, P.U. Environmental effects of nanosilver: Impact on castor seed germination, seedling growth, and plant physiology. Environ. Sci. Pollut. Res. 2013, 20, 8636–8648. [Google Scholar] [CrossRef]
- Wang, L.; Sun, J.; Lin, L.; Fu, Y.; Alenius, H.; Lindsey, K.; Chen, C. Silver nanoparticles regulate Arabidopsis root growth by concentration-dependent modification of reactive oxygen species accumulation and cell division. Ecotoxicol. Environ. Saf. 2020, 190, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Colman, B.P.; McGill, B.M.; Wright, J.P.; Bernhardt, E.S. Effects of silver nanoparticle exposure on germination and early growth of eleven wetland plants. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [Green Version]
- Pereira, S.P.P.; Jesus, F.; Aguiar, S.; de Oliveira, R.; Fernandes, M.; Ranville, J.; Nogueira, A.J.A. Phytotoxicity of silver nanoparticles to Lemna minor: Surface coating and exposure period-related effects. Sci. Total Environ. 2018, 618, 1389–1399. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Tang, H.; Deng, Z.; Liu, Y.; Chen, X.; Wang, H. Ag nanoparticles inhibit the growth of the bryophyte, Physcomitrella patens. Ecotoxicol. Environ. Saf. 2018, 164, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Guan, W.; Xu, L.; Ding, Z.; Ma, H.; Ma, A.; Terry, N. Effects of nanoparticles on algae: Adsorption, distribution, ecotoxicity and fate. Appl. Sci. 2019, 9, 1534. [Google Scholar] [CrossRef] [Green Version]
- Xia, B.; Chen, B.; Sun, X.; Qu, K.; Ma, F.; Du, M. Interaction of TiO2 nanoparticles with the marine microalga Nitzschia closterium: Growth inhibition, oxidative stress and internalization. Sci. Total Environ. 2015, 508, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Fang, J.; Cheng, H. Toxicity of silver nanoparticles to green algae M. aeruginosa and alleviation by organic matter. Environ. Monit. Assess. 2018, 190. [Google Scholar] [CrossRef]
- Miao, A.J.; Schwehr, K.A.; Xu, C.; Zhang, S.J.; Luo, Z.; Quigg, A.; Santschi, P.H. The algal toxicity of silver engineered nanoparticles and detoxification by exopolymeric substances. Environ. Pollut. 2009, 157, 3034–3041. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, F.; Gallego-Urrea, J.A.; Goodhead, R.M.; Van Gestel, C.A.M.; Moger, J.; Soares, A.M.V.M.; Loureiro, S. Uptake and elimination kinetics of silver nanoparticles and silver nitrate by Raphidocelis subcapitata: The influence of silver behaviour in solution. Nanotoxicology 2015, 9, 686–695. [Google Scholar] [CrossRef] [PubMed]
- Peulen, T.O.; Wilkinson, K.J. Diffusion of nanoparticles in a biofilm. Environ. Sci. Technol. 2011, 45, 3367–3373. [Google Scholar] [CrossRef] [PubMed]
- Flores-López, L.Z.; Espinoza-Gómez, H.; Somanathan, R. Silver nanoparticles: Electron transfer, reactive oxygen species, oxidative stress, beneficial and toxicological effects. Mini review. J. Appl. Toxicol. 2019, 39, 16–26. [Google Scholar] [CrossRef] [Green Version]
- Dayem, A.A.; Hossain, M.K.; Lee, S.B.; Kim, K.; Saha, S.K.; Yang, G.M.; Choi, H.Y.; Cho, S.G. The role of reactive oxygen species (ROS) in the biological activities of metallic nanoparticles. Int. J. Mol. Sci. 2017, 18, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khanna, P.; Ong, C.; Bay, B.H.; Baeg, G.H. Nanotoxicity: An interplay of oxidative stress, inflammation and cell death. Nanomaterials 2015, 5, 1163–1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallejo, M.J.; Salazar, L.; Grijalva, M. Oxidative stress modulation and ROS-mediated toxicity in cancer: A review on in vitro models for plant-derived compounds. Oxid. Med. Cell. Longev. 2017, 2017. [Google Scholar] [CrossRef] [Green Version]
- Wijnhoven, S.W.P.; Peijnenburg, W.J.G.M.; Herberts, C.A.; Hagens, W.I.; Oomen, A.G.; Heugens, E.H.W.; Roszek, B.; Bisschops, J.; Gosens, I.; Van De Meent, D.; et al. Nano-silver—A review of available data and knowledge gaps in human and environmental risk assessment. Nanotoxicology 2009, 3, 109–138. [Google Scholar] [CrossRef]
- McShan, D.; Ray, P.C.; Yu, H. Molecular toxicity mechanism of nanosilver. J. Food Drug Anal. 2014, 22, 116–127. [Google Scholar] [CrossRef] [Green Version]
- Panáček, A.; Kvítek, L.; Prucek, R.; Kolář, M.; Večeřová, R.; Pizúrová, N.; Sharma, V.K.; Nevěčná, T.; Zbořil, R. Silver colloid nanoparticles: Synthesis, characterization, and their antibacterial activity. J. Phys. Chem. B 2006, 110, 16248–16253. [Google Scholar] [CrossRef]
- Souza, I.R.; Silva, L.R.; Fernandes, L.S.P.; Salgado, L.D.; Silva de Assis, H.C.; Firak, D.S.; Bach, L.; Santos-Filho, R.; Voigt, C.L.; Barros, A.C.; et al. Visible-light reduced silver nanoparticles’ toxicity in Allium cepa test system. Environ. Pollut. 2019, 257, 113551. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.; Xia, T.; Madler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lekamge, S.; Miranda, A.F.; Trestrail, C.; Pham, B.; Ball, A.S.; Shukla, R.; Nugegoda, D. The toxicity of nonaged and aged coated silver nanoparticles to freshwater alga Raphidocelis subcapitata. Environ. Toxicol. Chem. 2019, 38, 2371–2382. [Google Scholar] [CrossRef]
- Szivák, I.; Behra, R.; Sigg, L. Metal-induced reactive oxygen species production in Chlamydomonas reinhardtii (Chlorophyceae). J. Phycol. 2009, 45, 427–435. [Google Scholar] [CrossRef]
- Galazzi, R.M.; Lopes Júnior, C.A.; de Lima, T.B.; Gozzo, F.C.; Arruda, M.A.Z. Evaluation of some effects on plant metabolism through proteins and enzymes in transgenic and non-transgenic soybeans after cultivation with silver nanoparticles. J. Proteom. 2019, 191, 88–106. [Google Scholar] [CrossRef]
- Oukarroum, A.; Samadani, M.; Dewez, D. Influence of pH on the toxicity of silver nanoparticles in the green alga Chlamydomonas acidophila. Water. Air. Soil Pollut. 2014, 225. [Google Scholar] [CrossRef]
- Nair, P.M.G.; Chung, I.M. Physiological and molecular level effects of silver nanoparticles exposure in rice (Oryza sativa L.) seedlings. Chemosphere 2014, 112, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Speranza, A.; Crinelli, R.; Scoccianti, V.; Taddei, A.R.; Iacobucci, M.; Bhattacharya, P.; Ke, P.C. In vitro toxicity of silver nanoparticles to kiwifruit pollen exhibits peculiar traits beyond the cause of silver ion release. Environ. Pollut. 2013, 179, 258–267. [Google Scholar] [CrossRef]
- Lu, G.; Yang, H.; Xia, J.; Zong, Y.; Liu, J. Toxicity of Cu and Cr nanoparticles to Daphnia magna. Water. Air. Soil Pollut. 2017, 228. [Google Scholar] [CrossRef]
- Khromykh, N.O.; Shupranova, L.V.; Lykholat, Y.V.; Bil’chuk, V.S.; Fedenko, V.S.; Boguslavs’ka, L.V.; Borysova, O.I. Physiological and biochemical reactions of Hordeum vulgare seedlings to the action of silver nanoparticles. Biosyst. Divers. 2015, 23, 100–104. [Google Scholar] [CrossRef] [Green Version]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinha, S.; Saxena, R. Effect of iron on lipid peroxidation, and enzymatic and non-enzymatic antioxidants and bacoside - A content in medicinal plant Bacopa monnieri L. Chemosphere 2006, 62, 1340–1350. [Google Scholar] [CrossRef] [PubMed]
- Chiang, H.-H.; Dandekar, A.M. Regulation of proline accumulation in Arabidopsis thaliana (L.) Heynh during development and in response to desiccation. Plant. Cell Environ. 1995, 18, 1280–1290. [Google Scholar] [CrossRef]
- Lee, S.; Pagoria, D.; Raigrodski, A.; Geurtsen, W. Effects of combinations of ROS scavengers on oxidative DNA damage caused by visible-light-activated camphorquinone/N,N-dimethyl-p-toluidine. J. Biomed. Mater. Res. 2007, 83, 391–399. [Google Scholar] [CrossRef]
- Ha, A.S.; Smith, A.P.; Howden, R.; Dietrich, W.M.; Bugg, S.; Connell, M.J.O.; Goldsbrough, P.B.; Cobbett, C.S.; The, S.; Cell, P.; et al. Phytochelatin synthase genes from Arabidopsis and the yeast Schizosaccharomyces pombe. Plant Cell 1999, 11, 1153–1163. [Google Scholar] [CrossRef]
- Havaux, M. Carotenoid oxidation products as stress signals in plants. Plant J. 2014, 79, 597–606. [Google Scholar] [CrossRef]
- Karimi, J.; Mohsenzadeh, S. Physiological effects of silver nanoparticles and silver nitrate toxicity in Triticum aestivum. Iran. J. Sci. Technol. Trans. A Sci. 2017, 41, 111–120. [Google Scholar] [CrossRef]
- Safafar, H.; Wagenen, J.V.; Møller, P.; Jacobsen, C. Carotenoids, phenolic compounds and tocopherols contribute to the antioxidative properties of some microalgae species grown on industrial wastewater. Mar. Drugs 2015, 13, 7339–7356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandra, R.; Bharagava, R.N.; Yadav, S.; Mohan, D. Accumulation and distribution of toxic metals in wheat (Triticum aestivum L.) and Indian mustard (Brassica campestris L.) irrigated with distillery and tannery effluents. J. Hazard. Mater. 2009, 162, 1514–1521. [Google Scholar] [CrossRef]
- Sharma, P.; Dubey, R.S. Lead toxicity in plants. Braz. J. Plant Physiol. 2005, 17, 35–52. [Google Scholar] [CrossRef] [Green Version]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Lovecká, P.; Macůrková, A.; Záruba, K.; Hubáček, T.; Siegel, J.; Valentová, O. Genomic damage induced in Nicotiana tabacum L. plants by colloidal solution with silver and gold nanoparticles. Plants 2021, 10, 1260. [Google Scholar] [CrossRef] [PubMed]
- Shigenaga, M.K.; Ames, B. Oxidants and mitogenesis as causes of mutation and cancer: The influence of diet. Basic Life Sci 1993, 61, 419–436. [Google Scholar] [PubMed]
- Kumari, M.; Mukherjee, A.; Chandrasekaran, N. Genotoxicity of silver nanoparticles in Allium cepa. Sci. Total Environ. 2009, 407, 5243–5246. [Google Scholar] [CrossRef]
- Hafez, R.M.; Fouad, A.S. Mitigation of genotoxic and cytotoxic effects of silver nanoparticles on onion root tips using some antioxidant scavengers. Egypt. J. Bot. 2020, 60, 133–145. [Google Scholar] [CrossRef]
- Casillas-Figueroa, F.; Arellano-García, M.E.; Leyva-Aguilera, C.; Ruíz-Ruíz, B.; Vázquez-Gómez, R.L.; Radilla-Chávez, P.; Chávez-Santoscoy, R.A.; Pestryakov, A.; Toledano-Magaña, Y.; García-Ramos, J.C.; et al. ArgovitTM silver nanoparticles effects on Allium cepa: Plant growth promotion without cyto genotoxic damage. Nanomaterials 2020, 10, 1386. [Google Scholar] [CrossRef]
- Pesnya, D.S. Cytogenetic effects of chitosan-capped silver nanoparticles in the Allium cepa test. Caryologia 2013, 66, 275–281. [Google Scholar] [CrossRef]
- Abbas, Q.; Liu, G.; Yousaf, B.; Ali, M.U.; Ullah, H.; Ahmed, R. Effects of biochar on uptake, acquisition and translocation of silver nanoparticles in rice (Oryza sativa L.) in relation to growth, photosynthetic traits and nutrients displacement. Environ. Pollut. 2019, 250, 728–736. [Google Scholar] [CrossRef]
- Qian, H.; Peng, X.; Han, X.; Ren, J.; Sun, L.; Fu, Z. Comparison of the toxicity of silver nanoparticles and silver ions on the growth of terrestrial plant model Arabidopsis thaliana. J. Environ. Sci. 2013, 25, 1947–1956. [Google Scholar] [CrossRef]
- Zou, X.; Li, P.; Lou, J.; Zhang, H. Surface coating-modulated toxic responses to silver nanoparticles in Wolffia globosa. Aquat. Toxicol. 2017, 189, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Dewez, D.; Goltsev, V.; Kalaji, H.M.; Oukarroum, A. Inhibitory effects of silver nanoparticles on photosystem II performance in Lemna gibba probed by chlorophyll fluorescence. Curr. Plant Biol. 2018, 16, 15–21. [Google Scholar] [CrossRef]
- Sosan, A.; Svistunenko, D.; Straltsova, D.; Tsiurkina, K.; Smolich, I.; Lawson, T.; Subramaniam, S.; Golovko, V.; Anderson, D.; Sokolik, A.; et al. Engineered silver nanoparticles are sensed at the plasma membrane and dramatically modify the physiology of Arabidopsis thaliana plants. Plant J. 2016, 85, 245–257. [Google Scholar] [CrossRef] [Green Version]
- Jansson, H.; Hansson, Ö. Competitive inhibition of electron donation to photosystem 1 by metal-substituted plastocyanin. Biochim. Biophys. Acta Bioenerg. 2008, 1777, 1116–1121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Q.S.; Hu, J.Z.; Xie, K.B.; Yang, H.Y.; Du, K.H.; Shi, G.X. Accumulation and acute toxicity of silver in Potamogeton crispus L. J. Hazard. Mater. 2010, 173, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Torrent, L.; Iglesias, M.; Marguí, E.; Hidalgo, M.; Verdaguer, D.; Llorens, L.; Kodre, A.; Kavčič, A.; Vogel-Mikuš, K. Uptake, translocation and ligand of silver in Lactuca sativa exposed to silver nanoparticles of different size, coatings and concentration. J. Hazard. Mater. 2020, 384, 121201. [Google Scholar] [CrossRef] [PubMed]
- Sadak, M.S. Impact of silver nanoparticles on plant growth, some biochemical aspects, and yield of fenugreek plant (Trigonella foenum-graecum). Bull. Natl. Res. Cent. 2019, 43. [Google Scholar] [CrossRef]
- Spinoso-Castillo, J.L.; Chavez-Santoscoy, R.A.; Bogdanchikova, N.; Pérez-Sato, J.A.; Morales-Ramos, V.; Bello-Bello, J.J. Antimicrobial and hormetic effects of silver nanoparticles on in vitro regeneration of vanilla (Vanilla planifolia Jacks. ex Andrews) using a temporary immersion system. Plant Cell. Tissue Organ Cult. 2017, 129, 195–207. [Google Scholar] [CrossRef]
- Bello-Bello, J.J.; Chavez-Santoscoy, R.A.; Lecona-Guzmán, C.A.; Bogdanchikova, N.; Salinas-Ruíz, J.; Gómez-Merino, F.C.; Pestryakov, A. Hormetic response by silver nanoparticles on in vitro multiplication of sugarcane (Saccharum spp. Cv. Mex 69-290) using a temporary immersion system. Dose-Response 2017, 15, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, P.; Bhatt, D.; Zaidi, M.G.H.; Saradhi, P.P.; Khanna, P.K.; Arora, S. Silver nanoparticle-mediated enhancement in growth and antioxidant status of Brassica juncea. Appl. Biochem. Biotechnol. 2012, 167, 2225–2233. [Google Scholar] [CrossRef] [PubMed]
- Larue, C.; Castillo-Michel, H.; Sobanska, S.; Cécillon, L.; Bureau, S.; Barthès, V.; Ouerdane, L.; Carrière, M.; Sarret, G. Foliar exposure of the crop Lactuca sativa to silver nanoparticles: Evidence for internalization and changes in Ag speciation. J. Hazard. Mater. 2014, 264, 98–106. [Google Scholar] [CrossRef]
- Castro-González, C.G.; Sánchez-Segura, L.; Gómez-Merino, F.C.; Bello-Bello, J.J. Exposure of stevia (Stevia rebaudiana B.) to silver nanoparticles in vitro: Transport and accumulation. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Salachna, P.; Byczyńska, A.; Zawadzińska, A.; Piechocki, R.; Mizielińska, M. Stimulatory effect of silver nanoparticles on the growth and flowering of potted oriental lilies. Agronomy 2019, 9, 610. [Google Scholar] [CrossRef] [Green Version]
- Gondikas, A.P.; Morris, A.; Reinsch, B.C.; Marinakos, S.M.; Lowry, G.V.; Hsu-Kim, H. Cysteine-induced modifications of zero-valent silver nanomaterials: Implications for particle surface chemistry, aggregation, dissolution, and silver speciation. Environ. Sci. Technol. 2012, 46, 7037–7045. [Google Scholar] [CrossRef]
- Kaveh, R.; Li, Y.S.; Ranjbar, S.; Tehrani, R.; Brueck, C.L.; Van Aken, B. Changes in Arabidopsis thaliana gene expression in response to silver nanoparticles and silver ions. Environ. Sci. Technol. 2013, 47, 10637–10644. [Google Scholar] [CrossRef]
- Pradas Del Real, A.E.; Vidal, V.; Carrière, M.; Castillo-Michel, H.; Levard, C.; Chaurand, P.; Sarret, G. Silver nanoparticles and wheat roots: A complex interplay. Environ. Sci. Technol. 2017, 51, 5774–5782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noori, A.; Bharath, L.P.; White, J.C. Type-specific impacts of silver on the protein profile of tomato (Lycopersicon esculentum L.). Int. J. Phytoremediation 2021, 1–13. [Google Scholar] [CrossRef]
- Schiavon, M.; Wirtz, M.; Borsa, P.; Quaggiotti, S.; Hell, R.; Malagoli, M. Chromate differentially affects the expression of a high-affinity sulfate transporter and isoforms of components of the sulfate assimilatory pathway in Zea mays (L.). Plant Biol. 2007, 9, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Waśkiewicz, A.; Beszterda, M.; Goliński, P. Nonenzymatic antioxidants in plants. In Oxidative Damage to Plants: Antioxidant Networks and Signaling; Parvaiz, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 201–234. ISBN 9780127999630. [Google Scholar]
- García-Sánchez, S.; Bernales, I.; Cristobal, S. Early response to nanoparticles in the Arabidopsis transcriptome compromises plant defence and root-hair development through salicylic acid signalling. BMC Genom. 2015, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, P.; Lombi, E.; Sun, S.; Scheckel, K.G.; Malysheva, A.; McKenna, B.A.; Menzies, N.W.; Zhao, F.J.; Kopittke, P.M. Characterizing the uptake, accumulation and toxicity of silver sulfide nanoparticles in plants. Environ. Sci. Nano 2017, 4, 448–460. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, H.; Yan, L.; Kerr, P.G.; Zhang, S.; Wu, Y. Electron transport, light energy conversion and proteomic responses of periphyton in photosynthesis under exposure to AgNPs. J. Hazard. Mater. 2021, 401, 123809. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, L.; Li, S.; Yin, L.; Huang, J.; Chen, C. Toxicity of silver nanoparticles to Arabidopsis: Inhibition of root gravitropism by interfering with auxin pathway. Environ. Toxicol. Chem. 2017, 36, 2773–2780. [Google Scholar] [CrossRef] [PubMed]




| Algae Species | AgNP Coating/ size (nm) | AgNP Concentration | Exposure Medium/Duration | Investigated Parameters | Findings | Reference |
|---|---|---|---|---|---|---|
| Chlamydomonas reinhardtii | carbonate/10 to 200; most particles around 25 | 10 to 100000 nmol L−1 | 10 mmol L−1 MOPS/ 1 and 2 h | photosynthesis | inhibitory effects on photosynthesis | [15] |
| carbonate/ 29 | 0.5–10 μmol L−1 | 10 mmol L−1 MOPS/ 1 h | bioaccumulation | Ag content increased with increasing exposure time and AgNP conc., reaching steady state conc. between 10−5 and 10−3 mol L−1 per cell | [16] | |
| uncoated/ 50 | 1, 5, and 10 mmol L−1 | HSM/ 6 h | photosynthesis | deteriorating effect on the structural and functional integrity of PSII | [17] | |
| PEG/ 80 ± 13 | 2 × 10–5 mol L−1 2 × 10–6 mol L−1 | Uspensky medium/ 24 h | photosynthesis | delayed fluorescence induction curves | [19] | |
| polyacrylate/ 5 | 0–100 μg L−1 | 4× diluted TAP medium/ 60 min | transcriptome | increased expression of transcript for copper transport protein 2 | [30] | |
| PEG/ 20 | 0.001–2200 µg L−1 | Woods Hole MBL medium/ 72 h | protein expression | majority of the proteins with differential expression were upregulated, the majority of which were those involved in thiamine biosynthesis, Calvin cycle, and photosynthesis | [28] | |
| carbonate/40 ± 0.5, chitosan/25 ± 1.7, citrate/17 ± 0.9, dexpanthenol/456 ± 200, gelatin/52 ± 2.8, lactate/ 35 ± 14.8, NDB/45 ± 3.3, PEG/70 ± 8.3, PVP/84 ± 40.0 | 0–1000 µmol L−1 | 10 mmol L−1 MOPS/ 1 and 2 h | photosynthesis | toxicity was related neither to particle size nor to the coatings | [20] | |
| PVP/ 11.7 ± 1.9 | 2 mg L−1 | tris-acetate-phosphate/ 4, 12, 24, 36, and 48 h | AgNP uptake, distribution, and morphology in algal cells | AgNPs enter the periplasmic space after cellular internalization and sequestration by sulfidation of Ag+ ions released from AgNPs by thiolates and sulfides | [14] | |
| uncoated/ 60–120 | 0, 1, 5, 10, 30, and 50 mg L−1 | SE medium/ 24, 48, 72, 96, and 120 h | growth, photosynthesis, and oxidative stress | damaged chloroplasts and inhibited photosynthetic pigments synthesis; inhibited growth; increased ROS production and MDA content; activated antioxidant enzymes | [31] | |
| Chlorella vulgaris | uncoated/ 50 | 0.1, 1, and 10 mg L−1 | BG-11 medium/ 24 h | viability; oxidative stress | strong decrease in chlorophyll content and cell viability; increased ROS formation and lipid peroxidation | [32] |
| citrate/10 PVP/10 PEG/10 | AgNP-citrate–9–140 nmol L−1 AgNP-PEG– 28–935 nmol L−1 AgNP-PVP– 28–93 nmol L−1 | Jaworski’s medium/ 72 h | growth; chlorophyll content; AgNP accumulation | citrate- and PVP-coated AgNPs showed similar uptake rate and toxicity; AgNP-PEG had the highest uptake rate but the lowest toxicity | [33] | |
| citrate/ 9–10 | 9.3, 93, 463, and 926 nmol L−1 | BG-11 medium/ 24, 48, 72, and 96 h | oxidative stress; gene and protein expression | induction of antioxidant enzymes, unabated photosynthesis at growth-inhibitory AgNP concentration | [21] | |
| uncoated/ 50 and 100 | 10, 50, 100, and 200 mg L−1 | f/2 medium/ 24, 48, 72, and 96 h | cell viability, chlorophyll a concentration, ROS formation | negative effect on cell viability and chlorophyll a content; increased ROS formation | [34] | |
| glucose/ 20 ± 5 | 0.1, 1, 10, 100 µg L−1 and 1 mg L−1 | BBM/ 24 h and 1 week | growth, chlorophyll a content, AgNP biodistribution, and subcellular localization | exposure time and dose-dependent growth reduction and decrease in chlorophyll a content; internalized AgNPs inside large vacuoles | [35] | |
| citrate/24, PEI/29 | AgNP-citrate -; 71.2 ± 13.6 μg L−1, AgNP-PEI -; 51.6 ± 9.6 μg L−1 | BG-11 medium/ 24 h | protein expression | AgNP-coating electrical property-dependent effects: negative AgNP-citrate regulated mitochondrial function-related proteins; positive AgNP-PEI targeted ribosome function-related proteins and interrupted pathways of protein synthesis and DNA genetic information transmission | [29] | |
| citrate/ 46.8 ± 3.3 | 90, 180, 360, 720, and 1440 μg L−1 | BBM/ 24, 48, 72, and 96 h | growth rate, cell diameter and volume; chlorophyll a and b, content of pheophytin, carotenoids carbohydrate, total lipids and proteins | altered growth kinetics and cell metabolism expressed in photosynthetic pigments and biochemical composition | [22] | |
| Raphidocelis subcapitata | PVP/96 | EC50 = 9.9 [7.4–13.2] µg L−1 | BBM/ 96 h | acute toxicity | AgNP-dose dependent toxicity | [36] |
| citrate/14 PVP/15 micron/2000–3500 | AgNP-citrate – 3.0 ± 0.7 µg L−1 AgNP-PVP – 19.5 ± 6.1 µg L−1 micron – 966 µg L−1 | modified USEPA medium/ 72 h | growth rate inhibition | AgNP-citrate was found to be more toxic than AgNP-PVP; micron-sized particles were less toxic than AgNPs; presence of natural organic matter stabilized AgNPs and reduced toxicity in freshwater | [37] | |
| alkane material/ 3–8 | 15 and 30 µg L−1 | MBL medium/ 48 h | kinetics of uptake and elimination of AgNP in comparison to AgNO3 | AgNP were not able to penetrate the cells, and Ag accumulation happens through the uptake of Ag ions | [38] | |
| PVP/ 20 | 0.1 to 1000 μmol L−1 | 1.36 mmol L−1 Ca(NO3)2, 0.73 mmol L−1 Mg(NO3)2, 1.19 mmol L−1 NaNO3, 0.20 mmol L−1 KNO3 in sterile Milli-Q water/4.5 h | photosynthetic efficiency | inhibited photosynthetic efficiency; humic substances alleviated AgNP-imposed toxicity in a dose-dependent matter | [23] | |
| uncoated/ NM300K–16 ± 5 NM302–176 ± 41 M-AgNP–11 ± 3 | NM300K– 2.56–25.6 µg L−1, NM302– 0.26–25.6 µg L−1, M-AgNP– 5–50 µg L−1 | modified OCED medium without Fe-EDTA/ 72 h | growth | reduced growth in the following order M-AgNP > NM300K > NM302 | [24] | |
| tyrosine/ 0.56 ± 2.27 epigallocatechin/ 9.27 ± 1.29 curcumin/13.68 ± 0.76 | 0.020, 0.050, 0.080, 0.110, 0.140, 0.170, 0.200, and 0.230 mg L−1 | MLA medium/ 24, 48, and 72 h | growth, antioxidant enzyme activities | physicochemical characteristics of the AgNP surface coating plays a major role in determining AgNP behavior in growth medium, toxicity, bioaccumulation, and antioxidant enzyme responses of algae | [25] | |
| Euglena gracilis | citrate/47 | 0–40 μmol L−1 for photosynthesis 5 μmol L−1 for cell morphology 0–10 μmol L−1 for uptake | 10 mmol L−1 MOPS/ 1 and 2 h for photosynthesis 1 h for cell morphology and uptake | photosynthetic yield cell morphology | photosynthetic yield decreased in a concentration-dependent manner; cell morphology was significantly altered: increased uptake with increasing AgNP concentration up to 2.5 μmol L−1 AgNPs | [39] |
| citrate/ 38–73 | 0–40 μmol L−1 | MOPS/ 1 and 2 h | silver uptake, photosynthetic yield | AgNPs adsorb onto the cell surface and can bind extracellular proteins | [40] | |
| Pithophora oedogonia | uncoated/ 10 to 15 | 0.5, 1, 3, and 5 mmol L−1 | BBM/ 5, 7, and 10 days | chlorophyll content, chromosomal aberrations | cell wall rupture and degradation, reduction in total chlorophyll content, cytological abnormalities | [27] |
| Chara vulgaris | uncoated/ 10 to 15 | 0.5, 1, 3, and 5 mmol L−1 | BBM/ 5, 7, and 10 days | chlorophyll content, chromosomal aberrations | reduction in total chlorophyll content, cytological abnormalities with disturbed metaphase | [27] |
| Scenedesmus sp. | PVA/ 6 to 10 | 5, 20, 50, 100, and 200 μg L−1 | COMBO medium/ 72 h | growth, chlorophyll a concentration, total lipids | change in cell diameter, reduction in chlorophyll a content, enhancement of total lipid production | [26] |
| Chlorella pyrenoidosa | citrate/19.3 ± 6.3 PVP/22.0 ± 6.1 | 10 mg L−1 | OECD medium/ various exposure times (0–24 h) | growth inhibition, bioaccumulation, interaction between EPS and AgNPs | AgNP-PVP strongly bind to EPS and have lower uptake and toxicity compared to AgNP-citrate; removal of EPS increases Ag uptake for both AgNP-PVP and AgNP-citrate | [41] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biba, R.; Košpić, K.; Komazec, B.; Markulin, D.; Cvjetko, P.; Pavoković, D.; Peharec Štefanić, P.; Tkalec, M.; Balen, B. Surface Coating-Modulated Phytotoxic Responses of Silver Nanoparticles in Plants and Freshwater Green Algae. Nanomaterials 2022, 12, 24. https://doi.org/10.3390/nano12010024
Biba R, Košpić K, Komazec B, Markulin D, Cvjetko P, Pavoković D, Peharec Štefanić P, Tkalec M, Balen B. Surface Coating-Modulated Phytotoxic Responses of Silver Nanoparticles in Plants and Freshwater Green Algae. Nanomaterials. 2022; 12(1):24. https://doi.org/10.3390/nano12010024
Chicago/Turabian StyleBiba, Renata, Karla Košpić, Bruno Komazec, Dora Markulin, Petra Cvjetko, Dubravko Pavoković, Petra Peharec Štefanić, Mirta Tkalec, and Biljana Balen. 2022. "Surface Coating-Modulated Phytotoxic Responses of Silver Nanoparticles in Plants and Freshwater Green Algae" Nanomaterials 12, no. 1: 24. https://doi.org/10.3390/nano12010024