Cytostatic and Cytotoxic Effects of Hollow-Shell Mesoporous Silica Nanoparticles Containing Magnetic Iron Oxide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
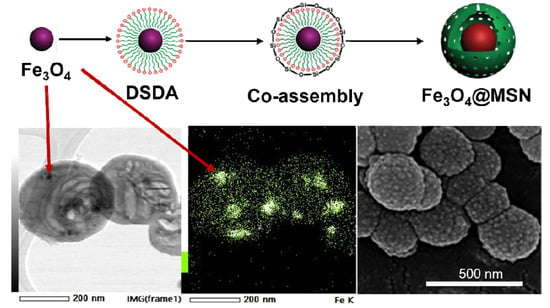
2.2. Synthesis and Functionalisation of Magnetic NPs (Fe3O4 NPs)
2.3. Synthesis of Magnetic MSNs (Fe3O4@MSNs)
2.4. Physicochemical Characterisation
2.5. DSDA Delivery and Cell Viability Studies
3. Results and Discussion
3.1. Synthesis and Functionalisation of Magnetic NPs (FeNPs)
3.2. Synthesis of Magnetic MSNs (Fe3O4@MSNs)
3.3. DSDA Delivery and Cell Viability
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- García-Muñoz, R.A.; Morales, V.; Linares, M.; González, P.E.; Sanz, R.; Serrano, D.P. Influence of the structural and textural properties of ordered mesoporous materials and hierarchical zeolitic supports on the controlled release of methylprednisolone hemisuccinate. J. Mater. Chem. B 2014, 2, 7996–8004. [Google Scholar] [CrossRef]
- Sun, M.; Chen, C.; Chen, L.; Su, B. Hierarchically Porous Materials: Synthesis Strategies and Emerging Applications. Front. Chem. Sci. Eng. 2016, 10, 301–347. [Google Scholar] [CrossRef]
- Tsou, C.J.; Hung, Y.; Mou, C.Y. Hollow Mesoporous Silica Nanoparticles with Tunable Shell Thickness and Pore Size Distribution for Application as Broad-Ranging pH Nanosensor. Microporous Mesoporous Mater. 2014, 190, 181–188. [Google Scholar] [CrossRef]
- Morales, V.; Villajos, J.A.; García, R.A. Simultaneous Synthesis of Modified Binol-Periodic Mesoporous Organosilica SBA-15 Type Material. Application as Catalysts in Asymmetric Sulfoxidation Reactions. J. Mater. Sci. 2013, 48, 5990–6000. [Google Scholar] [CrossRef]
- García-Muñoz, R.A.; Morales, V.; Linares, M.; Rico-Oller, B. Synthesis of Helical and Supplementary Chirally Doped PMO Materials. Suitable Catalysts for Asymmetric Synthesis. Langmuir 2014, 30, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Bustos, J.; Martín, A.; Morales, V.; Sanz, R.; García-Muñoz, R.A. Surface-Functionalization of Mesoporous SBA-15 Silica Materials for Controlled Release of Methylprednisolone Sodium Hemisuccinate: Influence of Functionality Type and Strategies of Incorporation. Microporous Mesoporous Mater. 2017, 240, 236–245. [Google Scholar] [CrossRef]
- García, R.A.; Morales, V.; Garcés, T. One-Step Synthesis of a Thioester Chiral PMO and Its Use as a Catalyst in Asymmetric Oxidation Reactions. J. Mater. Chem. 2012, 22, 2607–2615. [Google Scholar] [CrossRef]
- Wang, Y.; Li, B.; Zhang, L.; Song, H. Multifunctional Mesoporous Nanocomposites with Magnetic, Optical, and Sensing Features: Synthesis, Characterization, and Their Oxygen-Sensing Performance. Langmuir 2013, 29, 1273–1279. [Google Scholar] [CrossRef]
- Hola, K.; Markova, Z.; Zoppellaro, G.; Tucek, J.; Zboril, R. Tailored Functionalization of Iron Oxide Nanoparticles for MRI, Drug Delivery, Magnetic Separation and Immobilization of Biosubstances. Biotechnol. Adv. 2015, 33, 1162–1176. [Google Scholar] [CrossRef]
- Stigliano, C.; Key, J.; Ramirez, M.; Aryal, S.; Decuzzi, P. Radiolabeled Polymeric Nanoconstructs Loaded with Docetaxel and Curcumin for Cancer Combinatorial Therapy and Nuclear Imaging. Adv. Funct. Mater. 2015, 25, 3371–3379. [Google Scholar] [CrossRef]
- Marianecci, C.; Di Marzio, L.; Del Favero, E.; Cantù, L.; Brocca, P.; Rondelli, V.; Rinaldi, F.; Dini, L.; Serra, A.; Decuzzi, P.; et al. Niosomes as Drug Nanovectors: Multiscale pH-Dependent Structural Response. Langmuir 2016, 32, 1241–1249. [Google Scholar] [CrossRef]
- Morales, V.; Gutiérrez-Salmerón, M.; Balabasquer, M.; Ortiz-Bustos, J.; Chocarro-Calvo, A.; García-Jiménez, C.; García-Muñoz, R.A. New Drug-Structure-Directing Agent Concept: Inherent Pharmacological Activity Combined with Templating Solid and Hollow-Shell Mesostructured Silica Nanoparticles. Adv. Funct. Mater. 2016, 26, 7291–7303. [Google Scholar] [CrossRef]
- Okada, T.; Ozono, S.; Okamoto, M.; Takeda, Y.; Minamisawa, H.M.; Haeiwa, T.; Sakai, T.; Mishima, S. Magnetic Rattle-Type Core–Shell Particles Containing Iron Compounds with Acid Tolerance by Dense Silica. Ind. Eng. Chem. Res. 2014, 53, 8759–8765. [Google Scholar] [CrossRef]
- Han, L.; Zhang, X.-Y.; Wang, Y.-L.; Li, X.; Yang, X.-H.; Huang, M.; Hu, K.; Li, L.-H.; Wei, Y. Redox-Responsive Theranostic Nanoplatforms Based on Inorganic Nanomaterials. J. Control. Release 2017, 259, 40–52. [Google Scholar] [CrossRef]
- Alvarez-Berríos, M.P.; Vivero-Escoto, J.L. In Vitro Evaluation of Folic Acid-Conjugated Redox-Responsive Mesoporous Silica Nanoparticles for the Delivery of Cisplatin. Int. J. Nanomed. 2016, 11, 6251–6265. [Google Scholar] [CrossRef] [Green Version]
- Lu, A.-H.; Salabas, E.L.; Schüth, F. Magnetic Nanoparticles: Synthesis, Protection, Functionalization, and Application. Angew. Chem. Int. Ed. 2007, 46, 1222–1244. [Google Scholar] [CrossRef] [PubMed]
- Ling, D.; Lee, N.; Hyeon, T. Chemical Synthesis and Assembly of Uniformly Sized Iron Oxide Nanoparticles for Medical Applications. Acc. Chem. Res. 2015, 48, 1276–1285. [Google Scholar] [CrossRef]
- Dobson, J. Gene Therapy Progress and Prospects: Magnetic Nanoparticle-Based Gene Delivery. Gene Ther. 2006, 13, 283–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mou, X.; Xianbo, A.; Li, S.; He, N. Applications of Magnetic Nanoparticles in Targeted Drug Delivery System. J. Nanosci. Nanotech. 2015, 15, 54–62. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, K.; Zhang, R.; She, Z.; Tan, R.; Fan, Y.; Li, X. Magnetic nanoparticles applied in targeted therapy and magnetic resonance imaging: Crucial preparation parameters, indispensable pre-treatments, updated research advancements and future perspectives. J. Mater. Chem. B 2020, 8, 5973–5991. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, A.; Di Corato, R.; Kolosnjaj-Tabi, J.; Flaud, P.; Pellegrino, T.; Wilhelm, C. Duality of Iron Oxide Nanoparticles in Cancer Therapy: Amplification of Heating Efficiency by Magnetic Hyperthermia and Photothermal Bimodal Treatment. ACS Nano 2016, 10, 2436–2446. [Google Scholar] [CrossRef] [PubMed]
- Schweiger, C.; Pietzonka, C.; Heverhagen, J.; Kissel, T. Novel Magnetic Iron Oxide Nanoparticles Coated with Poly(ethylene Imine)-G-Poly(ethylene Glycol) for Potential Biomedical Application: Synthesis, Stability, Cytotoxicity and MR Imaging. Int. J. Pharm. 2011, 408, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.K.; Chan, P.S.; Fan, S.; Kwan, S.M.; Yeung, K.L.; Wáng, Y.-X.J.; Chow, A.H.L.; Wu, E.X.; Baum, L. Curcumin-Conjugated Magnetic Nanoparticles for Detecting Amyloid Plaques in Alzheimer’s Disease Mice Using Magnetic Resonance Imaging (MRI). Biomaterials 2015, 44, 155–172. [Google Scholar] [CrossRef]
- Mykhaylyk, O.; Sanchez-Antequera, Y.; Vlaskou, D.; Cerda, M.B.; Bokharaei, M.; Hammerschmid, E.; Anton, M.; Plank, C. Magnetic Nanoparticle and Magnetic Field Assisted siRNA Delivery In Vitro. In Methods in Molecular Biology; Humana Press: Clifton, NJ, USA, 2015; Volume 1218, pp. 53–106. [Google Scholar]
- Ma, M.; Chen, H.; Chen, Y.; Wang, X.; Chen, F.; Cui, X.; Shi, J. Au Capped Magnetic Core/mesoporous Silica Shell Nanoparticles for Combined Photothermo-/chemo-Therapy and Multimodal Imaging. Biomaterials 2012, 33, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.; Cervadoro, A.; Ramirez, M.R.; Stigliano, C.; Brazdeikis, A.; Colvin, V.L.; Civera, P.; Key, J.; Decuzzi, P. Assembly of Iron Oxide Nanocubes for Enhanced Cancer Hyperthermia and Magnetic Resonance Imaging. Nanomaterals 2017, 7, 72. [Google Scholar] [CrossRef] [Green Version]
- Estelrich, J.; Busquets, M. Iron Oxide Nanoparticles in Photothermal Therapy. Molecules 2018, 23, 1567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, F.C.; Hsu, C.H.; Lin, Y.Y. Nano-Therapeutic Cancer Immunotherapy Using Hyperthermia-Induced Heat Shock Proteins: Insights from Mathematical Modeling. Int. J. Nanomed. 2018, 13, 3529–3539. [Google Scholar] [CrossRef] [Green Version]
- Guoming, H.; Yuan, Q.; Feifei, Y.; Jiangao, X.; Xin, C.; Lili, W.; Huanghao, Y. Magnetothermally Triggered Free-Radical Generation for Deep Seated Tumor Treatment. Nano Lett. 2021, 21, 2926–2931. [Google Scholar]
- Xie, Y.X.; Liu, D.J.; Cai, C.L.; Chen, X.J.; Zhou, Y.; Wu, L.L.; Sun, Y.W.; Dai, H.L.; Kong, X.M.; Liu, P.F. Size-dependent cytotoxicity of Fe3O4 nanoparticles induced by biphasic regulation of oxidative stress in different human hepatoma cells. Int. J. Nanomed. 2016, 11, 3557–3570. [Google Scholar]
- Decuzzi, P.; Causa, F.; Ferrari, M.; Netti, P.A. The Effective Dispersion of Nanovectors Within the Tumor Microvasculature. Ann. Biomed. Eng. 2006, 34, 633–664. [Google Scholar] [CrossRef]
- Sherwood, J.; Lovas, K.; Rich, M.; Yin, Q.; Lackey, K.; Bolding, M.S.; Bao, Y. Shape-dependent cellular behaviors and relaxivity of iron oxide-based T1 MRI contrast agents. Nanoscale 2016, 8, 17506–17515. [Google Scholar] [CrossRef]
- Drummod, D.C.; Meyer, O.; Hong, K.; Kirpotin, D.B.; Papahadjopoulos, D. Optimizing Liposomes for Delivery of Chemotherapeutic Agents to Solid Tumors. Pharmacol. Rev. 1999, 51, 691. [Google Scholar]
- Patsula, V.; Kosinová, L.; Lovrić, M.; Ferhatovic Hamzić, L.; Rabyk, M.; Konefal, R.; Paruzel, A.; Šlouf, M.; Herynek, V.; Gajović, S.; et al. Superparamagnetic Fe3O4 Nanoparticles: Synthesis by Thermal Decomposition of Iron(III) Glucuronate and Application in Magnetic Resonance Imaging. ACS Appl. Mater. Interfaces 2016, 8, 7238–7247. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, X.; Rosenholm, J.M.; Gu, H. Synthesis and Characterization of Pore Size-Tunable Magnetic Mesoporous Silica Nanoparticles. J. Colloid Interface Sci. 2011, 361, 16–24. [Google Scholar] [CrossRef]
- Răcuciu, M.; Creangă, D.E.; Airinei, A. Citric-Acid-Coated Magnetite Nanoparticles for Biological Applications. Eur. Phys. J. E 2006, 21, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Villa, S.; Riani, P.; Locardi, F.; Canepa, F. Supplementary Materials: Functionalization of Fe3O4 NPs by Silanization: Use of Amine (APTES) and Thiol (MPTMS) Silanes and Their Physical Characterization. Materials 2016, 9, 826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Q.; Lan, F.; Yi, Q.; Wu, Y.; Gu, Z. A Colloidal Assembly Approach to Synthesize Magnetic Porous Composite Nanoclusters for Efficient Protein Adsorption. Nanoscale 2015, 7, 17617–17622. [Google Scholar] [CrossRef] [PubMed]
- Zaloga, J.; Janko, C.; Nowak, J.; Matuszak, J.; Knaup, S.; Eberbeck, D.; Tietze, R.; Unterweger, H.; Friedrich, R.P.; Duerr, S.; et al. Development of a Lauric Acid/albumin Hybrid Iron Oxide Nanoparticle System with Improved Biocompatibility. Int. J. Nanomed. 2014, 9, 4847–4866. [Google Scholar] [CrossRef] [Green Version]
- Chung, H.J.; Lee, H.; Bae, K.H.; Lee, Y.; Park, J.; Cho, S.-W.; Hwang, J.Y.; Park, H.; Langer, R.; Anderson, D.; et al. Facile Synthetic Route for Surface-Functionalized Magnetic Nanoparticles: Cell Labeling and Magnetic Resonance Imaging Studies. ACS Nano 2011, 5, 4329–4336. [Google Scholar] [CrossRef] [Green Version]
- Shaterabadi, Z.; Nabiyouni, G.; Soleymani, M. High Impact of in Situ Dextran Coating on Biocompatibility, Stability and Magnetic Properties of Iron Oxide Nanoparticles. Mater. Sci. Eng. C 2017, 75, 947–956. [Google Scholar] [CrossRef] [Green Version]
- Stephen, Z.R.; Dayringer, C.J.; Lim, J.J.; Revia, R.A.; Halbert, M.V.; Jeon, M.; Bakthavatsalam, A.; Ellenbogen, R.G.; Zhang, M. Approach to Rapid Synthesis and Functionalization of Iron Oxide Nanoparticles for High Gene Transfection. ACS Appl. Mater. Interfaces 2016, 8, 6320–6328. [Google Scholar] [CrossRef]
- Lojk, J.; Bregar, V.B.; Rajh, M.; Miš, K.; Kreft, M.E.; Pirkmajer, S.; Veranič, P.; Pavlin, M. Cell Type-Specific Response to High Intracellular Loading of Polyacrylic Acid-Coated Magnetic Nanoparticles. Int. J. Nanomed. 2015, 10, 1449–1462. [Google Scholar]
- Sivakumar, B.; Aswathy, R.G.; Nagaoka, Y.; Suzuki, M.; Fukuda, T.; Yoshida, Y.; Maekawa, T.; Sakthikumar, D.N. Multifunctional Carboxymethyl Cellulose-Based Magnetic Nanovector as a Theragnostic System for Folate Receptor Targeted Chemotherapy, Imaging, and Hyperthermia against Cancer. Langmuir 2013, 29, 3453–3466. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Jiang, C.; Ruotolo, A.; Pong, P.W.T. Amine-Functionalized Fe2O3–SiO2 Core–Shell Nanoparticles with Tunable Sizes. IEEE Trans. Nanotechnol. 2018, 17, 69–77. [Google Scholar] [CrossRef]
- Rühle, B.; Datz, S.; Argyo, C.; Bein, T.; Zink, J.I. A Molecular Nanocap Activated by Superparamagnetic Heating for Externally Stimulated Cargo Release. Chem. Commun. 2016, 52, 1843–1846. [Google Scholar] [CrossRef] [Green Version]
- Lin, F.C.; Zink, J.I. Probing the Local Nanoscale Heating Mechanism of a Magnetic Core in Mesoporous Silica Drug-Delivery Nanoparticles Using Fluorescence Depolarization. J. Am. Chem. Soc. 2020, 142, 5212–5220. [Google Scholar] [CrossRef]
- Lin, F.C.; Xie, Y.; Deng, T.; Zink, J.I. Magnetism, Ultrasound, and Light-Stimulated Mesoporous Silica Nanocarriers for Theranostics and Beyond. J. Am. Chem. Soc. 2021, 143, 6025–6036. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.Q.; Wu, J.; Doan, V.; Schwartz, B.J.; Tolbert, S.H. Control of energy transfer in oriented conjugated polymer-mesoporous silica composites. Science 2000, 288, 652–656. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.H.; Wang, C.C.; Hu, J.H.; Yang, W.L.; Fu, S.K. Investigation of Formation of Silica-Coated Magnetite Nanoparticles via Sol–gel Approach. Colloids Surf. A Physicochem. Eng. Asp. 2005, 262, 87–93. [Google Scholar] [CrossRef]
- Xuan, S.; Wang, F.; Lai, J.M.Y.; Sham, K.W.Y.; Wang, Y.-X.J.; Lee, S.-F.; Yu, J.C.; Cheng, C.H.K.; Leung, K.C.-F. Synthesis of Biocompatible, Mesoporous Fe 3 O 4 Nano/Microspheres with Large Surface Area for Magnetic Resonance Imaging and Therapeutic Applications. ACS Appl. Mater. Interfaces 2011, 3, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Riva, R.E.; Pastoriza-Santos, I.; Lak, A.; Pellegrino, T.; Pérez-Juste, J.; Mattoli, V. Plasmonic/magnetic Nanocomposites: Gold Nanorods-Functionalized Silica Coated Magnetic Nanoparticles. J. Colloid Interface Sci. 2017, 502, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yin, Y.; Mayers, B.T.; Xia, Y. Modifying the Surface Properties of Superparamagnetic Iron Oxide Nanoparticles through A Sol−Gel Approach. Nano Lett. 2002, 2, 183–186. [Google Scholar] [CrossRef]
- Morales, V.; McConnell, J.; Pérez-Garnes, M.; Almendro, N.; Sanz, R.; García-Muñoz, R.A. L-Dopa release from mesoporous silica nanoparticles engineered through the concept of drug-structure-directing agents for Parkinson’s disease. J. Mater. Chem. B 2021, 9, 4178–4189. [Google Scholar] [CrossRef]
- Guerrero-Martinez, A.; Perez-Juste, J.; Liz-Marzan, L.M. Recent Progress on Silica Coating of Nanoparticles and Related Nanomaterials. Adv. Mater. 2010, 22, 1182–1195. [Google Scholar] [CrossRef]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.V.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater. 2009, 8, 543. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.T.; Yu, M.H.; Hartono, S.B.; Yang, J.; Xu, H.Y.; Zhang, H.W.; Zhang, J.; Zou, J.; Dexter, A.; Gu, W.Y.; et al. Nanoparticles mimicking viral surface topography for enhanced cellular delivery. Adv. Mater. 2013, 25, 6233–6237. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Nor, Y.A.; Yu, M.H.; Yang, Y.N.; Zhang, J.; Zhang, H.W.; Xu, C.; Mitter, N.; Yu, C.Z. Silica Nanopollens Enhance Adhesion for Long-Term Bacterial Inhibition. J. Am. Chem. Soc. 2016, 138, 6455–6462. [Google Scholar] [CrossRef] [PubMed]
- Chithrani, B.D.; Chan, W.C. Elucidating the mechanism of cellular uptake and removal of proteincoated gold nanoparticles of different sizes and shapes. Nano Lett. 2007, 7, 1542–1550. [Google Scholar] [CrossRef] [PubMed]
- Yue, Q.; Zhang, Y.; Jiang, Y.; Li, J.; Zhang, H.; Yu, C.; Elzatahry, A.A.; Alghamdi, A.; Deng, Y.; Zhao, D. Nanoengineering of Core-Shell Magnetic Mesoporous Microspheres with Tunable Surface Roughness. J. Am. Chem. Soc. 2017, 139, 4954–4961. [Google Scholar] [CrossRef]
- Morales, V.; Pérez-Garnes, M.; Balabasquer, M.; González-Casablanca, J.; García-Muñoz, R.A. Oil-in-water synthesis of hollow-shell mesoporous peapod-like silicates: Electron microscopy insights. Microporous Mesoporous Mater. 2018, 264, 43–54. [Google Scholar] [CrossRef]
- Pérez-Garnes, M.; Gutiérrez-Salmerón, M.; Morales, V.; Chocarro-Calvo, A.; Sanz, R.; García-Jiménez, C.; García-Muñoz, R.A. Engineering hollow mesoporous silica nanoparticles to increase cytotoxicity. Mater. Sci. Eng. C 2020, 12, 110935–110945. [Google Scholar] [CrossRef] [PubMed]










| DSDA Removal | Sample | Vp (cm3/g) | SBET (m2/g) | PSD (nm) * | FeICP (wt%) |
|---|---|---|---|---|---|
| Extracted in EtOH/HCl | Fe3O4@MSN10-c | 0.232 | 227.17 | 2.1–47.1 (2.9) | |
| Fe3O4@MSN10-b | 0.266 | 394.32 | 2.0–43.1 (2.9) | ||
| Calcined | Fe3O4@MSN10-c | 0.389 | 392.3 | 1.7–4.0 (3.0) | 2.5 |
| Fe3O4@MSN10-b | 0.335 | 507.96 | 1.2–3.9 (3.0) | 2.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Garnes, M.; Morales, V.; Sanz, R.; García-Muñoz, R.A. Cytostatic and Cytotoxic Effects of Hollow-Shell Mesoporous Silica Nanoparticles Containing Magnetic Iron Oxide. Nanomaterials 2021, 11, 2455. https://doi.org/10.3390/nano11092455
Pérez-Garnes M, Morales V, Sanz R, García-Muñoz RA. Cytostatic and Cytotoxic Effects of Hollow-Shell Mesoporous Silica Nanoparticles Containing Magnetic Iron Oxide. Nanomaterials. 2021; 11(9):2455. https://doi.org/10.3390/nano11092455
Chicago/Turabian StylePérez-Garnes, Manuel, Victoria Morales, Raul Sanz, and Rafael A. García-Muñoz. 2021. "Cytostatic and Cytotoxic Effects of Hollow-Shell Mesoporous Silica Nanoparticles Containing Magnetic Iron Oxide" Nanomaterials 11, no. 9: 2455. https://doi.org/10.3390/nano11092455