Peptide-Based Electrospun Fibers: Current Status and Emerging Developments
Abstract
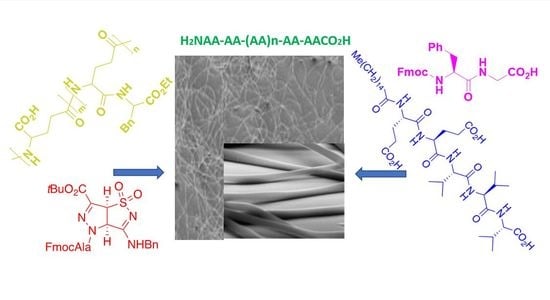
:1. Introduction
2. Peptides in Electrospinning
2.1. Short Peptides
2.1.1. Peptides Containing an Aromatic Moiety
2.1.2. Amphiphilic Peptides
2.2. Peptidomimetics
2.3. Polypeptides
2.4. Polypeptoids
3. Characterization
3.1. Optical Microscopy
3.2. Scanning Electron Microscopy
3.3. Absorption Spectroscopy Methods
3.4. Emission Spectroscopy and Microscopy (Fluorescence and Raman)
3.5. X-ray Diffraction
3.6. Atomic Force Microscopy
3.7. Mechanical Testing
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hosoyama, K.; Lazurko, C.; Muñoz, M.; McTiernan, C.D.; Alarcon, E.I. Peptide-Based Functional Biomaterials for Soft-Tissue Repair. Front. Bioeng. Biotechnol. 2019, 7, 205. [Google Scholar] [CrossRef] [PubMed]
- Chow, D.; Nunalee, M.L.; Lim, D.W.; Simnick, A.J.; Chilkoti, A. Peptide-based biopolymers in biomedicine and biotechnology. Mater. Sci. Eng. R Reports 2008, 62, 125–155. [Google Scholar] [CrossRef] [Green Version]
- Reneker, D.H.; Yarin, A.L. Electrospinning jets and polymer nanofibers. Polymer 2008, 49, 2387–2425. [Google Scholar] [CrossRef] [Green Version]
- Singer, J.C.; Ringk, A.; Giesa, R.; Schmidt, H.-W. Melt Electrospinning of Small Molecules. Macromol. Mater. Eng. 2015, 300, 259–276. [Google Scholar] [CrossRef]
- Raspa, A.; Pugliese, R.; Maleki, M.; Gelain, F. Recent therapeutic approaches for spinal cord injury. Biotechnol. Bioeng. 2016, 113, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.W.; Armgarth, A.; St-Pierre, J.-P.; Bertazzo, S.; Gentilini, C.; Aurisicchio, C.; McCullen, S.D.; Steele, J.A.M.; Stevens, M.M. Peptide-Directed Spatial Organization of Biomolecules in Dynamic Gradient Scaffolds. Adv. Healthc. Mater. 2014, 3, 1381–1386. [Google Scholar] [CrossRef] [PubMed]
- Yıldız, A.; Kara, A.A.; Acartürk, F. Peptide-protein based nanofibers in pharmaceutical and biomedical applications. Int. J. Biol. Macromol. 2020, 148, 1084–1097. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Xie, S.; Zeng, Y. Electric field distribution and jet motion in electrospinning process: From needle to hole. J. Mater. Sci. 2013, 48, 6647–6655. [Google Scholar] [CrossRef]
- DeFrates, K.G.; Moore, R.; Borgesi, J.; Lin, G.; Mulderig, T.; Beachley, V.; Hu, X. Protein-based fiber materials in medicine: A review. Nanomaterials 2018, 8, 457. [Google Scholar] [CrossRef] [Green Version]
- Ye, K.; Kuang, H.; You, Z.; Morsi, Y.; Mo, X. Electrospun nanofibers for tissue engineering with drug loading and release. Pharmaceutics 2019, 11, 182. [Google Scholar] [CrossRef] [Green Version]
- Yoo, H.S.; Kim, T.G.; Park, T.G. Surface-functionalized electrospun nanofibers for tissue engineering and drug delivery. Adv. Drug Deliv. Rev. 2009, 61, 1033–1042. [Google Scholar] [CrossRef]
- Min, B.-M.; Lee, G.; Kim, S.H.; Nam, Y.S.; Lee, T.S.; Park, W.H. Electrospinning of silk fibroin nanofibers and its effect on the adhesion and spreading of normal human keratinocytes and fibroblasts in vitro. Biomaterials 2004, 25, 1289–1297. [Google Scholar] [CrossRef]
- Rho, K.S.; Jeong, L.; Lee, G.; Seo, B.-M.; Park, Y.J.; Hong, S.-D.; Roh, S.; Cho, J.J.; Park, W.H.; Min, B.-M. Electrospinning of collagen nanofibers: Effects on the behavior of normal human keratinocytes and early-stage wound healing. Biomaterials 2006, 27, 1452–1461. [Google Scholar] [CrossRef]
- Rodríguez-Cabello, J.C.; Martín, L.; Girotti, A.; García-Arévalo, C.; Arias, F.J.; Alonso, M. Emerging applications of multifunctional elastin-like recombinamers. Nanomedicine 2011, 6, 111–122. [Google Scholar] [CrossRef]
- Khadka, D.B.; Haynie, D.T. Protein- and peptide-based electrospun nanofibers in medical biomaterials. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 1242–1262. [Google Scholar] [CrossRef]
- Lim, J.; Jun, I.; Lee, Y.B.; Kim, E.M.; Shin, D.; Jeon, H.; Park, H.; Shin, H. Fabrication of cell sheets with anisotropically aligned myotubes using thermally expandable micropatterned hydrogels. Macromol. Res. 2016, 24, 562–572. [Google Scholar] [CrossRef]
- Shin, Y.M.; Lee, Y.B.; Kim, S.J.; Kang, J.K.; Park, J.C.; Jang, W.; Shin, H. Mussel-inspired immobilization of vascular endothelial growth factor (VEGF) for enhanced endothelialization of vascular grafts. Biomacromolecules 2012, 13, 2020–2028. [Google Scholar] [CrossRef]
- Maione, S.; del Valle, L.J.; Pérez-Madrigal, M.M.; Cativiela, C.; Puiggalí, J.; Alemán, C. Antimicrobial electrospun fibers of polyester loaded with engineered cyclic gramicidin analogues. Fibers 2017, 5, 34. [Google Scholar] [CrossRef] [Green Version]
- Bucci, R.; Vaghi, F.; Erba, E.; Romanelli, A.; Gelmi, M.L.; Clerici, F. Peptide grafting strategies before and after electrospinning of nanofibers. Acta Biomater. 2021, 122, 82–100. [Google Scholar] [CrossRef]
- Ewaldz, E.; Brettmann, B. Molecular Interactions in Electrospinning: From Polymer Mixtures to Supramolecular Assemblies. ACS Appl. Polym. Mater. 2019, 1, 298–308. [Google Scholar] [CrossRef]
- Yoshida, H.; Klee, D.; Möller, M.; Akashi, M. Creation of Superhydrophobic Electrospun Nonwovens Fabricated from Naturally Occurring Poly(Amino Acid) Derivatives. Adv. Funct. Mater. 2014, 24, 6359–6364. [Google Scholar] [CrossRef]
- Zhang, W.; Yu, X.; Li, Y.; Su, Z.; Jandt, K.D.; Wei, G. Protein-mimetic peptide nanofibers: Motif design, self-assembly synthesis, and sequence-specific biomedical applications. Prog. Polym. Sci. 2018, 80, 94–124. [Google Scholar] [CrossRef]
- Nuansing, W.; Rebollo, A.; Mercero, J.M.; Zuñiga, J.; Bittner, A.M. Vibrational spectroscopy of self-assembling aromatic peptide derivates. J. Raman Spectrosc. 2012, 43, 1397–1406. [Google Scholar] [CrossRef]
- Singh, G.; Bittner, A.M.; Loscher, S.; Malinowski, N.; Kern, K. Electrospinning of Diphenylalanine Nanotubes. Adv. Mater. 2008, 20, 2332–2336. [Google Scholar] [CrossRef]
- Hamedani, Y.; Macha, P.; Evangelista, E.L.; Sammeta, V.R.; Chalivendra, V.; Rasapalli, S.; Vasudev, M.C. Electrospinning of tyrosine-based oligopeptides: Self-assembly or forced assembly? J. Biomed. Mater. Res. Part A 2020, 108, 829–838. [Google Scholar] [CrossRef]
- Levit, S.L.; Walker, R.C.; Pham, A.L.; Tang, C. 3. Polymer-free electrospinning. In Green Electrospinning; Horzum, N., Demir, M.M., Muniz-Espri, R., Crespy, D., Eds.; De Gruyter: Berlin, Germany; Boston, MA, USA, 2019; pp. 41–68. ISBN 978-3-11-056180-7. [Google Scholar]
- Locarno, S.; Eleta-Lopez, A.; Lupo, M.G.; Gelmi, M.L.; Clerici, F.; Bittner, A.M. Electrospinning of pyrazole-isothiazole derivatives: Nanofibers from small molecules. RSC Adv. 2019, 9, 20565–20572. [Google Scholar] [CrossRef] [Green Version]
- McMurtrey, R.J. Patterned and functionalized nanofiber scaffolds in three-dimensional hydrogel constructs enhance neurite outgrowth and directional control. J. Neural Eng. 2014, 11, 66009. [Google Scholar] [CrossRef]
- Gu, X.; Ding, F.; Williams, D.F. Neural tissue engineering options for peripheral nerve regeneration. Biomaterials 2014, 35, 6143–6156. [Google Scholar] [CrossRef]
- Yadav, N.; Chauhan, M.K.; Chauhan, V.S. Short to ultrashort peptide-based hydrogels as a platform for biomedical applications. Biomater. Sci. 2020, 8, 84–100. [Google Scholar] [CrossRef]
- Garanger, E.; Lecommandoux, S. Towards Bioactive Nanovehicles Based on Protein Polymers. Angew. Chemie Int. Ed. 2012, 51, 3060–3062. [Google Scholar] [CrossRef]
- Wu, S. Chain structure and entanglement. J. Polym. Sci. Part B Polym. Phys. 1989, 27, 723–741. [Google Scholar] [CrossRef]
- Isidro-Llobet, A.; Kenworthy, M.N.; Mukherjee, S.; Kopach, M.E.; Wegner, K.; Gallou, F.; Smith, A.G.; Roschangar, F. Sustainability Challenges in Peptide Synthesis and Purification: From R&D to Production. J. Org. Chem. 2019, 84, 4615–4628. [Google Scholar] [CrossRef] [Green Version]
- Fuse, S.; Otake, Y.; Nakamura, H. Peptide Synthesis Utilizing Micro-flow Technology. Chem. Asian J. 2018, 13, 3818–3832. [Google Scholar] [CrossRef]
- Pennington, M.W.; Zell, B.; Bai, C.J. Commercial manufacturing of current good manufacturing practice peptides spanning the gamut from neoantigen to commercial large-scale products. Med. Drug Discov. 2021, 9, 100071. [Google Scholar] [CrossRef]
- Gritsch, L.; Liverani, L.; Lovell, C.; Boccaccini, A.R. Polycaprolactone Electrospun Fiber Mats Prepared Using Benign Solvents: Blending with Copper(II)-Chitosan Increases the Secretion of Vascular Endothelial Growth Factor in a Bone Marrow Stromal Cell Line. Macromol. Biosci. 2020, 20. [Google Scholar] [CrossRef]
- Liverani, L.; Killian, M.S.; Boccaccini, A.R. Fibronectin functionalized electrospun fibers by using benign solvents: Best way to achieve effective functionalization. Front. Bioeng. Biotechnol. 2019, 7, 68. [Google Scholar] [CrossRef] [Green Version]
- Vogt, L.; Rivera, L.R.; Liverani, L.; Piegat, A.; El Fray, M.; Boccaccini, A.R. Poly(ε-caprolactone)/poly(glycerol sebacate) electrospun scaffolds for cardiac tissue engineering using benign solvents. Mater. Sci. Eng. C 2019, 103, 109712. [Google Scholar] [CrossRef]
- Unalan, I.; Endlein, S.J.; Slavik, B.; Buettner, A.; Goldmann, W.H.; Detsch, R.; Boccaccini, A.R. Evaluation of electrospun poly(ε-caprolactone)/gelatin nanofiber mats containing clove essential oil for antibacterial wound dressing. Pharmaceutics 2019, 11, 570. [Google Scholar] [CrossRef] [Green Version]
- Vigneswari, S.; Murugaiyah, V.; Kaur, G.; Abdul Khalil, H.P.S.; Amirul, A.A. Simultaneous dual syringe electrospinning system using benign solvent to fabricate nanofibrous P(3HB-co-4HB)/collagen peptides construct as potential leave-on wound dressing. Mater. Sci. Eng. C 2016, 66, 147–155. [Google Scholar] [CrossRef]
- Nuansing, W.; Frauchiger, D.; Huth, F.; Rebollo, A.; Hillenbrand, R.; Bittner, A.M. Electrospinning of peptide and protein fibres: Approaching the molecular scale. Faraday Discuss. 2013, 166, 209–221. [Google Scholar] [CrossRef]
- Lenci, E.; Trabocchi, A. Peptidomimetic toolbox for drug discovery. Chem. Soc. Rev. 2020, 49, 3262–3277. [Google Scholar] [CrossRef]
- Bucci, R.; Contini, A.; Clerici, F.; Beccalli, E.M.; Formaggio, F.; Maffucci, I.; Pellegrino, S.; Gelmi, M.L. Fluoro-Aryl Substituted α,β2,3-Peptides in the Development of Foldameric Antiparallel β-Sheets: A Conformational Study. Front. Chem. 2019, 7, 192. [Google Scholar] [CrossRef]
- Locarno, S.; Argentiere, S.; Ruffoni, A.; Maggioni, D.; Soave, R.; Bucci, R.; Erba, E.; Lenardi, C.; Gelmi, M.L.; Clerici, F. Self-assembled hydrophobic Ala-Aib peptide encapsulating curcumin: A convenient system for water insoluble drugs. RSC Adv. 2020, 10, 9964–9975. [Google Scholar] [CrossRef]
- Ruffoni, A.; Cavanna, M.V.; Argentiere, S.; Locarno, S.; Pellegrino, S.; Gelmi, M.L.; Clerici, F. Aqueous self-assembly of short hydrophobic peptides containing norbornene amino acid into supramolecular structures with spherical shape. RSC Adv. 2016, 6, 90754–90759. [Google Scholar] [CrossRef] [Green Version]
- Görbitz, C.H. The structure of nanotubes formed by diphenylalanine, the core recognition motif of Alzheimer’s β-amyloid polypeptide. Chem. Commun. 2006, 2332–2334. [Google Scholar] [CrossRef]
- Bonetti, A.; Pellegrino, S.; Das, P.; Yuran, S.; Bucci, R.; Ferri, N.; Meneghetti, F.; Castellano, C.; Reches, M.; Gelmi, M.L. Dipeptide Nanotubes Containing Unnatural Fluorine-Substituted beta2,3-Diarylamino Acid and l-Alanine as Candidates for Biomedical Applications. Org. Lett. 2015, 17, 4468–4471. [Google Scholar] [CrossRef]
- Reches, M.; Gazit, E. Casting Metal Nanowires Within Discrete Self-Assembled Peptide Nanotubes. Science 2003, 300, 625–627. [Google Scholar] [CrossRef] [Green Version]
- Görbitz, C.H. Nanotube formation by hydrophobic dipeptides. Chemistry 2001, 7, 5153–5159. [Google Scholar] [CrossRef]
- Min, K.-I.; Yun, G.; Jang, Y.; Kim, K.-R.; Ko, Y.H.; Jang, H.-S.; Lee, Y.-S.; Kim, K.; Kim, D.-P. Covalent Self-Assembly and One-Step Photocrosslinking of Tyrosine-Rich Oligopeptides to Form Diverse Nanostructures. Angew. Chemie Int. Ed. 2016, 55, 6925–6928. [Google Scholar] [CrossRef] [PubMed]
- Partlow, B.P.; Applegate, M.B.; Omenetto, F.G.; Kaplan, D.L. Dityrosine Cross-Linking in Designing Biomaterials. ACS Biomater. Sci. Eng. 2016, 2, 2108–2121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yuan, X.; Wu, L.; Han, Y.; Sheng, J. Study on morphology of electrospun poly(vinyl alcohol) mats. Eur. Polym. J. 2005, 41, 423–432. [Google Scholar] [CrossRef]
- Baker, S.R.; Banerjee, S.; Bonin, K.; Guthold, M. Determining the mechanical properties of electrospun poly-ε-caprolactone (PCL) nanofibers using AFM and a novel fiber anchoring technique. Mater. Sci. Eng. C 2016, 59, 203–212. [Google Scholar] [CrossRef]
- McKee, M.G.; Layman, J.M.; Cashion, M.P.; Long, T.E. Phospholipid Nonwoven Electrospun Membranes. Science 2006, 311, 353–355. [Google Scholar] [CrossRef] [Green Version]
- Hartgerink, J.D.; Beniash, E.; Stupp, S.I. Self-Assembly and Mineralization of Peptide-Amphiphile Nanofibers. Science 2001, 294, 1684–1688. [Google Scholar] [CrossRef] [Green Version]
- Boekhoven, J.; Stupp, S.I. 25th Anniversary Article: Supramolecular Materials for Regenerative Medicine. Adv. Mater. 2014, 26, 1642–1659. [Google Scholar] [CrossRef] [Green Version]
- Tysseling, V.M.; Sahni, V.; Pashuck, E.T.; Birch, D.; Hebert, A.; Czeisler, C.; Stupp, S.I.; Kessler, J.A. Self-assembling peptide amphiphile promotes plasticity of serotonergic fibers following spinal cord injury. J. Neurosci. Res. 2010, 88, 3161–3170. [Google Scholar] [CrossRef] [Green Version]
- Rajangam, K.; Behanna, H.A.; Hui, M.J.; Han, X.; Hulvat, J.F.; Lomasney, J.W.; Stupp, S.I. Heparin Binding Nanostructures to Promote Growth of Blood Vessels. Nano Lett. 2006, 6, 2086–2090. [Google Scholar] [CrossRef]
- Tayi, A.S.; Pashuck, E.T.; Newcomb, C.J.; McClendon, M.T.; Stupp, S.I. Electrospinning Bioactive Supramolecular Polymers from Water. Biomacromolecules 2014, 15, 1323–1327. [Google Scholar] [CrossRef]
- Saquing, C.D.; Tang, C.; Monian, B.; Bonino, C.A.; Manasco, J.L.; Alsberg, E.; Khan, S.A. Alginate-polyethylene oxide blend nanofibers and the role of the carrier polymer in electrospinning. Ind. Eng. Chem. Res. 2013, 52, 8692–8704. [Google Scholar] [CrossRef]
- Balogh, A.; Farkas, B.; Pálvölgyi, Á.; Domokos, A.; Démuth, B.; Marosi, G.; Nagy, Z.K. Novel Alternating Current Electrospinning of Hydroxypropylmethylcellulose Acetate Succinate (HPMCAS) Nanofibers for Dissolution Enhancement: The Importance of Solution Conductivity. J. Pharm. Sci. 2017, 106, 1634–1643. [Google Scholar] [CrossRef]
- Çakmak, S.; Çakmak, A.S.; Gumusderelioglu, M. RGD-bearing peptide-amphiphile-hydroxyapatite nanocomposite bone scaffold: An in vitro study. Biomed. Mater. 2013, 8. [Google Scholar] [CrossRef]
- Tambralli, A.; Blakeney, B.; Anderson, J.; Kushwaha, M.; Andukuri, A.; Dean, D.; Jun, H.W. A hybrid biomimetic scaffold composed of electrospun polycaprolactone nanofibers and self-assembled peptide amphiphile nanofibers. Biofabrication 2009, 1. [Google Scholar] [CrossRef] [Green Version]
- Oliva, F.; Bucci, R.; Tamborini, L.; Pieraccini, S.; Pinto, A.; Pellegrino, S. Bicyclic Pyrrolidine-Isoxazoline γ Amino Acid: A Constrained Scaffold for Stabilizing α-Turn Conformation in Isolated Peptides. Front. Chem. 2019, 7, 133. [Google Scholar] [CrossRef] [Green Version]
- Bucci, R.; Contini, A.; Clerici, F.; Pellegrino, S.; Gelmi, M.L. From glucose to enantiopure morpholino β-amino acid: A new tool for stabilizing γ-turns in peptides. Org. Chem. Front. 2019, 6, 972–982. [Google Scholar] [CrossRef] [Green Version]
- Bucci, R.; Giofré, S.; Clerici, F.; Contini, A.; Pinto, A.; Erba, E.; Soave, R.; Pellegrino, S.; Gelmi, M.L. Tetrahydro-4 H-(pyrrolo[3,4- d]isoxazol-3-yl)methanamine: A Bicyclic Diamino Scaffold Stabilizing Parallel Turn Conformations. J. Org. Chem. 2018, 83, 11493–11501. [Google Scholar] [CrossRef]
- Contini, A.; Ferri, N.; Bucci, R.; Lupo, M.G.; Erba, E.; Gelmi, M.L.; Pellegrino, S. Peptide modulators of Rac1/Tiam1 protein-protein interaction: An alternative approach for cardiovascular diseases. Biopolymers 2017, 110, e23089. [Google Scholar] [CrossRef] [Green Version]
- Bucci, R.; Bonetti, A.; Clerici, F.; Contini, A.; Nava, D.; Pellegrino, S.; Tessaro, D.; Gelmi, M.L. Tandem Tetrahydroisoquinoline-4-carboxylic Acid/β-Alanine as a New Construct Able To Induce a Flexible Turn. Chem. A Eur. J. 2017, 23, 10822–10831. [Google Scholar] [CrossRef]
- Srinath, D.; Lin, S.; Knight, D.K.; Rizkalla, A.S.; Mequanint, K. Fibrous biodegradable l-alanine-based scaffolds for vascular tissue engineering. J. Tissue Eng. Regen. Med. 2014, 8, 578–588. [Google Scholar] [CrossRef] [PubMed]
- del Valle, L.J.; Roa, M.; Díaz, A.; Casas, M.T.; Puiggalí, J.; Rodríguez-Galán, A. Electrospun nanofibers of a degradable poly(ester amide). Scaffolds loaded with antimicrobial agents. J. Polym. Res. 2012, 19, 9792. [Google Scholar] [CrossRef]
- Knight, D.K.; Gillies, E.R.; Mequanint, K. Biomimetic l-aspartic acid-derived functional poly(ester amide)s for vascular tissue engineering. Acta Biomater. 2014, 10, 3484–3496. [Google Scholar] [CrossRef] [PubMed]
- Curry, E.J.; Le, T.T.; Das, R.; Ke, K.; Santorella, E.M.; Paul, D.; Chorsi, M.T.; Tran, K.T.M.; Baroody, J.; Borges, E.R.; et al. Biodegradable nanofiber-based piezoelectric transducer. Proc. Natl. Acad. Sci. USA 2020, 117, 214–220. [Google Scholar] [CrossRef]
- Aghdam, R.M.; Najarian, S.; Shakhesi, S.; Khanlari, S.; Shaabani, K.; Sharifi, S. Investigating the effect of PGA on physical and mechanical properties of electrospun PCL/PGA blend nanofibers. J. Appl. Polym. Sci. 2012, 124, 123–131. [Google Scholar] [CrossRef]
- Haynie, D.T.; Khadka, D.B.; Cross, M.C. Physical Properties of Polypeptide Electrospun Nanofiber Cell Culture Scaffolds on a Wettable Substrate. Polymers 2012, 4, 1535–1553. [Google Scholar] [CrossRef] [Green Version]
- Khadka, D.B.; Haynie, D.T. Insoluble Synthetic Polypeptide Mats from Aqueous Solution by Electrospinning. ACS Appl. Mater. Interfaces 2010, 2, 2728–2732. [Google Scholar] [CrossRef]
- Khadka, D.B.; Cross, M.C.; Haynie, D.T. A Synthetic Polypeptide Electrospun Biomaterial. ACS Appl. Mater. Interfaces 2011, 3, 2994–3001. [Google Scholar] [CrossRef]
- Roach, P.; Shirtcliffe, N.J.; Newton, M.I. Progess in superhydrophobic surface development. Soft Matter 2008, 4, 224–240. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, W.; Su, B.-L. Superhydrophobic surfaces: From natural to biomimetic to functional. J. Colloid Interface Sci. 2011, 353, 335–355. [Google Scholar] [CrossRef]
- Hwang, S.-W.; Park, G.; Cheng, H.; Song, J.-K.; Kang, S.-K.; Yin, L.; Kim, J.-H.; Omenetto, F.G.; Huang, Y.; Lee, K.-M.; et al. 25th Anniversary Article: Materials for High-Performance Biodegradable Semiconductor Devices. Adv. Mater. 2014, 26, 1992–2000. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, L. Definition of Superhydrophobic States. Adv. Mater. 2007, 19, 3423–3424. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, Y.; Xi, J.; Zhu, Y.; Wang, N.; Xia, F.; Jiang, L. Petal Effect: A Superhydrophobic State with High Adhesive Force. Langmuir 2008, 24, 4114–4119. [Google Scholar] [CrossRef]
- Wang, Z.-H.; Chang, Y.-Y.; Wu, J.-G.; Lin, C.-Y.; An, H.-L.; Luo, S.-C.; Tang, T.K.; Su, W.-F. Novel 3D Neuron Regeneration Scaffolds Based on Synthetic Polypeptide Containing Neuron Cue. Macromol. Biosci. 2018, 18, 1700251. [Google Scholar] [CrossRef]
- Wang, Z.L.; Wu, W. Nanotechnology-Enabled Energy Harvesting for Self-Powered Micro-/Nanosystems. Angew. Chemie Int. Ed. 2012, 51, 11700–11721. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Luo, S.-C.; Yu, J.-S.; Chen, T.-C.; Su, W.-F. Peptide-Based Polyelectrolyte Promotes Directional and Long Neurite Outgrowth. ACS Appl. Bio Mater. 2019, 2, 518–526. [Google Scholar] [CrossRef]
- Pan, C.-T.; Yen, C.-K.; Lin, L.; Lu, Y.-S.; Li, H.-W.; Huang, J.C.-C.; Kuo, S.-W. Energy harvesting with piezoelectric poly(γ-benzyl-l-glutamate) fibers prepared through cylindrical near-field electrospinning. RSC Adv. 2014, 4, 21563–21570. [Google Scholar] [CrossRef]
- Sun, D.; Chang, C.; Li, S.; Lin, L. Near-Field Electrospinning. Nano Lett. 2006, 6, 839–842. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, D.-N.; Yu, S.M.; Moon, W. Electrospinning of poly(γ-benzyl–α,L-glutamate) microfibers for piezoelectric polymer applications. J. Appl. Polym. Sci. 2018, 135, 46440. [Google Scholar] [CrossRef]
- Saini, A.; Verma, G. Peptoids: Tomorrow’s therapeutics. In Nanostructures for Novel Therapy; Elsevier: Amsterdam, The Netherlands, 2017; pp. 251–280. ISBN 9780323461481. [Google Scholar]
- Sun, J.; Li, Z. Peptoid Applications in Biomedicine and Nanotechnology; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; ISBN 9780081007426. [Google Scholar]
- Thielke, M.W.; Secker, C.; Schlaad, H.; Theato, P. Electrospinning of Crystallizable Polypeptoid Fibers. Macromol. Rapid Commun. 2016, 37, 100–104. [Google Scholar] [CrossRef]
- Zou, Y.; Kaestner, M.; Reithmeier, E. Analysis of multiscale measurements of porous microstructures based on 3D optical microscopes. Meas. J. Int. Meas. Confed. 2016, 83, 1–9. [Google Scholar] [CrossRef]
- Maleki, M.; Natalello, A.; Pugliese, R.; Gelain, F. Fabrication of nanofibrous electrospun scaffolds from a heterogeneous library of co- and self-assembling peptides. Acta Biomater. 2017, 51, 268–278. [Google Scholar] [CrossRef]
- Pugliese, R.; Maleki, M.; Zuckermann, R.N.; Gelain, F. Self-assembling peptides cross-linked with genipin: Resilient hydrogels and self-standing electrospun scaffolds for tissue engineering applications. Biomater. Sci. 2019, 7, 76–91. [Google Scholar] [CrossRef]
- Nuansing, W.; Georgilis, E.; De Oliveira, T.V.A.G.; Charalambidis, G.; Eleta, A.; Coutsolelos, A.G.; Mitraki, A.; Bittner, A.M. Electrospinning of tetraphenylporphyrin compounds into wires. Part. Part. Syst. Charact. 2014, 31, 88–93. [Google Scholar] [CrossRef]
- Asenjo-Sanz, I.; Santos, J.I.; Bittner, A.M.; Pomposo, J.A.; Barroso-Bujans, F. Zwitterionic ring-opening polymerization for the facile, efficient and versatile grafting of functional polyethers onto graphene sheets. Eur. Polym. J. 2015, 73, 413–422. [Google Scholar] [CrossRef]
- Alonso, J.M.; Tatti, F.; Chuvilin, A.; Mam, K.; Ondarçuhu, T.; Bittner, A.M. The condensation of water on adsorbed viruses. Langmuir 2013, 29, 14580–14587. [Google Scholar] [CrossRef] [PubMed]
- Reich, G. Near-infrared spectroscopy and imaging: Basic principles and pharmaceutical applications. Adv. Drug Deliv. Rev. 2005, 57, 1109–1143. [Google Scholar] [CrossRef]
- Veras, F.F.; Ritter, A.C.; Roggia, I.; Pranke, P.; Pereira, C.N.; Brandelli, A. Natamycin-loaded electrospun poly(ε-caprolactone) nanofibers as an innovative platform for antifungal applications. SN Appl. Sci. 2020, 2, 1–14. [Google Scholar] [CrossRef]
- Yoshida, H.; Yanagisawa, K. Creation of superhydrophobic poly(L-phenylalanine) nonwovens by electrospinning. Polymers 2018, 10, 1212. [Google Scholar] [CrossRef] [Green Version]
- Brennan, D.A.; Conte, A.A.; Kanski, G.; Turkula, S.; Hu, X.; Kleiner, M.T.; Beachley, V. Mechanical Considerations for Electrospun Nanofibers in Tendon and Ligament Repair. Adv. Healthc. Mater. 2018, 7, 1–31. [Google Scholar] [CrossRef]
- Elsabee, M.Z.; Naguib, H.F.; Morsi, R.E. Chitosan based nanofibers, review. Mater. Sci. Eng. C 2012, 32, 1711–1726. [Google Scholar] [CrossRef]















| Peptide | Flow Rate (mL/h) | Concentration (wt.%) | Voltage (kV) |
|---|---|---|---|
| Phe-Tyr | 0.05 | 18%, 80/20:TFA/HFIP | 12 kV and 16 kV |
| Trp-Tyr | 0.1 | 18%, 30/70:TFA/HFIP | 12 kV and 16 kV |
| Tyr-Tyr | 0.02 | 18%, 70/30:TFA/HFIP | 12 kV and 16 kV |
| Acronym | Polypeptide |
|---|---|
| PGA | poly(l-glutamic acid) |
| PBG | poly(γ-benzyl-l-glutamate) |
| PLO | poly(L-ornithine) |
| PLEY | poly(L-glutamic acid4-co-L-tyrosine1) |
| γ-PGA | poly(γ-glutamic acid) |
| PBGA | poly(γ-benzyl-l-glutamate)-r-poly(l-glutamic acid) |
| PBGA20 | copolymer containing 20 mol% poly(l-glutamic acid) and 80 mol% poly(γ-benzyl-l-glutamate) |
| PBGA30 | copolymer containing 20 mol% poly(l-glutamic acid) and 80 mol% poly(γ-benzyl-l-glutamate) |
| PBGA20Na | Sodium salt of PBGA20 |
| Sequence | Solvent | Electrospinning Conditions | Comments | Ref. | |
|---|---|---|---|---|---|
| Short Peptides | Phe-Phe | HFIP | 17.5 wt.% solution 13.2 kV 13.8 cm distance from collector | Hydrophobic, crystalline fibers of infinite length and sub-micron diameter | [24] |
| Fmoc-Gly | >15 wt.% 10–30 kV 10–20 cm | Fibers of 100–300 nm in diameter | [23] | ||
| Fmoc-Phe-Gly | Long amorphous fibers composed of smaller needles. Diameter of the needles: 300–400 nm | [23,41] | |||
| Phe-Tyr | HFIP/TFA mixtures | 18 wt.% 12 and 16 kV 10–20 cm | Fibers of 50–200 nm average diameter | [25] | |
| Trp-Tyr | |||||
| Tyr-Tyr | |||||
| Amphiphilic Peptides | 1: Me(CH2)14-Glu2-Val3 | Water | 3 wt.% 10 kV 5 cm | Fibers of 3.8 μm wide composed of nanoribbons | [59] |
| 2: Me(CH2)14-Val3-Ala3-Glu3 | Fibers of 3.8 μm wide composed of cylindrical nanofibers | ||||
| Peptidomimetics | 3: tBuCO2-(pyrazole-isothiazole core)-Gly-Fmoc | HFIP | 30 wt.% 15 kV 15 cm | Continuous, fully filled fibers, 600 nm in diameter | [27] |
| Polypeptides | PLO | Water | 20–60% w/v 5–20 kV 5–15 cm | Fibers 0.5–1.5 μm in diameter | [74] |
| PLEY | Fibers 0.7–9 μm in diameter | [74] | |||
| γ-PGA modified with Phe | HFIP | 20 wt.% 20 kV 20 cm | Superhydrophobic Fibers 660 nm in diameter | [21] | |
| PGA | THF/DMAc mixture | 20 wt.% 20 kV | Fibers 1–2 μm in diameter | [82] | |
| PBG | |||||
| PBGA | |||||
| PBGA20 | |||||
| PBGA20Na | |||||
| PBGA30 | |||||
| Polypeptoids | PPGly (with PEG) | Methanol/Water mixture | 1.5 and 5 wt.%. of PEO (900 and 100 kDa, respectively) 10 wt.% PPGly 20 kV 25 cm | Fibers 680 nm in diameter, 520 nm after PEG annealing | [90] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bucci, R.; Georgilis, E.; Bittner, A.M.; Gelmi, M.L.; Clerici, F. Peptide-Based Electrospun Fibers: Current Status and Emerging Developments. Nanomaterials 2021, 11, 1262. https://doi.org/10.3390/nano11051262
Bucci R, Georgilis E, Bittner AM, Gelmi ML, Clerici F. Peptide-Based Electrospun Fibers: Current Status and Emerging Developments. Nanomaterials. 2021; 11(5):1262. https://doi.org/10.3390/nano11051262
Chicago/Turabian StyleBucci, Raffaella, Evangelos Georgilis, Alexander M. Bittner, Maria L. Gelmi, and Francesca Clerici. 2021. "Peptide-Based Electrospun Fibers: Current Status and Emerging Developments" Nanomaterials 11, no. 5: 1262. https://doi.org/10.3390/nano11051262